Abstract
Cerebral hypoxia and subsequent reoxygenation stress (H/R) is a component of several diseases. One approach that may enable neural tissue rescue after H/R is central nervous system (CNS) delivery of drugs with brain protective effects such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (i.e., statins). Our present in vivo data show that atorvastatin, a commonly prescribed statin, attenuates poly (ADP-ribose) polymerase (PARP) cleavage in the brain after H/R, suggesting neuroprotective efficacy. However, atorvastatin use as a CNS therapeutic is limited by poor blood–brain barrier (BBB) penetration. Therefore, we examined regulation and functional expression of the known statin transporter organic anion transporting polypeptide 1a4 (Oatp1a4) at the BBB under H/R conditions. In rat brain microvessels, H/R (6% O2, 60 minutes followed by 21% O2, 10 minutes) increased Oatp1a4 expression. Brain uptake of taurocholate (i.e., Oap1a4 probe substrate) and atorvastatin were reduced by Oatp inhibitors (i.e., estrone-3-sulfate and fexofenadine), suggesting involvement of Oatp1a4 in brain drug delivery. Pharmacological inhibition of transforming growth factor-β (TGF-β)/activin receptor-like kinase 5 (ALK5) signaling with the selective inhibitor SB431542 increased Oatp1a4 functional expression, suggesting a role for TGF-β/ALK5 signaling in Oatp1a4 regulation. Taken together, our novel data show that targeting an endogenous BBB drug uptake transporter (i.e., Oatp1a4) may be a viable approach for optimizing CNS drug delivery for treatment of diseases with an H/R component.
Keywords: blood–brain barrier, drug transport, hypoxia/reoxygenation, Oatp1a4, Statins, TGF-β signaling
Introduction
Cerebral hypoxia and subsequent reoxygenation (H/R) stress is a central component of several diseases, including traumatic brain injury, high altitude cerebral edema, acute mountain sickness, acute respiratory syndrome, obstructive sleep apnea, cardiac arrest, and ischemic stroke.1 It is directly associated with neuronal apoptosis, a proteolytic cascade characterized by cytochrome c release, caspase-3 activation, and internucleosomal DNA fragmentation.2 As neuronal cell damage transpires during H/R, neuroinflammation and apoptosis become more prevalent and dramatically affect brain tissue viability.1 Such pathophysiologic processes include activation of poly (ADP-ribose) polymerase (PARP), a family of nuclear enzymes involved in DNA repair, programmed cell death, and necrotic tissue damage. In vivo, hypoxic-ischemic insult and/or H/R stress results in increased PARP cleavage in the brain.3, 4 Indeed, genetic deletion of PARP has protected against in vivo DNA damage associated with ischemia-reperfusion injury, neuroinflammatory stress, and glutamate excitotoxicity.5 Taken together, these observations indicate that PARP cleavage is a reliable and sensitive early marker of central nervous system (CNS) damage and/or neuronal stress after H/R insult.
The critical need for novel therapeutic approaches to treat diseases with an H/R component is best illustrated by ischemic stroke. Stroke is the third most common cause of death and the number one cause of long-term morbidity in the United States.6 Ischemic stroke, which occurs due to restricted blood flow and oxygen supply to the brain, causes an irreversibly damaged ischemic core and salvageable surrounding tissue known as the penumbra. The primary goal of drug therapy for acute ischemic stroke is to salvage the penumbra as much as possible and as early as possible.7 Currently, only a single therapeutic agent is approved by the US Food and Drug Administration for acute ischemic stroke treatment: recombinant tissue plasminogen activator.1 The primary goal of recombinant tissue plasminogen activator therapy is to restore blood flow and oxygen supply to ischemic brain tissue; however, most cellular damage to the brain occurs when cerebral perfusion is reestablished (i.e., reoxygenation). Therefore, there is a critical need in stroke therapy for therapeutics that can be delivered to the brain for ‘rescue' of salvageable neural tissue. Currently, there is considerable interest in CNS protective properties of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (i.e., statins). Recent evidence suggests that statins can act as free-radical scavengers independent of their well-documented effects on cholesterol biosynthesis.8 The ability of statins to be effective neurotherapeutics after cerebral hypoxia requires efficient and precise CNS delivery. A recent comparative in vitro study that evaluated efficacy of statins as neuroprotectants by assessing their chemical structure, theoretical lipophilicity, and ability to protect against neuronal death induced by okadaic acid concluded that both atorvastatin and rosuvastatin were effective in mitigating neuron cell death.9 However, both these drugs had permeability values close to zero and blood–brain barrier (BBB) penetration estimates of <5%.9 This study illustrates the critical importance of identifying and characterizing endogenous transport mechanisms that can be targeted to facilitate CNS statin delivery.
One family of transporters that may have utility in brain delivery of statins is the organic anion transporting polypeptides (OATPs in humans and Oatps in rodents). OATPs/Oatps are a group of sodium-independent transporters classified within the larger solute carrier superfamily.1 In rodent brain, expression of organic anion transporting polypeptide 1a4 (Oatp1a4), Oatp1c1, and Oatp2a1 has been reported in capillary enriched fractions, capillary endothelial cells, and brain microvessels.10, 11, 12 Oatp1c1 primarily transports thyroxine and conjugated sterols11 while Oatp2a1 regulates BBB transport of prostaglandins.12 In contrast, Oatp1a4 is the primary drug transporting Oatp isoform expressed at the rat BBB.1 Studies in Oatp1a4(−/−) mice have shown reduced blood-to-brain transport of pitavastatin and rosuvastatin as compared with wild-type controls, which indicates involvement of Oatp1a4 in statin transport across the BBB.13 The human ortholog of Oatp1a4 is OATP1A2, which exhibits enrichment of mRNA expression in brain as compared with other tissues including liver, kidney, and gastrointestinal tract.14, 15 Immunofluorescence staining of human brain frontal cortex showed OATP1A2 localization at both the apical and basolateral sides of the microvascular endothelium.16 Although not directly studied at the BBB, OATP1A2 has been shown to transport rosuvastatin in isolated human hepatocytes and atorvastatin in human embryonic kidney cells (HEK293) stably transfected with OATP1A2.17, 18 Using our in vivo model of pain/inflammation, we have shown that Oatp1a4 is a BBB transporter that can be exploited to optimize CNS delivery of opioid peptide drugs;19 however, Oatp1a4-mediated delivery of statins across the brain microvascular endothelium under conditions of H/R has not been clearly elucidated.
Although pathophysiologic stressors can modulate endogenous BBB transporters, such changes must be effectively controlled to provide optimal CNS drug delivery. For example, studies in our in vivo inflammatory pain model showed increased BBB functional expression of Oatp1a4 only between 1 hour and 6 hours after induction of pain/inflammation.19 Therefore, if Oatp1a4 is to be utilized for effective delivery of therapeutics (i.e., statins) for treatment of diseases with an H/R component, its functional expression must be controlled over a more desirable time course than is possible by only relying on pathophysiologic processes. This objective can be accomplished by pharmacological targeting of signaling pathways that regulate Oatp1a4 such as the transforming growth factor-β (TGF-β) system.19 TGF-βs are cytokines that signal by binding to a heterotetrameric complex of type I and type II serine/threonine kinase receptors.20 The type I receptors, also known as activin receptor-like kinases (ALKs), propagate intracellular signals through phosphorylation of receptor-specific Smad proteins (i.e., receptor-regulated (R)-Smads). Phosphorylated (R)-Smads form complexes with the common Smad (i.e., Smad4), enabling nuclear translocation and subsequent changes in target gene transcription. At the BBB, only two ALK receptors (i.e., ALK1 and ALK5) have been identified.21 We have previously shown that pharmacological inhibition of TGF-β/ALK5 signaling can increase Oatp1a4 functional expression.19 Therefore, targeting of TGF-β/ALK5 signaling during H/R provides an opportunity to control Oatp1a4 expression/activity at the BBB for optimization of CNS drug delivery.
In the present study, we show that atorvastatin, a commonly prescribed statin, may have utility as a therapeutic for treatment of diseases with an H/R component. We show for the first time that increased Oatp1a4 functional expression occurs after an H/R insult. Furthermore, this increase in transport activity can be used to increase both brain delivery and neuroprotective efficacy of atorvastatin. We also provide evidence indicating that pharmacological targeting of the TGF-β-ALK5 signaling pathway is a potential mechanism that can be targeted for control of CNS drug delivery in animals subjected to H/R.
Materials and methods
Materials
[3H]Taurocholic acid (4.6 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA, USA). [3H]Atorvastatin (10 Ci/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St Louis, MO, USA). Rabbit polyclonal antibodies against Oatp1a4 were purchased from Millipore (anti-Oatp2; Temecula, CA, USA) and Santa Cruz Biotechnology, Inc. (M-50; Santa Cruz, CA, USA), respectively. The rabbit monoclonal anti-PARP antibody was obtained from Abcam (Cambridge, MA, USA). Atorvastatin calcium salt trihydrate, dimethyl sulfoxide (DMSO), estrone-3-sulfate potassium salt (E3S), fexofenadine (FEX), the selective ALK5 inhibitor SB431542, and sodium pentobarbital were all purchased from Sigma-Aldrich (St Louis, MO, USA).
Animals and Treatments
All animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines. Female Sprague-Dawley rats (200 to 250 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN, USA), housed under standard 12 hours light/12 hours dark conditions, and provided with food and water ad libitum. Animals were randomly assigned to each treatment group. Animals were subjected to hypoxic insult (i.e., 6% O2) for 1 hour as previously described.22 Rats were then subjected to reoxygenation (i.e., 21% O2) for 10 minutes, 30 minutes, 1 hour, 2 hours, 6 hours, or 24 hours. Animals subjected to H/R were compared with animals subjected to hypoxic insult only and with normoxic controls. For assessment of atorvastatin effects on PARP cleavage, atorvastatin calcium salt trihydrate was dissolved in 100% DMSO and was administered (20 mg/kg (1.0 mL/kg), intraperitoneally) 1 hour before hypoxic treatment, a time point that allowed atorvastatin to reach Tmax immediately before onset of hypoxia.23 To determine whether Oatp-mediated transport to the brain is critical for the effects of atorvastatin on PARP cleavage, we administered the known Oatp transport inhibitor E3S (2.5 mg/kg (1.0 mL/kg), dissolved in 100% DMSO, intraperitoneally) 30 minutes before atorvastatin administration. For experiments designed to evaluate involvement of ALK5-mediated signaling in regulation of Oatp1a4 functional expression, SB431542 (1.5 mg/kg (1.0 mL/kg), intraperitoneally), a selective ALK5 inhibitor, was dissolved in 100% DMSO and administered 30 minutes before initiation of hypoxia.
Rat Brain Microvessel Membrane Isolation
Brain microvessels were harvested as previously described by our laboratory.19, 21 After anesthesia with sodium pentobarbital (64.8 mg/mL (1.0 mL/kg), intraperitoneally), rats were decapitated and brains were removed. Meninges and choroid plexus were excised and cerebral hemispheres were homogenized in 4 mL of microvessel isolation buffer (103 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 15 mmol/L HEPES, pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich). After homogenization, 8 mL of 26% dextran at 4°C was added and homogenates were vortexed. Homogenates were then centrifuged (5,600 g; 4°C) for 10 minutes and the supernatant was aspirated. Pellets were resuspended in 10 mL of microvessel isolation buffer and passed through a 70-μm filter (Becton Dickinson, Franklin Lakes, NJ, USA). Filtered homogenates were pelleted by centrifugation at 3,000 g for 10 minutes. At this time, the supernatant was aspirated and the pellet was collected for use in western blot analyses or for preparation of plasma membrane isolates as described by our group.24 Plasma membrane preparations were obtained by resuspending the pellet in a modified radioimmunoprecipitation buffer consisting of 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1.0 mmol/L EGTA, 1.0 mmol/L sodium o-vanadate, 1% (v/v) Nonidet P-40, 0.25% (m/v) sodium deoxycholate, 0.1% (m/v) SDS, 200 μmol/L phenylmethylsulfonyl fluoride, and 0.1% protease inhibitor cocktail (Sigma-Aldrich). Samples were gently rocked for 15 minutes at 4°C to allow lysis to occur. Lysates were then centrifuged (3,000 g; 4°C) for 10 minutes and supernatants were collected. Denucleated supernatants were then centrifuged at 100,000 g (4°C) for 60 minutes. Pellets (i.e., plasma membranes) were resuspended in phosphate-buffered saline containing protease inhibitor cocktail and frozen at −20°C until use.
Western Blot Analysis
Rat brain microvessel samples were quantified for total protein using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL, USA) and analyzed for expression of PARP or Oatp1a4. Microvessel protein samples (10 μg) were resolved on 4% to 12% SDS-polyacrylamide gels (Bis-Tris Criterion XT; Bio-Rad, Hercules, CA, USA) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were incubated in Superblock (Pierce Biotechnology) containing 0.05% (v/v) Tween-20 for 1 hour at room temperature. Membranes were then incubated with primary antibody directed against PARP (1:1,000 dilution) or Oatp1a4 (anti-Oatp2, 1:1,000 dilution and M-50, 1:2,000 dilution) overnight at 4°C. The membranes were then washed in TBS-T ((15 mmol/L Tris-HCl and 150 mmol/L NaCl pH 7.6 containing 0.05% (v/v) Tween-20): (6 × 15 minutes)) and incubated with anti-rabbit IgG conjugated to horseradish peroxidase for 1 hour at room temperature. Membranes were developed using enhanced chemiluminescence (ECL, Amersham, Piscataway, NJ, USA) and were stained for total protein with Ponceau S. The optical density of each band was normalized to total protein in each sample (i.e., loading control) according to a previously published method.25 Ponceau S is a fast and fully reversible stain that, when applied and quantified before antibody staining, has been validated as an alternative means to immunoblotting of individual housekeeping/structural proteins (i.e., actin and Na+/K+-ATPase) in assessment of equal protein loading in western blot analysis. Since experimental manipulations (i.e., hypoxic stress) have been shown to alter expression of housekeeping genes such as glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and cyclophilin,26, 27 we chose to use total protein as our loading control. Bands were quantitated and corrected for background using the ImageJ densitometric software (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD). All data were normalized to normoxic control values that were matched to treated animals from the same experimental day and are reported as a percent of control (%control).
In Situ Brain Perfusion
Animals were anesthetized with sodium pentobarbital (64.8 mg/mL, intraperitoneally) and heparinized (10,000 U/kg, intraperitoneally). Body temperature was maintained at 37°C using a heating pad. The common carotid arteries were cannulated with silicone tubing connected to a perfusion circuit. The perfusate was an erythrocyte-free modified mammalian Ringer's solution consisting of 117 mmol/L NaCl, 4.7 mmol/L KCl, 0.8 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 10 mmol/L D-glucose, 3.9% (w/v) dextran (MW 60,000), and 1.0 g/L bovine serum albumin (type IV), pH 7.4, warmed to 37°C and oxygenated with 95% O2/5% CO2. Evan's blue dye (55 mg/L) was added to the perfusate to serve as a visual marker of BBB integrity. Perfusion pressure and flow rate were maintained at 95 to 105 mm Hg and 3.1 mL/min, respectively. Both jugular veins were severed to allow for drainage of the perfusate. Using a slow-drive syringe pump (0.5 mL/min per hemisphere; Harvard Apparatus, Holliston, MA, USA), [3H]taurocholic acid (1.0 μCi/mL; 10 mmol/L total concentration) or [3H]atorvastatin (0.5 μCi/mL; 0.013 μmol/L total concentration) was added to the inflowing perfusate. After perfusion, the rat was decapitated and the brain was removed. Meninges and choroid plexus were excised and cerebral hemispheres were sectioned and homogenized. At this time, TS2 tissue solubilizer (1 mL) was added and each sample was allowed to solubilize for 2 days at room temperature. To eliminate chemiluminescence, 100 μL of 30% glacial acetic acid was added, along with 2 mL Optiphase SuperMix liquid scintillation cocktail (PerkinElmer, Boston, MA, USA). Samples were measured for radioactivity on a model 1450 liquid scintillation counter (PerkinElmer). For inhibition studies, animals were perfused with erythrocyte-free modified mammalian Ringer's solution containing a known Oatp inhibitor (i.e., 100 μmol/L E3S and 100 μmol/L FEX) for 10 minutes before perfusion with [3H]taurocholic acid or [3H]atorvastatin.
Results were reported as picomoles of radiolabeled drug per gram of brain tissue (C; pmol/g tissue), which is equal to the total amount of radioisotope in the brain (CBrain; d.p.m./g tissue) divided by the amount of radioisotope in the perfusate (CPerfusate; d.p.m./pmol): C=CBrain/CPerfusate. The brain vascular volume in rats has been previously shown to range between 6 and 9 μL/g brain tissue.28 Because brain tissue was processed immediately after perfusion with radiolabeled substrate, all uptake values required correction for brain vascular volume. This was accomplished by subtracting the average vascular volume (i.e., 8.0 μL/g brain tissue as calculated from data reported by Takasato and colleagues) from whole-brain uptake data obtained for [3H]taurocholic acid or [3H]atorvastatin.
[3H]Atorvastatin Stability Studies
Venous outflow samples were collected during in situ brain perfusion studies and were analyzed for atorvastatin content using high performance liquid chromatography. Atorvastatin was extracted from venous outflow samples and was briefly centrifuged at 2,000 g for 15 minutes (25°C) to remove any precipitate. Samples (100 μL) were loaded onto a reverse phase C-18 column (5 μm, Altex Ultrasphere-I.P., 15 cm × 4.6 mm i.d.) using a Rheodyne 7125 manual injector (Rohnert Park, CA, USA). Atorvastatin was eluted using a mobile phase composed of 0.06 mol/L KH2PO4, adjusted to pH 3.2 with hydrochloric acid, and acetonitrile (50:50, v/v) isocratically pumped (Perkin-Elmer series 200 LC Pump) at 1.0 mL/min (37°C) for 20 minutes. UV absorbance (246 nm) was detected by an SPD-6A UV spectrophotometric detector (Shimadzu, Kyoto, Japan) while radioactivity associated with eluted compounds was determined by an LB 509 radioflow detector (Berthold Technologies, Bad Wildbad, Germany). Peaks corresponding to atorvastatin were identified by injecting known standards of both [3H]atorvastatin and nonradiolabeled atorvastatin calcium salt trihydrate. Retention times for atorvastatin were 3.9 to 4.2 minutes. All data were acquired and analyzed using the PowerChrom software (eDAQ Inc., Colorado Springs, CO, USA). [3H]Atorvastatin stability within venous outflow samples was determined by the appearance of a peak at the correct retention time, as well as its ability to coelute at the same retention time as unlabeled atorvastatin calcium salt trihydrate (1 μg/μL) spiked with [3H]atorvastatin under identical conditions.
Blood Gas and Electrolyte Analysis
Blood was collected from the descending aorta (∼700 μL aliquots) via a safePICO arterial blood gas syringe (Radiometer, Copenhagen, Denmark) and immediately analyzed using an ABL 77 blood gas analyzer (Radiometer). Measurements of pH, pCO2, pO2, O2 saturation, HCO3− concentration, anion gap, ion concentration (Na+, K+, Ca2+, Cl−), and hematocrit were performed in normoxic control animals, after the 1-hour hypoxic exposure (Hx), or after 1 hour hypoxic exposure followed by 10 minutes reoxygenation (H/R). Blood draws for the hypoxic (Hx) animals were done inside the hypoxic chamber to prevent inadvertent reoxygenation.
Statistical Analysis
Western blot image analysis data are reported as mean±s.d. from at least three separate experiments where each treatment group consists of pooled microvessels or pooled whole-brain lysates from three individual animals. In situ brain perfusion data are reported as mean±s.d. from six individual animals per treatment group. Blood gas analyses data are reported as mean±s.d. from six individual animals per treatment group. To determine statistical significance between treatment groups in western blot experiments, Student's t-test was used for unpaired experimental data. To determine the significance of brain [3H]taurocholate or [3H]atorvastatin accumulation, a repeated measures ANOVA and post hoc multiple-comparison Bonferroni t-test were used. To determine the significance of blood gas and electrolyte measurements, a one-way ANOVA was conducted for each parameter followed by Tukey's post hoc test when appropriate. A value of P<0.05 was accepted as statistically significant.
Results
Hypoxia/Reoxygenation Modulates Blood Gas and Circulating Electrolyte Levels
Evaluation of blood chemistries (pO2, pCO2, electrolyte concentrations, etc.) was conducted to validate the degree of hypoxic insult. Arterial blood samples were collected from control (i.e., normoxic) animals, animals exposed to hypoxic stress, and H/R animals that had been reoxygenated for 10 minutes. Analysis of blood gases (Table 1) illustrates significant decreases in pH, pCO2, pO2, and sO2% in hypoxic animals. In H/R animals, these values were still suppressed but approached recovery by the 10-minute reoxygenation time point. Additionally, the bicarbonate ion concentration was decreased in both hypoxic and H/R animals, causing an elevation in the anion gap. No differences in free ion concentrations (Na+, K+, Ca2+, Cl−) were observed. The hematocrit (i.e., erythrocyte volume fraction) of the hypoxic animals was increased compared with the normoxic and H/R animals. Taken together, these data indicate that our global model of oxygen deprivation achieved an acute state of hypoxic acidosis that is recoverable, an observation that is supported by blood gas and electrolyte levels in H/R animals.
Table 1. Blood gas and electrolyte measurements from animals subjected to normoxia (Nx), hypoxia (Hx; 6% O2 for 1 hour) or H/R (6% O2 for 1 hour followed by Nx for 10 minutes).
| Normoxic | Hypoxic | H/R | |
|---|---|---|---|
| pH | 7.45±0.01 | 7.31±0.01** | 7.35±0.03* |
| pCO2, mm Hg | 37.0±2.65 | 29.3±0.58* | 39.0±2.0 |
| pO2, mm Hg | 102±4.6 | 5.0±3.0** | 115±5.5* |
| Hct, % | 40±3.0 | 46±2.0* | 41±1.0 |
| Na+, mmol/L | 140±0.58 | 138±1.0 | 139±0.58 |
| K+, mmol/L | 3.70±0.35 | 3.93±0.40 | 3.57±0.21 |
| Ca2+, mmol/L | 1.33±0.02 | 1.37±0.03 | 1.38±0.03 |
| Cl−, mmol/L | 109±1.5 | 106±1.5 | 109±1.0 |
| HCO3−, mmol/L | 25.1±1.59 | 14.3±0.61** | 20.8±2.08* |
| Anion gap | 8.90±0.89 | 20.9±1.12** | 13.1±2.06* |
| sO2, % | 98.1±0.31 | 3.60±2.56** | 98.2±0.29 |
H/R, hypoxia/reoxygenation.
Blood was collected from the descending aorta via heparinized syringe and immediately analyzed. Blood draws for the hypoxic (Hx) animals were done within the hypoxic chamber. Values are means±s.d. of six animals per treatment group.
*P<0.05, **P<0.01.
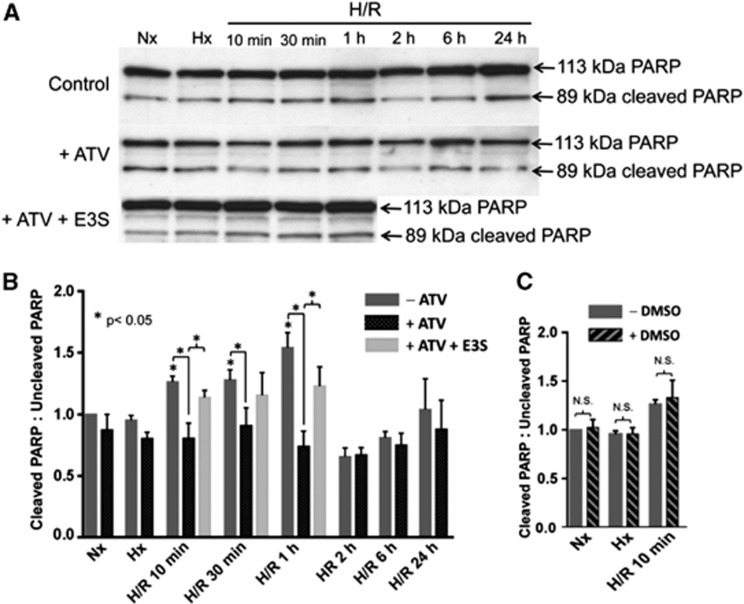
Atorvastatin Attenuates Increases in Cleaved Poly (ADP-ribose) Polymerase-to-Uncleaved Poly (ADP-ribose) Polymerase Ratio after Hypoxia/Reoxygenation Stress
The ratio of cleaved-to-uncleaved PARP is an established early indicator of end-stage cell death.29 PARP can be cleaved by executioner caspases (i.e., caspases-3 and -7), calpains, cathepsins, granzymes, and matrix metalloproteinases, which are all associated with various cellular death pathways (i.e., caspase-dependent apoptosis, caspase-independent apoptosis, production of pro-inflammatory mediators, and energy-failure-induced necrosis).5, 29 The molecular weights of resulting PARP fragments are indicative of participation of a specific protease. Consistent with previous findings,30 we found elevated cleaved-to-uncleaved PARP ratios in whole-brain lysates prepared from animals subjected to hypoxic insult (1 hour, 6% O2) and reoxygenated for 10 minutes, 30 minutes, and 1 hour as compared with these same ratios in brain lysates from normoxic animals (Figures 1A and 1B). The PARP fragments detected (i.e., 89 kDa) were consistent with those produced by executioner caspases.29 No significant change in cleaved PARP-to-uncleaved PARP ratios was identified in animals subjected to hypoxia only, or those subjected to H/R with reoxygenation times of 2, 6, or 24 hours. Atorvastatin treatment 1 hour before hypoxia challenge attenuated the H/R-induced increase in the cleaved-to-uncleaved PARP ratio at the 10-minute, 30-minutes, and 1-hour reoxygenation time points (Figures 1A and 1B). Administration of E3S reversed the atorvastatin-induced attenuation in PARP cleavage in the H/R 10 minutes and H/R 1 hour treatment groups (Figures 1A and 1B), which suggests that Oatp-mediated delivery of atorvastatin to the brain is required for these neuroprotective effects. For these experiments, we focused only on those time points where atorvastatin was shown to significantly affect PARP cleavage in the brain. Estrone-3-sulfate did not cause any significant changes in PARP cleavage in normoxic control animals or animals subjected to hypoxia only (Figure 1A). Since both atorvastatin and E3S were dissolved in 100% DMSO, we also measured the cleaved PARP-to-uncleaved PARP ratio in animals dosed with DMSO and subjected to H/R (H=6% O2, 1 hour; R=21% O2, 10 minutes) as well as in animals subjected to hypoxia only and in normoxic control animals. Figure 1C shows that DMSO did not cause any significant vehicle effect on the cleaved PARP-to-uncleaved PARP ratio in any of these treatment groups. Taken together, these data suggest that H/R treatment activates PARP-mediated cellular stress mechanisms, which can be attenuated by treatment with atorvastatin, a drug that is delivered to the brain via an Oatp-dependent transport mechanism.
Figure 1.
Atorvastatin (ATV) attenuates hypoxia/reoxygenation (H/R)-induced increase in ratio of cleaved PARP-to-uncleaved PARP. (A) Western blot analysis of whole-brain lysates isolated from normoxic (Nx), hypoxic (Hx), and H/R-treated rats dosed with ATV (20 mg/kg). Animals receiving ATV (20 mg/kg, intraperitoneally) were dosed 1 hour before hypoxia treatment (1 hour, 6% O2). Animals receiving the organic anion transporting polypeptide 1a4 (Oatp1a4) transport inhibitor estrone-3-sulfate (E3S; 2.5 mg/kg, intraperitoneally) were dosed 30 minutes before ATV administration. Brain samples (10 μg) were resolved on a 4% to 12% SDS-polyacrylamide gel, transferred onto a polyvinylidene difluoride (PVDF) membrane, and analyzed for expression of cleaved and uncleaved PARP. (B) Relative cleaved-to-uncleaved PARP ratios determined by densitometric analysis and expressed as fold change over control. Results are expressed as mean±s.d. of three separate experiments where each group consists of pooled whole-brain lysates from three individual animals. Asterisks represent data points that are significantly different from control. (C) Dimethyl sulfoxide (DMSO) vehicle control does not alter the ratio of cleaved PARP-to-uncleaved PARP in Nx, Hx, or H/R 10-minute treatments (NS, not significant). PARP, poly (ADP-ribose) polymerase.
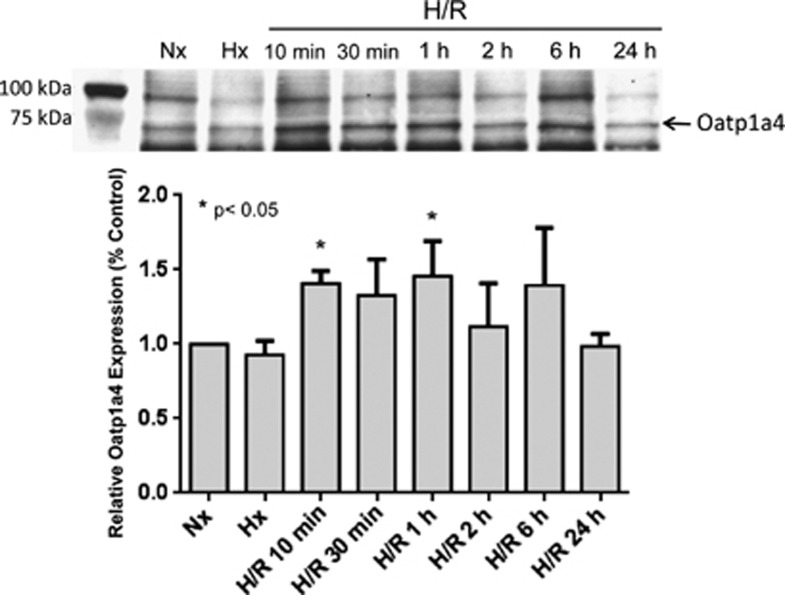
Hypoxia/Reoxygenation Stress Increases Organic Anion Transporting Polypeptide 1a4 Membrane Expression
Use of atorvastatin as a CNS therapeutic is limited by poor BBB permeation.9 Since our data show that atorvastatin exhibits protective effects in the brain, we sought to study a biologic mechanism that could be targeted to deliver atorvastatin to the CNS. One potential mechanism that could facilitate CNS statin delivery is Oatp1a4-mediated transport. To evaluate both BBB expression of Oatp1a4 and how this transporter is affected by H/R, we examined Oatp1a4 expression in plasma membrane fractions of rat brain microvessels by western blot analysis. H/R animals (H=1 hour, 6% O2; R=10 minutes, 30 minutes, 1 hour, 2 hours, 6 hours, or 24 hours, 21% O2) were compared with those subjected to hypoxic insult (1 hour, 6% O2) only and with normoxic control animals. Using the anti-Oatp2 antibody, western blot analysis of rat brain microvessel membrane fractions isolated from animals subjected to H/R revealed a significant increase in Oatp1a4 expression at reoxygenation time points of 10 minutes and 1 hour (Figure 2). No significant alteration in Oatp1a4 expression was detected in control animals and those subjected to hypoxic insult only. Similar results on Oatp1a4 expression in rat brain microvessels after H/R, hypoxia only, and in normoxic controls were obtained using the M-50 antibody (data not shown). The anti-Oatp2 antibody recognizes a 12 amino acid epitope near the C-terminus of Oatp1a4 while the M-50 antibody recognizes a different epitope corresponding to amino acids 611 to 660 on the Oatp1a4 protein sequence. All subsequent hypoxia/reoxygenation experiments were performed at the 10-minute time point because this was the time point where the earliest changes in Oatp1a4 expression induced by H/R were observed.
Figure 2.
Hypoxia/reoxygenation (H/R) increases expression of organic anion transporting polypeptide 1a4 (Oatp1a4) in brain microvessel membranes. Western blot analysis of brain microvessel plasma membrane preparations isolated from Hx and H/R-treated rats compared with normoxic control. Brain samples (10 μg) were resolved on a 4% to 12% SDS-polyacrylamide gel, transferred onto a polyvinylidene difluoride (PVDF) membrane, and analyzed for expression of Oatp1a4 (using the anti-Oatp2 polyclonal antibody). Relative levels of Oatp1a4 determined by densitometric analysis. Results are expressed as mean±s.d. of three separate experiments where each group consists of pooled microvessels from three individual animals. Asterisks represent data points that are significantly different from control.
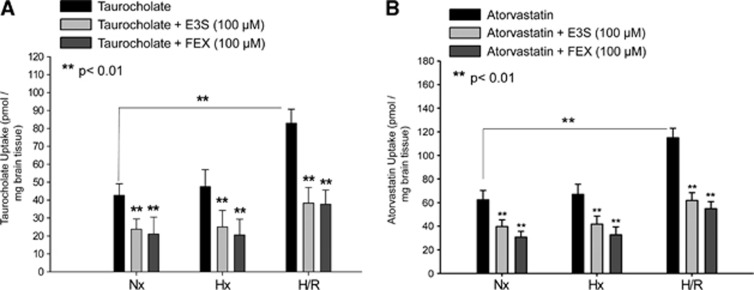
Hypoxia/Reoxygenation Increases Organic Anion Transporting Polypeptide 1a4-Mediated Taurocholate and Atorvastatin Transport
To determine whether the increase in membrane Oatp1a4 expression during H/R corresponded to altered Oatp1a4-mediated transport at the BBB, the in situ perfusion technique was utilized. Brain uptake of [3H]taurocholate, a soluble bile salt and established probe drug for Oatp-mediated transport, was evaluated in control animals, those exposed to hypoxic insult (1 hour, 6% O2) and those exposed to H/R. After a 10-minute perfusion, [3H]taurocholate (10 μmol/L) was significantly increased (2.0-fold) in the H/R treatment group as compared with normoxic and hypoxic treatment groups. To confirm that observed changes in [3H]taurocholate brain permeation were attributable to changes in Oatp-mediated transport, control animals and those subjected to hypoxia and H/R treatment were perfused in the presence and absence of known Oatp inhibitors (i.e., E3S and FEX) for 10 minutes before perfusion with [3H]taurocholate. Both E3S and FEX significantly decreased [3H]taurocholate accumulation in all treatment groups (Figure 3A), suggesting that H/R increases the blood-to-brain transport of taurocholate via an Oatp-dependent process. Using high performance liquid chromatography analysis of inflow and outflow perfusate, we have previously shown that [3H]taurocholate remains metabolically intact throughout our perfusion experiments.19
Figure 3.
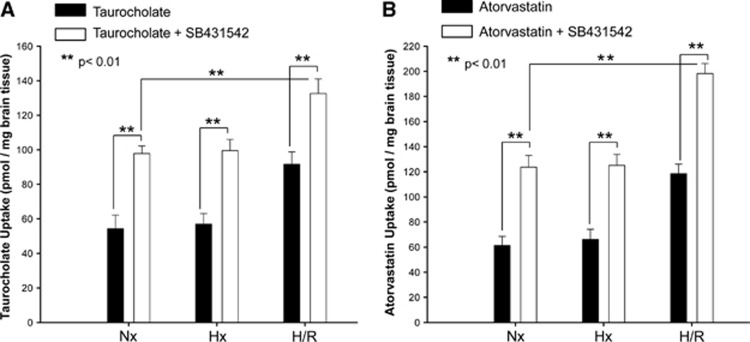
Increased brain uptake of organic anion transporting polypeptide (Oatp) substrates after hypoxia/reoxygenation (H/R). Uptake of taurocholate (A) and atorvastatin (B) is increased in rat brain after H/R as determined by in situ brain perfusion. Animals were perfused with [3H]taurocholic acid (1.0 μCi/mL) or [3H]atorvastatin (0.5 μCi/mL) for 10 minutes in the presence and absence of Oatp inhibitors (E3S=estrone-3-sulfate; FEX=fexofenadine) (H=6% O2 for 1 hour and R=21% O2 for 10 minutes). Perfusions including Oatp1a4 inhibitors (i.e., 100 μmol/L E3S and 100 μmol/L FEX) were done 10 minutes before perfusion with [3H]taurocholic acid or [3H]atorvastatin. Animals were then perfused with [3H]taurocholic acid (1.0 μCi/mL; 10 mmol/L total concentration) or [3H]atorvastatin (0.5 μCi/mL; 0.013 μmol/L total concentration). Nx=Normoxia; Hx=Hypoxia. Results are expressed as mean±s.d. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
To examine the relevance of this finding to delivery of a therapeutic drug, brain uptake of [3H]atorvastatin was examined in control animals, those exposed to hypoxic insult (1 hour, 6% O2), and in H/R animals. After a 10-minute perfusion, [3H]atorvastatin brain accumulation was significantly increased (1.7-fold) in the H/R treatment group as compared with both the normoxic and hypoxic treatment groups (Figure 3B). Similar to our data with taurocholate, perfusion in the presence of E3S or FEX reduced [3H]atorvastatin uptake in all treatment groups, suggesting an Oatp1a4 contribution to atorvastatin brain uptake. High performance liquid chromatography analysis of inflow and outflow perfusate revealed a single peak (RT=4 minutes) corresponding to atorvastatin retention, thereby confirming that atorvastatin remained metabolically intact throughout the course of our in situ perfusion experiments (data not shown). Brain accumulation of both [3H]taurocholate and [3H]atorvastatin in the presence of transport inhibitors E3S or FEX was slightly enhanced in the H/R treatment group compared with the normoxic treatment group; however, this increase was not statistically significant.
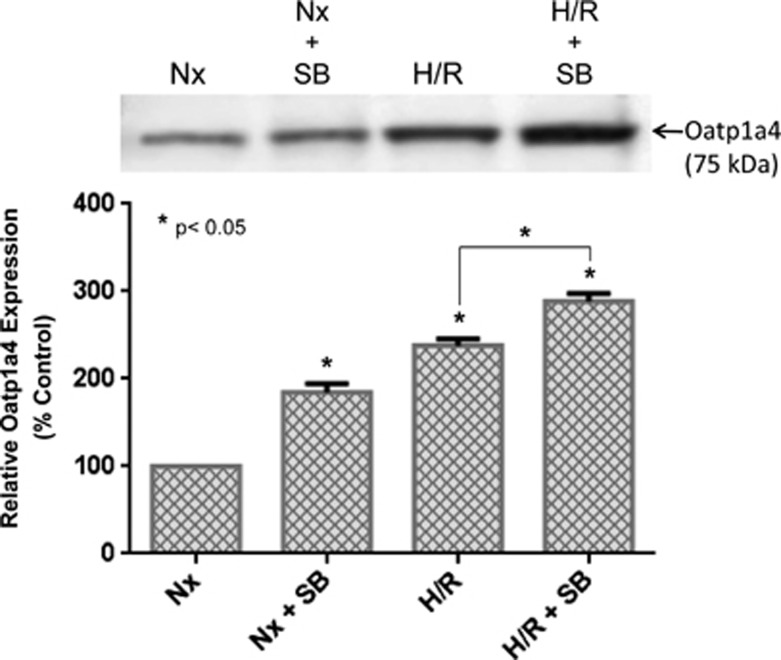
Effect of Transforming Growth Factor-β/Activin Receptor-Like Kinase 5 Signaling on Organic Anion Transporting Polypeptide 1a4 Functional Expression
Although the above data show that H/R alters Oatp1a4 functional expression, the biologic mechanism underlying these changes in our in vivo H/R model has not been elucidated. Our group has previously reported that Oatp1a4 is regulated by TGF-β/ALK5 signaling in an in vivo rodent model of peripheral inflammatory pain.19 Therefore, we hypothesized that pharmacological inhibition of TGF-β/ALK5 signaling may also control Oatp1a4 functional expression in the context of H/R. To investigate the role of TGF-β signaling in Oatp1a4 regulation after H/R, the highly specific ALK5 receptor inhibitor SB431542 (1.5 mg/kg) was administered 30 minutes before hypoxia. Our group has shown that this dose and dosing interval significantly blocks TGF-β/ALK5 signaling at the in vivo BBB.21 Administration of SB431542 before hypoxia treatment resulted in an increase in Oatp1a4 expression in whole rat brain microvessels as detected by the M-50 antibody (Figure 4). This increase in Oatp1a4 expression was observed in both normoxic controls and H/R animals, which further suggests that expression of the ‘influx' transporter Oatp1a4 at the BBB can be increased by targeting TGF-β/ALK5 signaling. Inhibition of the ALK5 receptor with SB431542 also resulted in enhanced [3H]taurocholate (Figure 5A) and [3H]atorvastatin (Figure 5B) brain uptake in normoxic animals, hypoxic animals and in animals subjected to H/R. Additionally, accumulation of [3H]taurocholate and [3H]atorvastatin in H/R rats treated with SB431542 was significantly increased as compared with normoxic animals administered SB431542 (Figure 5B), further emphasizing that TGF-β/ALK5 signaling is prominently involved in regulation of Oatp1a4 transport activity at the BBB.
Figure 4.
SB431542, a pharmacological transforming growth factor-β (TGF-β)/activin receptor-like kinase 5 (ALK5) inhibitor, increases organic anion transporting polypeptide 1a4 (Oatp1a4) expression in rats subjected to hypoxia/reoxygenation (H/R). Western blot analysis of rat brain microvessels isolated from H/R rats treated with SB431542 and compared with normoxic controls. Brain microvessel membrane samples (10 μg) were resolved on a 4% to 12% SDS-polyacrylamide gel, transferred onto a polyvinylidene difluoride (PVDF) membrane, and analyzed for expression of Oatp1a4 (using the M-50 polyclonal antibody). Nx=Normoxia; Hx=Hypoxia; SB=SB431542. Results are expressed as mean±s.d. of three separate experiments where each group consists of pooled microvessels from three individual animals. Asterisks represent data points that are significantly different from control.
Figure 5.
Uptake of taurocholate (A), a selective organic anion transporting polypeptide (Oatp) substrate, and atorvastatin (B) after hypoxia/reoxygenation (H/R), in hypoxic (Hx) animals, and in normoxic (Nx) controls in the presence and absence of SB431542, a pharmacological transforming growth factor-β (TGF-β)/activin receptor-like kinase 5 (ALK5) inhibitor. Results are expressed as mean±s.d. of six animals per treatment group. Animals were perfused with [3H]taurocholate (1.0 μCi/mL; 10 mmol/L total concentration) or [3H]atorvastatin (0.5 μCi/mL; 0.013 μmol/L total concentration) for 10 minutes. Nx=Normoxia; Hx=Hypoxia. Results are expressed as mean±s.d. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
Discussion
Our laboratory is focused on characterizing regulation and functional expression of Oatp1a4 under H/R conditions and determining its role in CNS drug delivery. A critical factor in translational applicability of this research objective is selection of an appropriate in vivo H/R model. For our studies, we have selected a nonocclusive global model of H/R where animals are exposed to an oxygen-depleted environment (i.e., 6% O2) for 1 hour, an insult that confers an acute, moderate to severe, hypoxic insult.31 This well-established model offers several advantages including (1) maintenance of interactions between BBB endothelium and other cell types/structures of the neurovascular unit (i.e., astrocytes, microglia, pericytes, neurons, and extracellular matrix); (2) preservation of interactions between the BBB and circulating systemic mediators; and (3) the degree of hypoxic stress does not induce necrotic damage of the BBB endothelium, which is often associated with other in vivo hypoxia/ischemia models. Therefore, our H/R model permits study of a dynamically regulated and recoverable BBB. Additionally, our model reduces cerebral oxygen availability without altering cerebral blood flow.32 This allows nutrients within the systemic circulation to reach the brain and reduces hydrostatic reperfusion pressures that can alter BBB integrity.32 The recoverable nature of our model is emphasized by our blood gas and electrolyte measurements (Table 1). This analysis reveals decreases in pH, pCO2, pO2, and sO2% in hypoxic animals. Of particular note, these parameters approach recovery by the 10-minute reoxygenation time point. The hematocrit of hypoxic animals was also increased as compared with the normoxic and reoxygenated animals, a physiologic compensatory response that typically occurs in an effort to increase oxygen carrying capacity under conditions of low blood-oxygen levels. Our global model of cerebral hypoxia has been used previously to study changes in BBB architecture and intracellular signaling after hypoxic insult.22, 32, 33, 34 However, it has not been used to examine regulation and functional expression of putative BBB drug transporters until the present study.
Cerebral hypoxia and subsequent reoxygenation is a central component of several CNS conditions.1 There are very few therapeutics with clinical utility for treatment of diseases with such a cerebral H/R component. One particularly intriguing class of drugs is statins, which have shown antioxidant and/or neuroprotective efficacy in several in vitro and in vivo studies.35, 36, 37, 38, 39 For example, statin treatment has reduced cerebral expression of oxidative stress markers such as nitrotyrosine and F2-isoprostanes, even in clinical investigations.35, 36 Studies in an in vivo rodent model of subarachnoid hemorrhage showed that atorvastatin reduced brain caspase-3 activity and DNA fragmentation, suggesting an ability to attenuate neuronal apoptosis.37, 38 Statin-induced neuroprotection has been reported in a concentration range between 100 nmol/L and 1 μmol/L within the CNS, while neuronal toxicity has only been reported at concentrations above 1 μmol/L.39 We therefore sought to determine whether pretreatment with the commonly prescribed statin atorvastatin could attenuate neuronal damage induced by H/R insult in our global oxygen deprivation model. We show for the first time that the ratio of cleaved PARP-to-uncleaved PARP is decreased after a single dose of atorvastatin (20 mg/kg). Poly (ADP-ribose) polymerase is a downstream target of caspase-3 and has a critical role in cellular stress signaling after a pathologic insult such as hypoxia. Poly (ADP-ribose) polymerase-1 is the dominant member of the PARP family and is critical in the detection and repair of damaged DNA. Its binding to specific DNA motifs such as single- and double-strand breaks, supercoils, cruciforms, and crossovers activates its catalytic domain. Activated PARP then utilizes NAD+ to poly (ADP-ribosyl)ate itself, other transcription-related factors (i.e., p53, nuclear factor-κB, activator protein 1, and E2F-1),5, 29 and DNA repair machinery. Neurons with extensive DNA damage, such as is observed during cerebral ischemia, will experience depletion of nuclear and cytosolic pools of NAD+ due to PARP-1 overactivation.5 To prevent energy failure-induced necrosis, activated caspases-3 and -7 will cleave PARP between aspartic acid 214 and glycine 215, yielding fragments of 24 and 89 kDa.29 This cleavage effectively terminates PARP's ability to initiate DNA repair, an event that causes DNA fragmentation and subsequent apoptosis. Therefore, pharmacological interventions that decrease PARP activation and cleavage in the brain are indicative of a potentially protective therapy that can attenuate neural apoptosis. Indeed, our data with atorvastatin imply that this drug can elicit protective effects in the brain after H/R stress as indicated by a reduction in the cleaved PARP-to-uncleaved PARP ratio. Furthermore, our data emphasize that the atorvastatin-induced neuroprotective effects are dependent on Oatp-mediated drug delivery.
If atorvastatin is to act efficaciously as a neurotherapeutic, then it must be able to effectively accumulate in brain parenchyma. That is, the utility of atorvastatin to treat diseases with an H/R component requires identification and characterization of a biologic mechanism that can facilitate its CNS delivery. Our in vivo data provide evidence for increased BBB functional expression of Oatp1a4, a known transporter for atorvastatin, in response to hypoxia/reoxygenation (H/R) stress. Increased expression and/or activity of an endogenous BBB drug uptake transporter has not been reported until the present study. This increase in Oatp1a4 expression at the plasma membrane of BBB endothelial cells may itself be a CNS protective mechanism, allowing for enhanced blood-to-brain delivery of endogenous substances that are known Oatp1a4 substrates, such as prostaglandin E1, which prevents neural apoptosis.40 It is important to consider that changes in Oatp1a4 expression at the BBB may result from altered transporter trafficking as opposed to de novo synthesis of new transporter protein. Previously, our group has shown, in vivo, that changes in functional expression of the drug efflux transporter P-glycoprotein at the BBB may result from relocation of this transporter from ‘storage pools' within cerebral microvascular endothelial cells in response to a pathologic insult.41 Furthermore, Oatp transporters that possess a consensus PDZ motif at their C-terminus (i.e., Oatp1a1) have been shown to exhibit subcellular trafficking properties in human embryonic kidney cells that were transfected with the transporter of interest.42 While Oatp1a4 lacks a consensus PDZ domain, we believe that protein trafficking is likely involved in regulating Oatp1a4 expression at the brain microvascular endothelium after an H/R insult. Studies to assess ‘pools' of ‘relocating Oatp1a4 transporter' in the context of H/R stress are currently being undertaken in our laboratory. In terms of pharmacotherapy, we have previously shown in vivo that Oatp1a4 can mediate blood-to-brain drug transport and therefore be a critical determinant of CNS accumulation of the opioid peptide, [D-penicillamine2,5]-enkephalin.19 Here, we expand on these critical findings by showing that Oatp1a4 is a key determinant of atorvastatin delivery to the brain, both in normoxic animals and after H/R stress. As previously described, Oatp1a4-mediated transport of statins has been shown in knock-out animals and in cell culture;13, 17, 18 however, we are the first to show changes in this transporter at the BBB after a pathophysiologic insult. Therefore, our present data indicate that this transport system may have considerable utility as a delivery target to treat diseases with an H/R component.
If Oatp1a4 is to be utilized for effective delivery of drugs (i.e., statins), then its functional expression must be precisely controlled over a more desirable time course than can be achieved by relying solely on pathophysiologic changes. We show that TGF-β/ALK5 signaling has a key role in the regulation of Oatp1a4. The observation that a highly selective ALK5 antagonist induces an increase in Oatp1a4 protein expression and transport activity implies that TGF-β/ALK5 signaling can be targeted for regulation of Oatp1a4 functional expression at the BBB. Our group has previously shown that SB431542 effectively blocks TGF-β/ALK5 signaling as demarcated by reduced nuclear expression of phosphorylated Smad2 and Smad3.21 Furthermore, we have shown that SB431542 does not affect TGF-β/ALK1 signaling, data that emphasize the specificity of this small molecule for the TGF-β/ALK5 signaling pathway.21 Our current study also identifies TGF-β/ALK5 signaling as a target for pharmacological manipulation of brain microvascular permeability to neuroprotective and/or antioxidant drugs for treatment of diseases with an H/R component. Targeting the TGF-β signaling pathway may enable ‘tighter control' of Oatp1a4 functional expression, thereby enabling increased delivery of drugs to the brain. A greater understanding of this pathway under H/R conditions will enable novel therapeutic strategies for improved treatment of diseases with an H/R component such as ischemic stroke. Such studies are presently being undertaken in our laboratory.
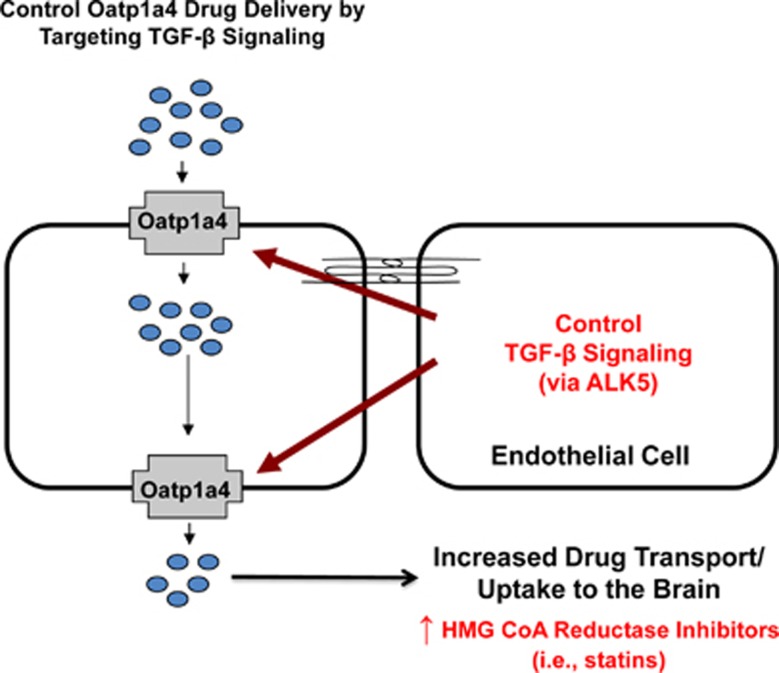
In summary, this study describes, in vivo, increased functional expression of Oatp1a4 during H/R stress. Our data also show that H/R can directly modulate specific BBB drug transporters and suggest involvement of TGF-β/ALK5 signaling in regulation of Oatp1a4 functional expression (Figure 6). Additionally, these observations imply that ALK5 may represent a novel target to control brain delivery of Oatp1a4 substrate drugs such as statins. Overall, these results indicate that changes in Oatp1a4 expression and/or activity can be exploited in an effort to enhance CNS delivery of statins, a class of therapeutics whose CNS utility requires effective BBB penetration.
Figure 6.
Overall summary. Data from this study show that targeting organic anion transporting polypeptide (Oatp) transporters during pathophysiologic stress may modify central nervous system (CNS) drug delivery. We propose that organic anion transporting polypeptide 1a4 (Oatp1a4) facilitates brain delivery of drugs that may exhibit efficacy in treatment of peripheral inflammatory pain or cerebral hypoxia such as statins and opioid peptide analgesics. The transforming growth factor-β (TGF-β) signaling pathway regulates Oatp1a4 functional expression via signaling mediated by TGF-β receptors (i.e., activin receptor-like kinase 5 (ALK5)). Targeting TGF-β receptors with small molecules (i.e., SB431542) may offer an opportunity to ‘control' Oatp1a4 expression/activity for optimization of CNS drug delivery.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants from the American Heart Association (12 BGIA 9850007) and the Pharmaceutical Research and Manufacturers' Association Foundation to PTR.
References
- Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev. 2013;65:291–314. doi: 10.1124/pr.112.005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobysheva NV, Tonshin AA, Selin AA, Yaguzhinsky LS, Nartsissov YR. Diversity of neurodegenerative processes in the model of brain cortex tissue ischemia. Neurochem Int. 2009;54:322–329. doi: 10.1016/j.neuint.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Martinez-Romero R, Canuelo A, Martinez-Lara E, Javier Oliver F, Cardenas S, Siles E. Poly(ADP-ribose) polymerase-1 modulation of in vivo response of brain hypoxia-inducible factor-1 to hypoxia/reoxygenation is mediated by nitric oxide and factor inhibiting HIF. J Neurochem. 2009;111:150–159. doi: 10.1111/j.1471-4159.2009.06307.x. [DOI] [PubMed] [Google Scholar]
- Tu YF, Lu PJ, Huang CC, Ho CJ, Chou YP. Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke. 2012;43:491–498. doi: 10.1161/STROKEAHA.111.629931. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying' NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Baldwin K, Orr S, Briand M, Piazza C, Veydt A, McCoy S. Acute ischemic stroke update. Pharmacotherapy. 2010;30:493–514. doi: 10.1592/phco.30.5.493. [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I—from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3:47–55. doi: 10.6030/1939-067x-3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, et al. Atorvastatin treatment in a dog preclinical model of Alzheimer's disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15:981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23:307–318. doi: 10.3233/JAD-2010-101179. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2:1015–1041. doi: 10.4155/tde.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology. 2009;150:1025–1032. doi: 10.1210/en.2008-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol (1985) 2006;100:1392–1399. doi: 10.1152/japplphysiol.01259.2005. [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38:168–176. doi: 10.1124/dmd.109.029454. [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, et al. Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem. 2004;89:403–417. doi: 10.1046/j.1471-4159.2004.02336.x. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, et al. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Mandery K, Sticht H, Bujok K, Schmidt I, Fahrmayr C, Balk B, et al. Functional and structural relevance of conserved positively charged lysine residues in organic anion transporting polypeptide 1B3. Mol Pharmacol. 2011;80:400–406. doi: 10.1124/mol.111.071282. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther. 2011;336:827–839. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29:1084–1098. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92:203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]
- Reddy GD, Reddy AG, Rao GS, Kumar MV. Pharmacokinetic interaction of garlic and atorvastatin in dyslipidemic rats. Indian J Pharmacol. 2012;44:246–252. doi: 10.4103/0253-7613.93860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, Thompson BJ, Sanchez-Covarrubias L, Zhang Y, Laracuente ML, Vanderah TW, et al. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84:774–786. doi: 10.1124/mol.113.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Yamaji R, Fujita K, Takahashi S, Yoneda H, Nagao K, Masuda W, et al. Hypoxia up-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse brain capillary endothelial cells: involvement of Na+/Ca2+ exchanger. Biochim Biophys Acta. 2003;1593:269–276. doi: 10.1016/s0167-4889(02)00397-x. [DOI] [PubMed] [Google Scholar]
- Zhong H, Simons JW. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun. 1999;259:523–526. doi: 10.1006/bbrc.1999.0815. [DOI] [PubMed] [Google Scholar]
- Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984;247 (3 Pt 2:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS ONE. 2013;8:e57735. doi: 10.1371/journal.pone.0057735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Krieglstein J. Cerebral circulation, metabolism, and blood-brain barrier of rats in hypocapnic hypoxia. Am J Physiol. 1987;252 (3 Pt 2:H504–H512. doi: 10.1152/ajpheart.1987.252.3.H504. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15:251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HC, Yang DY, Ou YC, Ho SP, Cheng FC, Chen CJ.Neuroprotective effect of atorvastatin in an experimental model of nerve crush injury Neurosurgery 201067376–388.discussion 388-9. [DOI] [PubMed] [Google Scholar]
- Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: a prescription to move the field forward. Ann NY Acad Sci. 2010;1199:69–76. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Akira T, Watanabe M, Kagitani Y. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997;36:1023–1030. doi: 10.1016/s0028-3908(97)00096-8. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122:962–975. doi: 10.1111/j.1471-4159.2012.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Murray JW, Wolkoff AW. PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G384–G393. doi: 10.1152/ajpgi.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]