Abstract
After administration of the 99mTc complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester (99mTc-ECD), a brain perfusion imaging agent, the radioactive metabolite is trapped in primate brain, but not in mouse and rat. Here, we investigate the involvement of metabolite extrusion by organic anion transporter 3 (OAT3), which is highly expressed at the blood–brain barrier in mice, in this species difference. The efflux rate of radioactivity in the cerebrum of Oat3−/− mice at later phase was 20% of that of control mice. Thus, organic anion transporters in mouse brain would be involved in the low brain retention of radioactivity after 99mTc-ECD administration.
Keywords: blood–brain barrier, brain imaging, pharmacokinetics, SPECT
Introduction
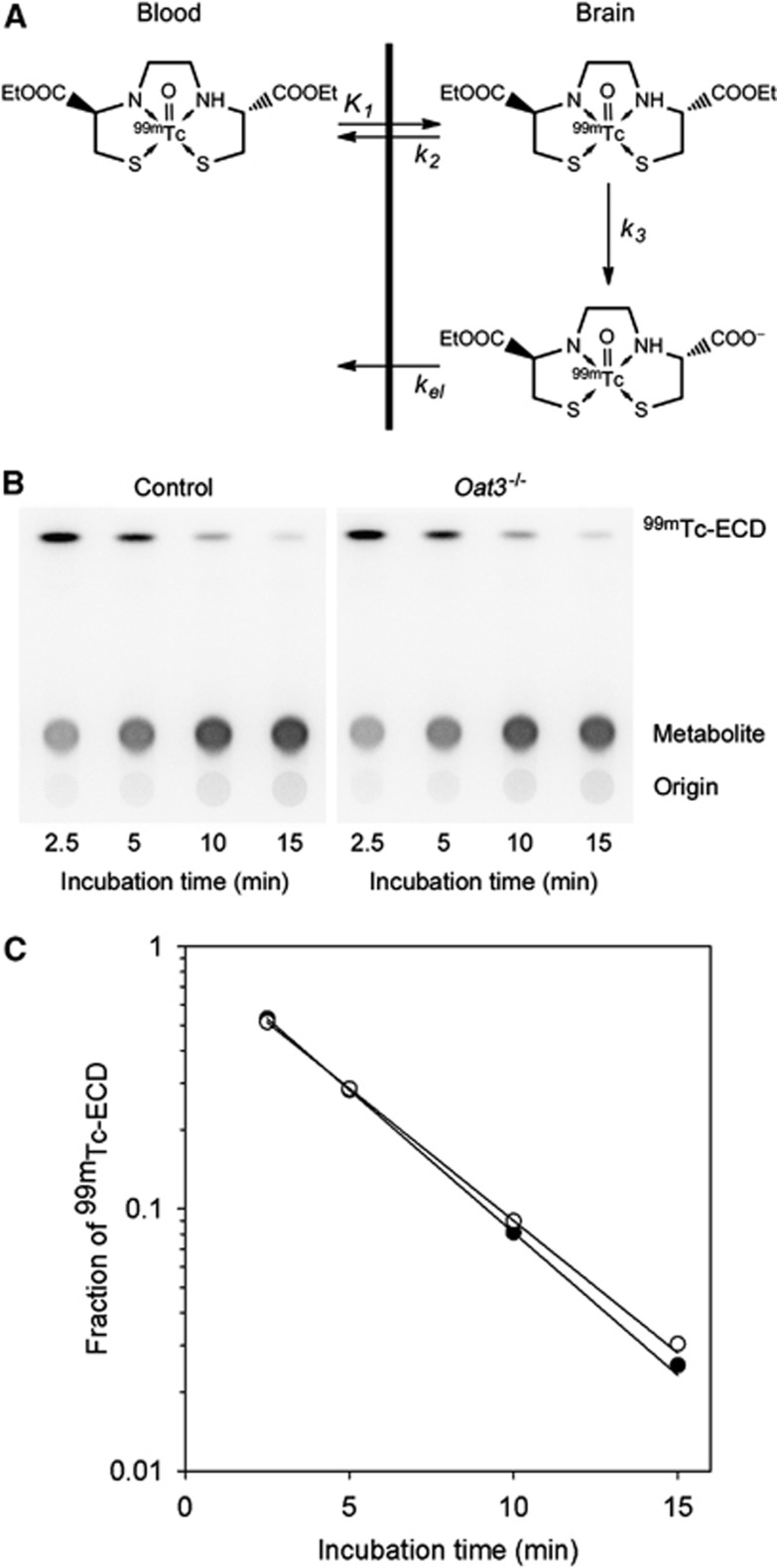
Technetium-99m complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester (99mTc-ECD) has been clinically used for brain perfusion imaging.1 Administrated 99mTc-ECD readily enters the brain with K1, a parameter that represents cerebral blood flow multiplied by extraction fraction (Figure 1A). Then, 99mTc-ECD in the brain diffuses back to the blood with k2 or it is metabolized to a monoacid form in the brain with k3.2, 3 Because k3 of 99mTc-ECD is much higher than k2, the distribution of radioactivity reflects regional cerebral blood flow in the brain regions in which the extraction fraction of 99mTc-ECD is equivalent and the elimination of metabolite (kel) is negligible. In primates, including humans, radioactivity in the brain is trapped for a long period after 99mTc-ECD administration, whereas radioactivity is not retained in the brain of mouse and rat.2, 3, 4, 5, 6, 7 The reason for this species difference has not been clarified, but is considered to be due to lower esterase activity in the brain of mouse and rat compared with the primates.7, 8 However, theoretically, the kinetics of radioactivity in the brain responds poorly to the reduction in metabolic rate (k3) under flow-limitation conditions (k2<<k3).9 Even if the kinetics of radioactivity in the brain responded to the reduction in metabolic rate, radioactivity would remain constant at a later phase after 99mTc-ECD injection under negligibly low kel conditions.
Figure 1.
The kinetics of 99mTc-ECD in the brain and the metabolic rate of 99mTc-ECD in mice brain homogenates. (A) The kinetics of 99mTc-ECD in the brain. (B) Typical analytical image of radioactivity on a thin-layer chromatography (TLC) plate in the measurement of metabolic rate (control, 0.243 g/mL; Oat3−/−, 0.237). (C) Time-dependent metabolism of 99mTc-ECD in mice brain homogenates shown in (B) (closed, control; open, Oat3−/−). Regression curves based on first-order kinetics are presented. 99mTc-ECD, 99mTc complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester; Oat3, organic anion transporter 3.
The reduction of radioactivity in the rat brain after 99mTc-ECD injection was inhibited by probenecid, an inhibitor of organic anion transporters.3 However, the extent of involvement of organic anion transporters in the extrusion of the 99mTc-ECD metabolite in the brain of mouse and rat has yet been unclear. We inferred from these considerations that the species difference in the retention of radioactivity may be attributed to the species difference in the expression of efflux transporters that extrude the 99mTc-ECD metabolite. The expression of transporters that extrude organic anions from brain to blood has been found in brain capillary endothelial cells. Among the transporters, organic anion transporter 3 (OAT3) is highly expressed in the endothelial cells in mice, in contrast to the low expression in the primate brain.10 Thus, we investigated the involvement of OAT3 in the efflux of 99mTc-ECD metabolite from mice brains using Oat3 knockout mice.
Materials and methods
Reagents
The injectable solution of 99mTc-ECD (Neurolite) was purchased from Fujifilm RI Pharma Co., Ltd. (Tokyo, Japan). Other chemicals were of reagent grade or better, and were available commercially.
Animals
The Oat3+/− mice were obtained from Deltagen, Inc. (San Mateo, CA, USA). The Oat3+/− mice and C57BL/6J were mated to increase the number of Oat3+/− mice, and the first generation of the homozygous mice (Oat3−/−) was generated from the pairs of Oat3+/− mice followed by breeding of the Oat3−/− mice in Charles River Laboratories Japan, Inc. (Kanagawa, Japan). After purchasing the male Oat3−/− mice, the mice were kept under conventional laboratory conditions of temperature, humidity, and light, and allowed free access to food and water in our institute, and mice (25 to 42 weeks) were used in this study (n=9 in total). The C57BL/6J mice were obtained from Charles River Laboratories Japan, Inc., and the male mice (10 to 16 weeks) were used as control mice (n=9 in total). The animals were treated and handled according to the publication ‘Recommendations for Handling of Laboratory Animals for Biomedical Research', compiled by the Committee on Safety and Ethical Handling Regulations for Laboratory Animal Experiments, National Institute of Radiological Sciences (Chiba, Japan), and this study was approved by the committee.
Metabolic Rate of 99mTc Complex with N,N'-1,2-Ethylenediylbis-L-Cysteine Diethyl Ester in Mice Cerebrum Homogenates
The metabolic rates of 99mTc-ECD in the homogenate of the cerebrum of control and Oat3−/− mice (n=3 each, 0.17 to 0.25 g/mL) were measured as follows: 0.5 mL of homogenate was placed in tubes and preincubated at 37°C for 30 minutes. The solution of 99mTc-ECD (10 μL, 2 MBq) was added to initiate the reaction. At 2.5, 5, 10, and 15 minutes after 99mTc-ECD addition, 50 μL of the reaction solution was added to ethanol (0.1 mL) to stop the reaction followed by centrifugation for 10 minutes at 10 000 × g. Then, 5 μL of the supernatant was applied to a silica gel thin-layer chromatography (TLC) plate (silica gel 60 F254; Merck Ltd., Tokyo, Japan), and the TLC plate was developed with a mixture of ethyl acetate/2-propanol/acetic acid (30/10/1). The TLC plate was covered with a 5-μm thick film and placed in a cassette in contact with the imaging phosphor plate for 60 minutes. Radioactivity was quantified using an imaging plate reader (BAS-5000; Fujifilm Corporation, Tokyo, Japan). The first-order metabolic rate was calculated using regression analysis based on the fraction of 99mTc-ECD at each time point.
Uptake and Chemical Form in Mice Cerebrums
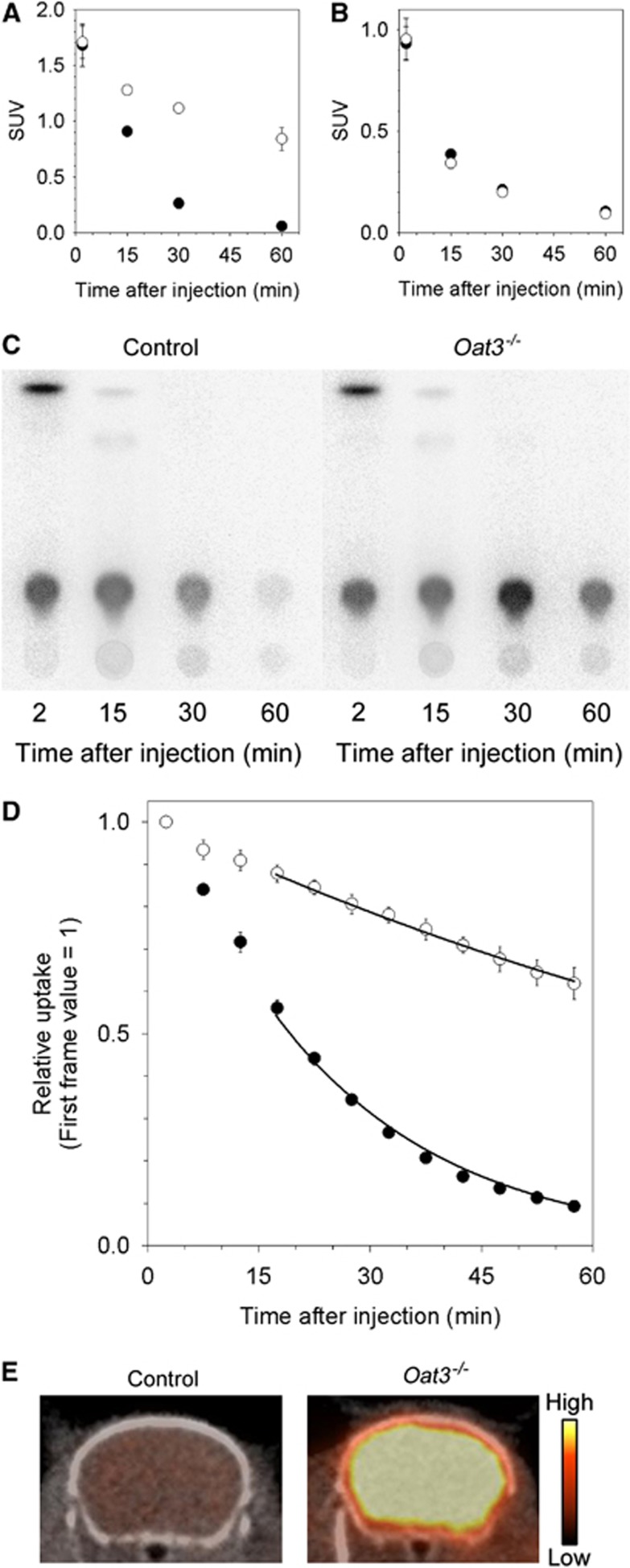
After intravenous administration of 99mTc-ECD (37 MBq) to controls and Oat3−/− mice (n=3 at each time point), they were decapitated at 2, 15, 30, and 60 minutes. The heads were immediately frozen in liquid nitrogen to stop further metabolism, and the frozen cerebrums were removed. The unilateral cerebrum was homogenized in ice-cooled ethanol (1 mL) followed by centrifugation at 10 000 × g for 10 minutes, and radioactivity in an aliquot of the supernatant was analyzed by TLC, as described above. The residual of the cerebrum was weighed followed by measurement of radioactivity using a gamma counter (Wizard, PerkinElmer Japan Co., Ltd., Kanagawa, Japan). Blood samples were also collected from the stump, and radioactivity in the blood was measured. The uptake values corrected for body weight (standardized uptake value: %ID/g tissue × g body weight/100) were used for analysis.
Single-Photon Emission Computed Tomography Study
Dynamic single-photon emission computed tomography (SPECT) was performed using VECTor/CT (MILabs BV, Utrecht, The Netherlands) with a rat collimator. The control and Oat3−/− mice (n=3 each) were anesthetized with 1.7% isoflurane, and body temperature was maintained within the normal range using a heating system equipped with the SPECT/computed tomography (CT) system. After the intravenous injection of 99mTc-ECD (∼37 MBq/100 μL) to control and Oat3−/− mice, the SPECT images of the brain were acquired every 5 minutes for 60 minutes. Without attenuation correction, the data were reconstructed using pixel-based ordered-subset expectation maximization with 16 subsets and 6 iterations on a 0.8-mm voxel grid.
Volumes of interest of the cerebrum were placed on the region with >60% of the maximum count in a coronal view of the summed SPECT images and transferred to all frames of the images to generate time-activity curves for cerebrum. X-ray CT imaging was performed immediately after SPECT imaging. Calibration of detection efficiency was not performed.
Statistical Analysis
The statistical analysis of the metabolic rate was performed using Student's t-test. The analysis of time-activity curves was performed using two-way repeated measures analysis of variance. The uptake values of control and Oat3−/− mice at each time point were compared using the Holm–Sidak method. The significance level was set at P<0.01.
Results
Metabolic Rate of 99mTc Complex with N,N'-1,2-Ethylenediylbis-L-Cysteine Diethyl Ester in Mice Cerebrum Homogenates
Radioactivity corresponding to 99mTc-ECD was decreased and radioactivity of a single metabolite was increased as the incubation time increased (Figure 1B). 99mTc complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester was metabolized in a first-order manner (r2>0.99) in mice cerebrum homogenates (Figure 1C). No significant difference was found in the first-order rate of cerebrum homogenates between control (mean±s.d., 1.01±0.03 minutes/g per mL) and Oat3−/− mice (1.03±0.06 minutes/g per mL) (P=0.67).
Uptake and Chemical Form in Mice Cerebrums
The initial uptake value (standardized uptake value at 2 minutes after injection) in the cerebrum of control mice (mean±s.d., 1.45±0.11) was not different compared with Oat3−/− mice (1.49±0.08, P=0.73). Thereafter, radioactivity in the cerebrum of control mice was significantly lower compared with Oat3−/− mice (P<0.01) (Figure 2A). No difference in the kinetics of radioactivity in the blood was observed between control and Oat3−/− mice (P=0.69) (Figure 2B). 99mTc complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester observed in the cerebrum at 2 minutes after injection was scarcely observed at 15 minutes after injection (Figure 2C). Radioactivity in the cerebrum after 15 minutes postinjection almost entirely consisted of a single metabolite.
Figure 2.
Uptake and chemical forms after 99mTc-ECD injection. Uptake in (A) the cerebrum and (B) the blood (closed, control; open, Oat3−/−). (C) Typical analytical images of radioactivity on a thin-layer chromatography (TLC) plate in the chemical form analysis. (D) Time-activity curves of the cerebrum measured using single-photon emission computed tomography (SPECT) (closed, control; open, Oat3−/−). Regression curves based on first-order kinetics during 15 to 60 minutes are presented. (E) Typical summed SPECT/computed tomography (CT) images (0 to 60 minutes) of the cerebrum. The color bar represents relative radioactivity ranging 0% to 60% of the maximum value in the corresponding first frame. 99mTc-ECD, 99mTc complex with N,N'-1,2-ethylenediylbis-L-cysteine diethyl ester; Oat3, organic anion transporter 3; SUV, standardized uptake value.
Single-Photon Emission Computed Tomography Study in Mice
The time-activity curves and SPECT images after 99mTc-ECD injection into control and Oat3−/− mice are shown in Figures 2D and 2E. Radioactivity in the cerebrum rapidly decreased after initial uptake in control mice, while the reduction in Oat3−/− mice was gradual (P<0.01). Radioactivity in the cerebrums of control and Oat3−/− mice was reduced in a first-order manner (r2>0.99) after 15 minutes postinjection. The first-order rate (mean±s.d.) in the cerebrums of control and Oat3−/− mice was 0.046±0.00067/min and 0.0089±0.0012/min (∼20% of the rate in control mice), respectively.
Discussion
The reduction of radioactivity in the mice cerebrums after 15 minutes postinjection of 99mTc-ECD predominantly reflects the extrusion of the monoacid metabolite from the cerebrum. Because the hydrolysis rate and the initial uptake in the cerebrum and the kinetics of radioactivity in the blood were comparable between control and Oat3−/− mice after 99mTc-ECD injection, the difference in the kinetics of radioactivity in the cerebrum between control and Oat3−/− mice was considered to be due to a difference in the transporter mediated extrusion of the 99mTc-ECD metabolite from the cerebrum. The half-lives of the reduction of radioactivity in the cerebrums of control mice calculated from the SPECT study (15.2±0.2 minutes) were in agreement with those reported by Vanbilloen et al6 (11.0 minutes) and Apostolova et al7 (24±7 minutes). The efflux rate in the cerebrums of Oat3−/− mice was ∼20% of that of control mice. The remaining efflux of the 99mTc-ECD metabolite from the cerebrums of Oat3−/− mice would be mediated by other transporters expressed in brain capillary endothelial cells, choroid plexus epithelial cells, and by bulk flow of extracellular fluid.10 In the route of efflux of the 99mTc-ECD metabolite via brain capillary endothelial cells, 99mTc-ECD metabolite generated in the brain parenchymal cells should sequentially pass through the membrane of parenchymal cells and the abluminal and luminal membranes of brain capillary endothelial cells to enter the blood stream. As OAT3 is localized in the abluminal membrane of brain capillary endothelial cells in mice,11 other transporters that mediate 99mTc-ECD metabolite extrusion likely exist in the membrane of brain parenchymal cells and the luminal membranes of brain capillary endothelial cells in mice. In rat, OAT3 is expressed on both luminal and abluminal membranes in the endothelial cells;12 therefore, other transporters that mediate 99mTc-ECD metabolite extrusion might exist in the membrane of brain parenchymal cells.
In addition to OAT3, other transporters that mediate transport of organic anions were also highly expressed in the brain capillary endothelial cells in mice, but not in primates.10 And, it was suggested that a portion of 99mTc-ECD could be hydrolyzed in the extracellular space in the human brain.13 Thus, the species difference in retention of radioactivity in the brain after 99mTc-ECD injection between mouse and human would be mainly attributable to either or both of the species differences in the expression level of the organic anion transporters in brain capillary endothelial cells and the substrate recognition of the transporters, rather than a species difference in the hydrolysis rate of 99mTc-ECD in the brain. However, if the 99mTc-ECD metabolite is predominantly generated in the brain parenchymal cells in mouse and primates and it is retained in the cells in primates, organic anion transporters expressed in the cells of mouse brain would be involved in the species difference.
Organic anion transporter 3 mediates the extrusion of anionic substances from the brain in mouse and rat.14, 15 However, the present study showed that an organic anion that is not extruded from the primate brain is extruded by OAT3 from the mouse brain. Thus, further studies are required to determine the transporters involved in regulating the kinetics of organic anions in the primate brain.
Conclusion
Organic anion transporter 3 mediated the extrusion of the 99mTc-ECD metabolite in the mouse brain. The retention of radioactivity for a long period in the human brain after 99mTc-ECD administration would be due to the absence of an efflux transporter in either or both of brain capillary endothelial cells and brain parenchymal cells that mediates the extrusion of the 99mTc-ECD metabolite in mouse.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by the Takeda Science Foundation and JSPS KAKENHI (24591826).
References
- Kapucu ÖL, Nobili F, Varrone A, Booij J, Vander Borght T, Någren K, Darcourt J, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2093–2102. doi: 10.1007/s00259-009-1266-y. [DOI] [PubMed] [Google Scholar]
- Walovitch RC, Franceschi M, Picard M, Cheesman EH, Hall KM, Makuch J, et al. Metabolism of 99mTc-L,L-Ethyl cysteinate dimer in healthy volunteers. Neuropharmacology. 1991;30:283–292. doi: 10.1016/0028-3908(91)90156-6. [DOI] [PubMed] [Google Scholar]
- Walovitch RC, Cheesman EH, Maheu LJ, Hall KM. Studies of the retention mechanism of the brain perfusion imaging agent 99mTc-bicisate (99mTc-ECD) J Cereb Blood Flow Metab. 1994;14 (Suppl 1:S4–S11. [PubMed] [Google Scholar]
- Holman BL, Hellman RS, Goldsmith SJ, Mena IG, Leveille J, Gherardi PG, et al. Biodistribution, dosimetry, and clinical evaluation of technetium-99m ethyl cysteinate dimer in normal subjects and in patients with chronic cerebral infarction. J Nucl Med. 1989;30:1018–1024. [PubMed] [Google Scholar]
- Walovitch RC, Hill TC, Garrity ST, Cheesman EH, Burgess BA, O'Leary DH, et al. Characterization of technetium-99m-L,L-ECD for brain perfusion imaging, Part 1: Pharmacology of technetium-99m ECD in nonhuman primates. J Nucl Med. 1989;30:1892–1901. [PubMed] [Google Scholar]
- Vanbilloen HP, Cleynhens BJ, Verbruggen AM. Importance of the two ester functions for the brain retention of 99mTc-labelled ethylene dicysteine diethyl ester (99mTc-ECD) Nucl Med Biol. 1998;25:569–575. doi: 10.1016/s0969-8051(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Apostolova I, Wunder A, Dirnagl U, Michel R, Stemmer N, Lukas M, et al. Brain perfusion SPECT in the mouse: normal pattern according to gender and age. Neuroimage. 2012;63:1807–1817. doi: 10.1016/j.neuroimage.2012.08.038. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Momose T, Ohtake T, Nishikawa J, Sasaki Y, Waritani T, et al. Metabolism of technetium-99m- L,L-ethyl cysteinate dimer in rat and cynomolgus monkey tissue. J Nucl Med. 1997;38:1731–1737. [PubMed] [Google Scholar]
- Koeppe RA, Frey KA, Mulholland GK, Kilbourn MR, Buck A, Lee KS, et al. [11C]Tropanyl benzilate-binding to muscarinic cholinergic receptors: methodology and kinetic modeling alternatives. J Cereb Blood Flow Metab. 1994;14:85–99. doi: 10.1038/jcbfm.1994.13. [DOI] [PubMed] [Google Scholar]
- Deo AK, Theil FP, Nicolas JM. Confounding parameters in preclinical assessment of blood-brain barrier permeation: an overview with emphasis on species differences and effect of disease states. Mol Pharm. 2013;10:1581–1595. doi: 10.1021/mp300570z. [DOI] [PubMed] [Google Scholar]
- Jacquier-Sarlin MR, Polla BS, Slosman DO. Cellular basis of ECD brain retention. J Nucl Med. 1996;37:1694–1697. [PubMed] [Google Scholar]
- Ohtsuki S, Kikkawa T, Mori S, Hori S, Takanaga H, Otagiri M, et al. Mouse reduced in osteosclerosis transporter functions as an organic anion transporter 3 and is localized at abluminal membrane of blood-brain barrier. J Pharmacol Exp Ther. 2004;309:1273–1281. doi: 10.1124/jpet.103.063370. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Sugiyama D, Sugiyama Y. Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood-brain barrier. J Pharmacol Exp Ther. 2003;306:51–58. doi: 10.1124/jpet.103.049197. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, et al. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83:57–66. doi: 10.1046/j.1471-4159.2002.01108.x. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos. 2011;39:814–819. doi: 10.1124/dmd.110.036863. [DOI] [PubMed] [Google Scholar]