Abstract
The automated touchscreen operant chamber for rats and mice allows for the assessment of multiple cognitive domains within the same testing environment. This protocol presents the Location Discrimination task (LD) and the Trial-Unique delayed Nonmatching-to-Location task (TUNL), which both assess memory for location. On these tasks, animals are trained to a pre-defined criterion during ~20-40 daily sessions. In LD-sessions, touching the same location on the screen is rewarded on consecutive trials, followed by a reversal of location-reward contingencies. TUNL, a working memory task, requires animals to “non-match” to a sample location after a delay. In both LD and TUNL spatial similarity can be varied, allowing assessment of “pattern separation” ability, a function thought to be performed by the dentate gyrus. These tasks are therefore particularly useful in animal models of hippocampal, and specifically dentate gyrus function, but additionally permit discernment of changes in pattern separation from those in working memory.
INTRODUCTION
The touchscreen operant chamber platform for rats and mice enables presentation of a variety of cognitive tests, which in many cases are highly similar to touchscreen tasks used in human cognitive testing. Within this platform, several tasks have been developed that involve learning and memory of locations on the screen1-4. Of these, the trial-unique delayed nonmatching-to-location (TUNL) task3 and the location discrimination (LD) task1 are described here.
Assessing Spatial Learning and Memory
The acquisition and demonstration of memory for locations has been extensively modeled in rodents. The focus has been on hippocampal function and pathology, which is often assessed using mazes such as the Morris water maze5, Barnes maze6, T-maze and radial arm maze7, 8. In these tasks animals are trained to navigate, using distal cues, towards a place that is associated with a reinforcer (i.e. means of escape or food reward). Alternatively, automated tests in operant chambers have addressed memory for location using paradigms such as delayed matching or nonmatching-to-position (DMTP or DNMTP9, 10). In these tests, animals are trained to sample one of two fixed locations by pressing a lever. After the so-called sample phase, a delay is imposed, during which the sample lever is retracted. At the end of the delay the two levers are presented during the choice phase, and in order to obtain a food reward, a matching or non-matching rule needs to be applied. The touchscreen tasks described here are based on the same principle of using automated operant chambers to assess spatial learning and memory. It should be noted that operant chamber tests may not rely on spatial navigation per se, at least not in the same way as maze tasks. However, operant chamber tests can be a valuable tool to detect changes in the neural substrates underlying memory for location, such as the hippocampus11-13.
Advantages and Limitations of the Touchscreen Platform
Advantages of the use of the touchscreen platform have been discussed in detail elsewhere14-16 but a particular important benefit of this method is that performance can be compared with other tasks carried out in the same testing environment, which facilitates comparison between tasks14, 16-18. In addition, automated tests offer advantages such as standardization of test procedure, minimization of experimenter involvement and stress, lowering of motor/mobility demands, simultaneous assessment of animals and improved sensitivity of measures such as response latencies.
With respect to potential limitations, touchscreen operant chamber tasks as presented here and elsewhere16, 18, make use of visual stimuli and are reward based, which may confound testing in certain animal models. More specifically, the use of visual stimuli precludes the use of certain subjects, such as mice with genetic alterations that cause rapid retinal degeneration. In addition, common albino rat or mouse strains differ in visual acuity when compared to pigmented strains,19, 20 although albino rodents appear to have sufficient vision to perform as well in the touchscreen as pigmented animals15. Finally, as with most appetitive operant paradigms, the use of food reward may introduce possible problems; for example, an experimental treatment may affect appetite or interact with the physiological effects of food restriction.
A particular advantage of the touchscreen, which is utilized in the TUNL and LD tasks, is the flexible nature of the screen which allows for the manipulation of the distance between, and therefore similarity of, response locations. This provides a useful tool with respect to the functional contribution of the hippocampal dentate gyrus (DG), as it has been suggested that this region is a “pattern separator” of spatial information21-23. Pattern separation refers to the ability of neural circuits to orthogonalize or decorrelate similar input patterns into distinct representations24, 25, to avoid memory interference. Behavioral evidence for the role of the DG as a pattern separator in spatial memory has been found in rats and mice23, 26-28, and was initially obtained using a delayed matching-to-sample paradigm in a holeboard maze, in which the distance between choice locations was varied and DG lesions (but not lesions of the CA1, a different hippocampal subregion) resulted in impairments in recognizing similar, but not dissimilar locations24. In TUNL and LD, a comparable approach is used with respect to measuring pattern separation. Specifically, both tests compare performance on a dissimilar condition (large separation) to a similar condition (small separation), aiming to place a variable demand on pattern separation capacity and designed to provide a behavioral read-out of this computation. The dissimilar condition in particular, allows for the dissociation of changes in pattern separation from general changes in location-memory and rule learning. These tasks may therefore be particularly useful in (disease) models that show changes in the DG, such as altered levels of adult hippocampal neurogenesis. Indeed, aberrant adult neurogenesis has been implicated in psychopathological and neurodegenerative diseases29, 30 such as depression31, 32, schizophrenia33, 34 and Alzheimer’s disease35-38. It is changed by acute, chronic and developmental stress39-43, ageing44, 45, exercise46 and hormone levels47, 48, amongst other factors, and has been suggested to play a role in cognitive functions, such as hippocampus-dependent learning and memory49-52.
Trial-unique delayed Nonmatching-to-Location (TUNL)
TUNL, developed by John Talpos and colleagues3, is a working memory task based on the operant DNMTP paradigm with an important alteration: the pre-defined sample and novel choice location can be randomly selected from multiple response locations and vary between trials within a session, rendering this task more “trial-unique”. Whilst the DNMPT task in a 2-lever operant chamber has been found to be sensitive to both prefrontal and hippocampal lesions 11, 53, 54 cf. 55, it has been criticized for allowing mediating strategies. Specifically, the requirement to retain spatial information across the delay can be reduced by taking advantage of the predictability of the to-be-correct location56-59. The TUNL task circumvents this issue by using an array of spatial locations making the to-be-correct choice location less predictable, and extensive analysis of putative mediating behaviors has shown that animals are unlikely to make use of such strategies3. An advantage of using multiple spatial locations is the ability to manipulate the distance between the sample and the to-be-correct location, so that in addition to the assessment of working memory, effects on pattern separation can be measured. Drawbacks of the TUNL task include a high task difficulty level, resulting in a slow acquisition rate. Furthermore, the task has so far been developed only for rats. Mice, in our experience, perform poorly on the TUNL task in its current form. However, we are addressing task-difficulty through the development of a continuous-version of TUNL that is acquired more rapidly (C.A.O., unpublished data) and we are currently developing a version for mice. The TUNL task as presented here has been validated as sensitive to hippocampal dysfunction, as lesioning this structure impairs performance in both a delay- and separation-dependent manner3. In contrast, lesions to the prefrontal cortex impair performance when the delay, but not the separation is manipulated, resulting in impairments under long-, but not short-, delay conditions60. In addition to variations in delay, working memory capacity in TUNL may also be assessed by manipulating the degree of interference. In the current task setup, memory of earlier events may interfere with the memory of more recent events, causing proactive interference61. Inter-trial interference may be increased by shortening the inter-trial interval (ITI)62-64, and performance on increased interference probe sessions in TUNL was recently shown to be dependent on an intact prefrontal cortex60. To conclude, the TUNL task offers a paradigm in which both working memory and pattern separation requirements can be assayed, with sensitivity to detect deficits in isolated processes. In light of this, the TUNL task may be particularly interesting for use in rodent models of disorders in which working memory deficits are a core feature of the phenotype, such as animal models of schizophrenia.65, 66 Furthermore, structural and functional changes in -and connectivity between- the hippocampus and prefrontal cortex play an important part in the pathophysiology of schizophrenia67-70.
Location Discrimination
The LD task, developed by Stephanie McTighe and colleagues, was originally devised as a simplified procedure for studying pattern separation in rats and mice, and was shown to be sensitive to hippocampal lesions1. The protocol has been successfully implemented in both rats1 and (male, female and aged) mice28, 71, 72. In LD, two locations are presented on each trial, and the subject learns across trials to respond to the correct location. Using the LD task, pattern separation was demonstrated to be dependent on adult neurogenesis in the DG28, and further studies have shown that the ability to “pattern separate” in this task correlates with voluntary exercise levels and neurogenesis71, and that it is associated with glutamate receptor regulation and signaling72. Moreover, the current protocol includes a spatial reversal component, which could permit the discernment of changes in executive function from pattern separation per se. Potential issues with this task include the difficulty some subjects (depending on species and strain) may have in completing acquisition and reversal within a single session. However, the basic location discrimination protocol is easily amenable to modifications in experimental design to flexibly address the limitations of such subjects (see EXPERIMENTAL DESIGN and TROUBLESHOOTING).
Experimental design
Experimental details for TUNL and LD are described below in separate sections. In the first section, some general principles are discussed. First, it should be noted that the protocols for both TUNL and LD as described here, are based on the original publications1, 3, but as a result of ongoing method- and task development15, minor modifications to improve task acquisition and performance have been made since. For example, in both tasks the number and dimensions of response locations presented here may differ from published work. This protocol describes the standard as currently used in our lab.
General considerations
With respect to choosing the appropriate task, we propose that the two tasks presented in this paper are used as alternatives, rather than complementary measures to assess changes in location memory and pattern separation. For example, if the priority is to assess working memory, TUNL represents a suitable choice. Alternatively, for a study focusing mainly on pattern separation, the LD task provides a more rapidly acquired alternative. In addition, these tasks may be combined with other touchscreen tasks16, 18 in a “flexible battery”14, to address specific hypotheses and research requirements. For a discussion of optional batteries, see16. This approach may be particularly appropriate when there are no specific a priori hypotheses regarding the domains of cognition that will be affected by a given manipulation.
With respect to the experimental design, it is important to recognize that the timing of the experimental manipulation determines the exact procedure. Both tasks require initial pretraining, during which animals learn to touch the screen for a reward. This procedure preceeds most touchscreen tasks and experimental details will be briefly described in the following section, but for an extensive description of pretraining stages, including flowcharts, see16. For subsequent TUNL and LD training, we describe four relatively common experimental designs (Cases 1-4) that may result in a somewhat different implementation of either the TUNL or LD protocol. In subsequent sections these cases will be referred to as appropriate. In Case 1, the subject receives treatment before onset of the experiment (e.g., transgenic models, developmental manipulations). In Case 2, the subject receives treatment before task acquisition, but after initial pretraining (e.g., subchronic drug treatment, neurotoxic lesions). In Case 3, differences between groups are assessed after animals have reached an asymptotic performance level. This can be done using a between-subject design after acquisition (e.g., neurotoxic lesion, sub-chronic drug treatment). Finally, in Case 4, a within-subject manipulation after acquisition may be applied, such as in transient systemic drug studies or infusion procedures.
As mentioned, these experimental “cases” determine how TUNL and LD (and other touchscreen tasks16, 18) are to be implemented. To assess differences in acquisition curves between groups, all animals may be trained continuously (i.e. 5-7 days per week) until performance asymptotes. In this scenario some animals will be relatively “over-trained” compared to others, due to variation in acquisition speed. Even though TUNL and LD can be used in this way, it may be less useful to assess the acquisition of the learning rule per se, and in both tasks post-acquisition probes are likely to be of interest, in order to assess delay-dependent performance (TUNL only), or pattern separation-dependent performance (TUNL and LD). If a particular research question is aimed at addressing differences in performance on post-acquisition behavioural probes (combined with Cases 1 and 2) or in the case of post-acquisition experimental manipulations (Cases 3 and 4), variation due to over-training on the initial acquisition phase, as mentioned above, is not desirable.
Therefore, several options exist as to how (a group of) animals should be advanced through acquisition training, as an alternative to continuous training until (beyond) criterion. First, each animal in the group may be trained until it reaches criterion, then individually advanced to the manipulation of interest. Whilst this avoids over-training and variations in performance level, the group is not synchronised.
Second, a group of animals may all be tested for a prespecified number of acquisition sessions (e.g., based on previous data), and then all advanced to the post-acquisition manipulation regardless of performance level. An advantage is that all animals in the group will be synchronised and the manipulation will begin for all animals on the same day which minimises variability due to external factors and is ideal for pharmacological studies. For example, injections may be conducted on the same day(s) for all animals and decisions concerning the number of days to run a manipulation, can be made ad hoc based on the group’s mean performance level. This is also particularly important when subjects must be the same age at the start of each testing phase, for example, when testing a progressive disease model. However, there will be some variation in the performance levels of the animals at the end of training, and some may not have acquired the initial task to a sufficient baseline level from which to assess alterations in performance due to a manipulation.
We often apply a third option, in which each animal in the group is trained daily (5-7 days per week, as recommended) until it reaches criterion, upon which it is “rested” without daily training (although food restriction continues). Subjects on rest are usually given one or two “reminder” training sessions per week unless it is anticipated that all subjects will reach criterion within a few days of each other. If an animal’s performance falls below criterion in a reminder session, that animal is trained daily until criterion is re-attained. When all animals have reached criterion (at least) once, they are rebaselined as a group, i.e. all animals are trained daily. Post-acquisition manipulations may begin when performance of all subjects has been stable at criterion for at least two days. Whilst subjects receive a different number of training days, precluding plotting of a complete acquisition curve, the animals are synchronised, with minimal variation in their performance levels, and over-training is minimised.
Pretraining
Following the introduction of mild food restriction, the first stage of the protocol is habituation of the animals to the chambers and food rewards (see PROCEDURE; step 5). In Stage 2, the relationship between offset of a visual stimulus on the screen and delivery of reward is introduced. During this stage, a stimulus is presented on the screen which, in the case of TUNL and LD, is a white square (or squares). If not touched, offset occurs after 30 s and a reward is delivered, along with illumination of the magazine and a tone (conditioned reinforcer). Touches to stimuli on the screen are encouraged with immediate offset, a triple reward delivery, tone and magazine illumination. When the reward is retrieved, an ITI begins, after which the next trial is automatically initiated. Please note that TUNL and LD pretraining differ during this stage, with respect to the number of response windows active (see PROCEDURE, step 6).
Stage 3 is similar to Stage 2, but stimulus offset is dependent upon the subject touching it. A stimulus is presented, and remains there until touched, upon which the stimulus disappears and a reward is delivered accompanied by a tone and magazine illumination. When the animal retrieves the reward and exits the magazine, the ITI begins, after which the next trial begins automatically. Please note that TUNL and LD pretraining differ during this stage, with respect to the number of response windows active (see PROCEDURE, step 7A, B).
Stage 4 is similar to Stage 3, but subjects are required to trigger stimulus presentation, referred to as trial initiation. The session begins with a “free” reward delivery and magazine illumination, indicating that a trial may be initiated by magazine entry. When the animal nose pokes into the magazine, the magazine light is extinguished and a click sounds, and when the animal withdraws from the magazine, stimuli are presented on the screen. Initiation is also required after each ITI (see PROCEDURE, step 8).
Stage 5 is similar to Stage 4, but subjects are discouraged from touching blank response windows during stimulus presentation, with stimulus removal and a time out period in which the houselight is inverted. After the time out, an ITI begins, after which the next trial can be initiated. However, in the pretraining preceding the tasks in this paper, a correction trial is given instead of a new trial, in which the same stimulus location is presented. This stage also serves to introduce the subject to the cue signalling incorrect responses (the time out). By the end of pretraining, subjects should be completing a sufficient number of trials per session (as specified in PROCEDURE, step 9), to promote completion of sessions in the subsequent task.
Trial-unique delayed non matching-to-location (TUNL)
The trial structure of the TUNL task is depicted in a flowchart (see Fig. 1). Each session starts with a “free” food pellet delivered into the food magazine, coinciding with illumination of the magazine. When the animal nose pokes into the magazine to collect the pellet, the magazine light is extinguished, a click sounds (0.2 s) and the first trial is initiated. A sample location (i.e. a white square, see EQUIPMENT SETUP) is presented the moment the animal exits the magazine. During the sample phase, the animal is required to respond by touching the sample location. Upon touching, the stimulus disappears and the delay starts. One in three sample-touches is rewarded with a pellet, delivery coinciding with a tone and illumination of the magazine light, which extinguishes upon reward collection. At the end of the delay, the magazine light is turned on to indicate that the choice phase can be initiated. As soon as the animal exits the magazine after choice phase initiation, the incorrect sample location and a different, correct choice location are simultaneously presented on the screen. Depending on the choice of animals, two scenarios are possible. In the case of a correct response to the novel location, touching the screen leads to reward delivery accompanied by a tone (standard: 1 s, 3 kHz), both stimuli are removed from the screen, and the magazine light is turned on. Upon subsequent magazine entry during reward collection the magazine light is extinguished and the inter-trial interval (ITI, 20 s) is started. At the end of the ITI the magazine light is turned on, signaling that the animal can initiate a new trial (i.e. a newly selected distance, sample location and choice location). However, in the case of an incorrect response during the choice phase (a touch to the sample location), the animal is presented with a time out (5 s) signaled by houselight illumination. Please note that our current standard procedure is to have the houselight off during stimulus presentation and inter-trial intervals (and on for “time out” periods). However, published work on TUNL3, 60 has been performed with houselight settings inverted, and we do not have conclusive evidence that these variations affect task performance. After the time out the ITI is started, at the end of which the animal can initiate a correction trial (i.e. presentation of the same sample location and choice location). There is typically no limit to the number of correction trials, and further correction trials are presented until a correct response is given. Correction trials are implemented to minimize the formation of biases towards certain locations. After an incorrect response, subsequent correction trials are not added to the total trial number and are not used when calculating percentage correct.
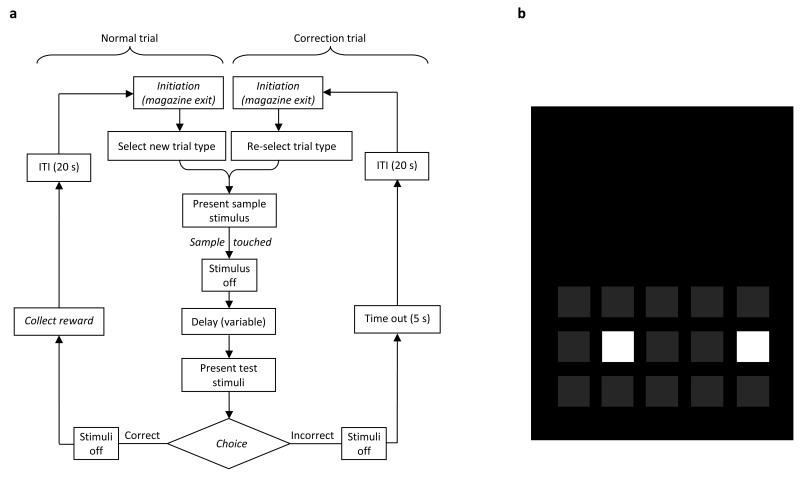
Figure 1.
Flowchart overview of the main features of the touchscreen Trial-Unique delayed Nonmatching-to-Location (TUNL) task (a). The animal initiates a trial by a nose poke into the magazine. The program pseudo-randomly selects a trial type and presents one sample stimulus on the screen. The rat is required to touch the sample after which the delay starts. At the end of the delay, a choice between the previously illuminated sample location and a novel choice location is presented. If the animal responds correctly (touches the choice location), it is rewarded. If it responds incorrectly (touches the sample location), it is punished with a time out (5 s). Either response is further followed by an ITI of 20 s, after which the animal is required to initiate the next trial. If the previous response was incorrect, the same sample and choice locations will be presented. This loop will continue until a correct response has been made. The labels in italics indicate steps in which the animal is required to perform an action. (b) As an example (please refer to the text for actual dimensions), the TUNL mask with 15 locations is depicted. The choice phase is shown with two response windows illuminated at “separation 2” (separation is defined as the number of response windows separating two active locations).
We recommend that initial acquisition of TUNL is performed under constant, relatively short delay conditions (e.g., 2 s). To further investigate the nature of potential changes in working memory, specific post-acquisition “probe sessions” can be implemented with variation in delay, ITI and separation. Trials during probe sessions are the same as those in regular sessions, with the exception that particular delay conditions or separation conditions (or combinations thereof) are used. Several options can be considered when designing probe sessions. First, it is possible to test animals on sessions in which the combination of delay (short, e.g., 0 s, or long, e.g., 6 s) and separation (small or large) is fixed within each session. We recommend testing all four possible permutations, i.e. short-small, short-large, long-small, long-large. It should be noted that, in our experience, animals perform near chance on the most difficult condition (6 s delay; small separation). Therefore, in studies that assess impairments, this condition could be omitted or adapted in order to avoid a floor effect.
Second, probe sessions can be designed that alternate selected distances (e.g., small, medium, large) and delays (e.g., 3, 6 and 9 s) within a session to assess whether animals perform differently under these varying conditions of task demand (C.A.O., unpublished data).
Third, recent work indicates that by minimizing both the delay and the ITI, conditions of increased proactive interference can be created, which may be particularly interesting in models of prefrontal pathology, as prefrontal cortex lesions impaired performance on this condition60. In this particular study, increased interference was measured in sessions in which the separation was fixed at maximum distance.
Location Discrimination and reversal (LD)
During LD training (see Fig. 2), each session starts with a “free” food pellet delivered into the food magazine, coinciding with illumination of the magazine. When the animal nose pokes into the magazine to collect the pellet, the magazine light is extinguished, a click sounds (0.2 s), the first trial is initiated and the stimuli appear on the screen. On each trial, animals are presented with two illuminated locations. Initial acquisition of LD is performed using a so-called “intermediate separation” condition, in which response windows 2 and 5 (or in rats: windows 2 and 6) are illuminated (if window 1 is at the extreme left and window 6 is at the extreme right). One location is designated “correct” (CS+) and a nose poke to this location results in reward delivery accompanied by a tone (standard: 1s, 3 kHz) and magazine illumination. Upon reward collection, as the animal enters the magazine, the magazine light is extinguished. Alternatively, a nose poke response to the other (“incorrect”) illuminated location results in a time out period (standard: 5 s) indicated by houselight illumination. Please note that our current standard procedure is to have the houselight off during stimulus presentation and inter-trial intervals (and on for “time out” periods). However, published work on LD has been performed with houselight settings inverted, and we do not have conclusive evidence that these variations affect task performance. Stimuli are removed from the screen immediately after either type of response. Following magazine activation during reward collection or at the end of the time out period, an inter-trial interval (ITI, 10 s) commences, after which the magazine is illuminated to indicate the next trial can be initiated. The animal must continue responding until 7 correct responses have been made in 8 consecutive trials, which constitutes the acquisition criterion for this task. At this point the reward contingency is reversed, with the “correct” location becoming “incorrect”, and vice versa. The animal must then acquire this reversed contingency as demonstrated by again performing 7 correct responses in 8 consecutive trials. This intermediate training phase is continued until the animal is able to attain the initial location-reward contingency, as well as the subsequent reversal within one session (fixed number of trials/ 60 minutes), on 3 out of 4 consecutive sessions. The initial correct/incorrect response window designation is counterbalanced across animals. The window designation is also maintained across training sessions (i.e. if window 2 was correct for a particular animal at the end of a training session, it is used as the correct location at the beginning of the subsequent session for that animal).
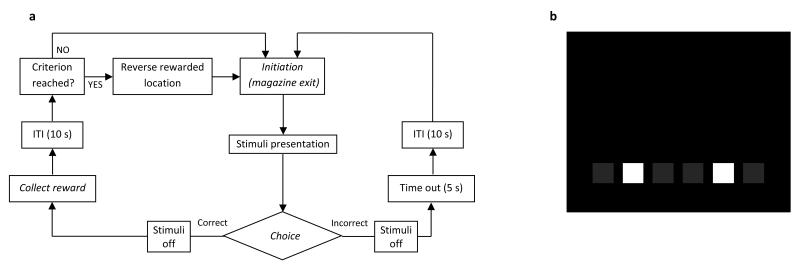
Figure 2.
Flowchart overview of the intermediate training and performance probe phases of the location discrimination (LD) task (a). Following initiation, two stimuli are simultaneously presented; one is designated correct and the other incorrect. If a nose poke response to the stimulus in the correct location is made, a reward is delivered. A nose poke response to the incorrect stimulus results in a time out (5 s). Either response is further followed by an ITI of 10 s, after which the animal is required to initiate the next trial. If an animal makes 7 correct responses in 8 consecutive trials, the reward-location contingency is reversed and trials are repeated until the same level of performance is achieved. The labels in italics indicate steps in which the animal is required to perform an action. (b) As an example (please refer to the text for actual dimensions), the LD mask for mice is shown during the “intermediate” training phase (see PROTOCOL, Step 11B-ii).
Following completion of the intermediate training phase, the LD performance probe sessions are conducted. The trial structure of these sessions is identical to the intermediate training phase, except that the animal is either presented with response windows 1 and 6 (rat 7) illuminated (maximal inter-stimulus distance; the “large” separation condition) or windows 3 and 4 (rat 5) illuminated (minimal inter-stimulus distance; the “small” separation condition). Subjects receive an unlimited number of trials per session, with the session terminating after 60 minutes or after an animal is able to attain the initial location-reward contingency, as well as the subsequent reversal, whichever comes first. All presentations in a given session are either all large or all small and the two session types are counterbalanced within animal groups. Each animal receives two consecutive sessions of the same probe type and then, irrespective of performance, receives two consecutive sessions of the other probe type. This alternating paired pattern is repeated for multiple sessions (e.g., 20). Performance on the intermediate training phase may be assessed from the number of sessions or trials required to reach overall criterion (acquisition and reversal within one session, on 3 out of 4 sessions)71. The primary measure of probe performance is the average number of trials required to attain acquisition criterion for each probe separation (large, small)28, 71, 72, see PROCEDURE, data analysis.
MATERIALS
REAGENTS
Rats or mice (see REAGENT SETUP)
Animal housing (see REAGENT SETUP)
Husbandry: rodent food pellets (e.g., Rodent Pellets, Special Diets Services, UK)
-
Reward: we use solid (e.g., Bio-Serv® purified rodent Dustless Precision Pellets®, 45 mg (rat)/14 mg (mouse), through Sandown Scientific, Esher, UK) or liquid (Yazoo® strawberry milkshake, FrieslandCampina UK Ltd) food rewards.
Caution When filling reward dispenser with Dustless Precision Pellets®, take care to discard dust, as this can potentially clog dispensers.
Cleaning materials (e.g., TriGene®, 70% ethanol solution, stiff brush)
EQUIPMENT
Touchscreen operant chambers (from, e.g., Campden Instruments Ltd., Med Associates Inc., other commercial suppliers; or custom-made operant system). Note that these are species-specific. See EQUIPMENT SETUP.
Sound- and light-attenuating box with ventilation system, enclosing an operant chamber and reward delivery system.
Camera above chamber, connected to closed circuit monitor and digital video recording device, to monitor and record the animals’ behavior (optional but recommended)
Controlling software and devices which are generally available from operant chamber supplier
Black plastic masks with response windows (the number and size of which differ between tasks and species – see EQUIPMENT SETUP)
Shelves for rat chambers (for some tasks, see EQUIPMENT SETUP)
Appropriate data analysis software
Personal protection equipment (e.g., disposable medical gloves, lab coat or coverall, FFP2 mask) should always be worn when handling or working near animals, to minimize allergen exposure.
REAGENT SETUP
Rats or mice
Laboratory-bred or commercially available rats or mice are generally used for testing. There are some advantages to testing male rats and mice, such as avoiding potential estrus cycle-related performance variability in females and potentially increased inter-male aggression when males must be tested in the same apparatus as females. Most commonly for touchscreen operant chamber tests, male Lister Hooded rats and male mice on the C57BL/6 or 129 substrain genetic backgrounds have been used. For LD, female-28 and aged71 mice have been tested in addition to young male rats and mice. Critical TUNL, as mentioned earlier, can be acquired by rats but not mice. A version for mice is currently under development.
Caution All experiments using live animals must be approved by national and institutional bodies, and performed according to their regulations.
Caution If animals are not fully grown when food restriction begins, they must be allowed to gain sufficient weight as they continue to grow. Standard strain growth curves are usually available from the supplier for guidance (e.g., http://jaxmice.jax.org/support/weight/index.html).
Animal housing
Rats and mice should be housed in groups of 2-5 animals, with sawdust, bedding and (optional, although recommended) shelter, with cages cleaned regularly. The housing room should be maintained at a constant temperature (21±2 °C) and humidity (55 ± 10%). Lighting is usually on a 12-hour light-dark cycle, and we favor testing rats and mice in the active period of their circadian cycle as this may enhance activity and potentially learning and memory73-75. We advise that researchers consider light-phase conditions, as it can potentially interact with sex, strain, experimental manipulations, etc. to influence performance. When shifting or inverting the light cycle, allow sufficient time for rats and mice to habituate before commencing behavioral testing (see, e.g.,76). We tend to allow one day per hour of shift.
Rewards
Two types of reward are typically used – liquid or solid (see MATERIALS). Pellets seem to work well for rats. We use either liquid or solid for mice; liquid rewards may be a better choice in some cases (for mice in particular), e.g., when using manipulations that result in motoric changes that could affect chewing, cause dry mouth, or reduce motivation. Introduce reward (pellets or milkshake) inside the home cage to habituate the animals for 1-3 days before the start of testing. Solid rewards may be scattered on the cage floor; liquid rewards should be put into a shallow, wide-based dish.
EQUIPMENT SETUP
Touchscreen operant chambers for rats and mice
The specific model or make of touchscreen operant chamber used depends on the experimenter’s needs and preference. We will describe mouse and rat chambers used in our lab, from Campden Instruments Ltd. and our in-house assembled boxes. Both TUNL and LD, as well as the majority of other tests16, 18 have been performed in both.
Campden chambers: Housed inside a dense fiberboard box, these are equipped with a fan, touchscreen monitor (rat: 15.0 inch, screen resolution 1024 × 768 (rotated); mouse: 12.1 inch, screen resolution 600 × 800), tone and click generator, houselight (LED), magazine unit (with light and infrared beam to detect entries; in the standard configuration this is outside the testing arena, on the wall opposite the touchscreen) and pellet dispenser and/or pump connected to bottles of liquid reward. The chambers have a trapezoidal shape (rat: 30 h × 33 l (screen-magazine) × 25 w (at screen) or 13 w (at magazine) cm; mouse 20 h × 18 l × 24 or 6 w cm), composed of 3 black plastic walls opening on to the touchscreen, intended to help focus the animal’s attention to the touchscreen and reward delivery area. The touchscreen uses infrared photocells, and therefore does not require the subject to exert any pressure in order for responses to be registered. Our experience is that rats and mice work most readily and learn fastest with these IR beams, and not when they have to exert any pressure on the screen, although we have not done a properly controlled experiment to test this. We typically observe rats and mice responding to the screen with their noses (see Supplementary Video 1 of the accompanying paper by Horner et al16). Access to the chamber is through a transparent lid, which can be secured to the trapezoidal walls with latches during animal testing. The floor is perforated stainless steel, raised above a tray lined with filter paper. Two additional photobeams extend between the side walls of the arena, parallel to the screen, to detect the movement of an animal in the front (rat: ~6 cm from the screen; mouse: ~7 cm) or the rear (rat: ~5 cm from the magazine; mouse: ~3.5 cm) parts of the arena. A small infrared camera can be installed above the chamber to monitor animals’ behavior (optional, but recommended). In Campden rat chambers, a spring-hinged shelf (24 w × 6 l cm) can be attached 15 cm above the floor at a 90° angle to the screen and mask. In general, attaching a shelf to the mask may reduce impulsive responding and improve attention directed to stimuli, which we found specifically occurs in rats, as this forces the rat to rear up before making a choice77. Until recently, a shelf has been used in rat LD and TUNL studies1, 3, 60. However, recent task-development in our lab has shown that rats acquire TUNL significantly faster when locations are presented on the lower half of the screen without the use of a shelf (C.A.O., unpublished data), which we therefore recommend in this protocol. We continue to recommend the use of a shelf for the LD task.
Our in-house chambers: Housed inside a melamine box, chambers (modified in our lab from Med Associates operant chambers) are equipped with a fan, infrared touchscreen monitor (rat: 29.0 h × 23.0 w cm; mouse: 16.0 h × 21.2 w cm; Craft Data Limited, Chesham, UK), tone generator, click generator, houselight (~3 W), magazine and pellet dispenser. The touchscreen does not require the subject to exert any pressure in order for touches to be registered. The chambers have a rectangular shape, consisting of a metal frame with clear Perspex walls (rat: 29 h × 31 l × 24 w cm; mouse: 13 h × 25 l × 19 w cm; excluding space below floor). Access is through a hinged side wall, secured with a latch during testing. The floor consists of stainless steel bars spaced 1 cm apart, above a tray lined with filter paper. The magazine is equipped with a light and a photocell nose poke detector. A spring-hinged shelf (20.5 w × 6 l cm) can be fitted in these rat chambers, 14.0 cm above the floor, at a 90° angle to the screen and mask.
Mask and stimulus dimensions: In both LD and TUNL, animals are trained to touch white (rectangular or square) stimuli on the screen, which serve as response locations. A black plastic mask (rat in-house: 38.7 h × 30.0 w cm; rat Campden: 35.8 h × 28.0 w cm; mouse in-house: 11.8 h × 22.8 w cm; mouse Campden: 24.3 h × 28.0 w cm) with windows that delineate the response locations, is fitted in front of the touchscreen to reduce accidental screen touches and make response locations clearly identifiable from the background. In LD and TUNL, the size of the (white, square or rectangular) stimuli presented on the screen is similar to the size of the response windows in the mask. Please note that, particularly for LD, dimensions may vary between box-type and species.
For LD in mice: we recommend using a single row of 6 response locations located on the bottom half of the screen (no shelf). Response-window and stimuli dimensions are as follows: 3 cm w × 2 cm h (Campden boxes); or 2.5 w × 2.5 h (in-house boxes); spaced apart by 1 cm (Campden boxes) or 0.5 cm (in-house boxes); located 2 cm (Campden boxes) or 1.5 cm (in-house boxes) above the floor of the chamber. Variation in separation is accomplished by using locations 1 and 6 (large separation); locations 2 and 5 (intermediate separation) or locations 3 and 4 (small separation).
For LD in rats: data thus far1 were collected using a single row of 7 response locations (2 cm w × 2 cm h, in-house boxes); spaced apart by 1 cm. Response windows were located 1.5 cm above a hinged shelf, 16.5 cm above the floor of the chamber. Variation in separation is accomplished by using locations 1 and 7 (large separation); locations 2 and 6 (intermediate separation) or locations 3 and 5 (small separation).
For TUNL (only rats), varying response location dimensions and positions have been used3, 60. We recommend using a mask in which response locations are organized in 3 rows of 5 locations (3.3 cm w × 3.3 cm h), spaced apart by 1.5 cm, positioned on the bottom half of the screen (bottom row 1.5 cm from the grid floor), without the use of a spring-hinged shelf.
Controlling software and devices
Controlling software can be purchased from the suppliers of the operant chambers, e.g., “Whisker Server”78; ELO software (ELO Touchsystems Inc.). Multiple chambers may be controlled by a single computer, although it is important to check that minimum system requirements are met (e.g., memory and graphics cards) to prevent delays in stimuli presentation and chamber responses. All task software is based on earlier publications and is available (excluding, in some cases, recent modifications) from Campden Instruments Ltd., and in some cases from Med Associates Inc. (K-Limbic) or other suppliers. Alternatively, software may be programmed using common programming languages, e.g., Visual Basic 6.0 (Microsoft, Redmond, WA).
PROCEDURE
Preparation for pretraining
1. If animals are obtained from an outside source (i.e. different from the animal facility in which behavioural testing will occur), allow animals to acclimatize after transport without any procedures, with food and water ad libitum, for a minimum of 7 days. Begin handling and weighing the animals after 2 days of acclimatization.
Critical step We advise consulting with your institutional animal care regulatory body when planning and designing experiments, regarding matters including food restriction and housing.
Critical step In general, considerations such as group size should -ideally- be based on previous work using comparable animals (i.e. species, strain, age, background) and type of behavioral assay.
Critical step If subjects have previously been tested on another instrumental touchscreen task, maintain food restriction and start at pretraining Step 9. As discussed in EXPERIMENTAL DESIGN, touchscreen tasks (e.g., 11A, B, also see16, 18) may be employed in flexible combinations and orders using a “battery” approach.
2. Weigh each animal for three consecutive days with ad libitum food and water and calculate the mean free-feeding weight of each animal.
Critical step Ensure that each animal can be reliably identified.
3. After the 7 day acclimatization period, begin food restriction, adhering to institutional and national guidelines. Slowly reduce (e.g., over 3-7 d) the weight of individual animals down to the goal weight, which will be a percentage of the measured free-feeding weight (e.g., we use 85-95%) by controlling the daily amount of food they are given (e.g., for rats, ~7 g food per 100 g body weight; for mice, ~2-3 g food per 25-35g mouse). Start Step 4 when animals are close to their goal weights.
Critical Step Maintain food restriction throughout touchscreen testing.
Critical step It is important to check the weight of animals daily (mice) or twice a week (rats) throughout the experiment, which, in our experience, is particularly important for performance in mice. This also helps habituate the animals to being handled. Aim to avoid weight reduction of greater than 5% per day, and weight reduction below 85% of free-feeding.
4. Introduce reward (pellets or milkshake) inside the cage to habituate the animals for 1-3 days. Solid rewards may be scattered on the cage floor; liquid rewards should be put into a shallow, wide-based dish.
Pretraining
5. Stage 1: habituation. Start pretraining with a habituation stage, for this, set up the apparatus (see MATERIALS) as appropriate for the planned task (i.e. LD or TUNL) using the corresponding mask and, optionally, a spring-hinged shelf (as recommended here, only in the case of LD in rats). Turn on all electronic components so that subjects can habituate to these. Place ~10 reward pellets or 0.2 ml liquid reward in the reward magazine. Place each rodent into its assigned chamber for 30 minutes. No task-related software is active during habituation, but when available (e.g., Campden Instruments boxes), we recommend recording baseline activity. Remove the animal from the chamber and return it to its homecage. Check whether rewards are consumed at the end of the session. Test all subjects on this habituation stage for a minimum of two sessions. The criterion for advancing animals to the next stage of pretraining is consuming all rewards in one session.
Critical step Animals require fewer standard rodent food pellets when receiving rewards during training; adjust daily food allowance as appropriate to maintain goal weight.
Critical step Aim to train, weigh and feed each animal at approximately the same time each day and use the same operant box for each animal during training. Always counterbalance chambers and testing times across experimental groups. We recommend training animals for one session per day, 5-7 days per week.
Critical step Advance individual subjects to the next pretraining stage when they reach criterion, even if some animals in the group remain on previous stage(s).
Critical step Operant chambers should be cleaned regularly (e.g., once a week, or more) to avoid context change during sensitive task phases, to ensure the touchscreen and infrared photobeams retain maximum sensitivity, and to prevent accumulation of dirt and excrement. We typically dismantle inner chambers and clean with surface disinfectants (e.g., TriGene® and 70% ethanol) using paper towels or a stiff brush.
6. Stage 2: Training to associate stimuli on the screen with a reward. Set up the apparatus as detailed in MATERIALS, with choice of mask and shelf suitable for the planned behavioral task and use the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN. Place each subject in its assigned chamber, and start the session. The session finishes after 60 min or 100 trials (rat)/30 trials (mouse) are completed, whichever comes first. After session termination, return each animal to their respective home cage. Advance individual subjects to the next training phase when they achieve a criterion of completing all trials (mice) or 60 trials (rats) within 60 min.
Critical step Please note that rats are typically given the opportunity to complete more trials per session than mice, e.g., 100 as opposed to 30 during pretraining. Rats readily complete a greater number of trials per session than mice, perhaps because the mouse:rat body mass ratio is smaller than the mouse:rat reward pellet size ratio (14mg:45mg).
Critical step Please note that pretraining Stage 2 is different for LD and TUNL with respect to the number of response windows active on any given trial. In LD, a single response window is illuminated (and responsive to touches) throughout all pretraining stages. In TUNL, we recommend having all 15 locations illuminated simultaneously during Stage 2 (step 6) and -initially- Stage 3 (step 7) of pretraining, to facilitate acquisition. During Stage 3 of TUNL-pretraining the size of the location is scaled down to a single response window.
Critical step At the end of each session, record critical data for each subject such as number of correct responses and number of trials completed. Most software programs will log many other measures (see EXPERIMENTAL DESIGN).
Critical step If testing the effects of a manipulation conducted before onset of the experiment (Case 1, see EXPERIMENTAL DESIGN), ensure that animals in experimental and control groups complete comparable numbers of trials per session from Stage 3 (step 7) onwards. Cap the number of trials given per session to accommodate the lowest responders.
7. Stage 3: Training to touch stimuli on the screen for a reward. Follow option A for LD pretraining and option B for TUNL pretraining.
-
A. For LD pretraining.
-
i. Repeat procedure as detailed in Step 6/Stage 2 using the appropriate software program for Stage 3 (see EXPERIMENTAL DESIGN). When animals have reached criterion on Stage 3, transfer animals to Stage 4.
-
i.
-
B. For TUNL pretraining.
-
i. Repeat procedure as detailed in Step 6/Stage 2 using the appropriate software program for Stage 3 (see EXPERIMENTAL DESIGN). Start this Stage by subjecting animals initially to this task with all 15 response windows activated simultaneously, until criterion is reached.
-
ii. Then repeat Stage 3 using an adapted version of the task where instead of all 15 windows, a smaller response location is used, consisting of 4 (2×2) adjacent locations. The location of this square on the screen should be selected pseudorandomly across trials.
-
iii. After animals have reached criterion, repeat Stage 3 a third time, using a single window as the active response location. Proceed to Stage 4 when animals have reached criterion.
-
i.
8. Stage 4: Training to initiate trials. Repeat procedure as detailed in Step 6/Stage 2 using the appropriate software program for Stage 4 (see EXPERIMENTAL DESIGN). For this stage, ensure that at the start of each session, subjects are provided with a single free reward to encourage initiation of the first trial.
9. Stage 5: Punishment for incorrect responses. Repeat procedure as detailed in Step 8/Stage 4 using the appropriate software program for Stage 5 (see EXPERIMENTAL DESIGN). The criterion for this stage is completing all trials with ≥ 80% correct (not including correction trials) within 60 min (rat), or with ≥ 75% correct within 35 min (mouse), on 2 consecutive sessions.
Critical step It is critical that pretraining Stage 5 is carried out including correction trials, to minimize the formation of biases towards certain locations.
Critical step There is likely to be variation in the number of days that animals require to complete pretraining. We suggest “resting” individual animals when they reach criterion early on this final stage of pretraining, with “reminder” sessions once or twice per week (and continued food restriction), until the entire group has achieved criterion, please refer to Experimental design. Then rebaseline all subjects so that the entire group can advance to a specific touchscreen task on the same day. This also ensures that subsequent performance differences on the task cannot be attributed to differences in pretraining performance (relevant for Case 1 only, see EXPERIMENTAL DESIGN).
10. If subjects are scheduled to receive experimental treatments after pretraining but prior to task acquisition (Case 2, see EXPERIMENTAL DESIGN), perform these now, counterbalancing control and experimental groups according to the number of sessions required to complete pretraining. Then rebaseline on Step 9/Stage 5 before task-specific training.
Task
11. Proceed to Trial-Unique delayed Nonmatching-to-Location (TUNL) (Option A), or Location Discrimination and reversal (LD) (Option B).
Critical step We propose TUNL and LD as alternatives to assess memory for location and pattern separation in the touchscreen. For details on rationale and task differences, please see INTRODUCTION. As mentioned earlier, mice are not able to reliably acquire TUNL using the current protocol (in our experience).
A. TUNL
-
i.
Train subjects on once-daily sessions of TUNL, 5-7 days per week. Provide a single free reward at the start of each session (if your program does not do so automatically). Set up the apparatus as detailed for this task in MATERIALS and the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN. We recommend using a short (e.g., 2 s), fixed delay for initial acquisition. Place each subject in its assigned chamber, and start the session. The session usually finishes either after 60 min or after all trials (e.g., 84) are completed, whichever comes first.
Critical step Counterbalance chambers and testing times (time of day) across experimental groups.
Critical step Given that performance will be at chance at the start of training (resulting in a relatively high number of additional correction trials), limit sessions to 42 trials in 60 minutes initially. Continue until subjects can complete this in 30 min, then advance to 84-trial sessions. Give each subject an even number of these reduced sessions, such that they can be combined into full 84-trial sessions for analysis. If the subject completes fewer trials than required, the missed trials may be added on to the trials required in the next session (if less than ~10). If this situation occurs frequently, consider capping the number of trials per session further, to accommodate the slowest responders. It is important that animals complete sessions, so that all animals are exposed, in a counterbalanced fashion, to the same number of trials and trial types (which is particularly important in Cases 1 and 2).
Critical step At the end of each session, record critical data for each subject such as number of correct responses, number of trials completed. Most software programs will log many other measures (see Data analysis).
-
ii.
When all animals have reached criterion at least once, proceed to the next step. Criterion during acquisition of TUNL can be set to a certain performance level across all trial types (i.e. all separations), or can be measured for different separation conditions individually. We recommend a criterion of 80% correct, on two consecutive days, on the two largest separation conditions only. These separations include all trials in which response locations are -horizontally- separated by two or three inactive locations. In our experience this corresponds to a stable overall performance of approximately 70-75% correct.
Critical step It is essential to perform data analysis daily, to determine which animals have reached criterion (see Step 11A-iv).
Critical step Refer to the TROUBLESHOOTING section for solutions in the case of incompletion of sessions, or when animals are unable to reach criterion.
Critical step Consider, depending on the experiment, how to proceed when individual animals reach criterion. In TUNL, probe sessions that include variation in delay and/or separation are often applied in which case we recommend “resting” individual animals when they reach criterion (with regular reminder sessions) until all animals have acquired the task (for discussion of scenarios, see EXPERIMENTAL DESIGN, General considerations).
-
iii.
In the case of investigating the effects of post-acquisition treatment (Case 4, see EXPERIMENTAL DESIGN) on task manipulations (i.e. probe sessions) that are not part of regular task acquisition, expose animals to these conditions before treatment to avoid confounds due to novelty or contextual change, and to allow for a within-subject pre- and post-treatment comparison of performance level. Expose animals to these probe sessions until performance is stable.
-
iv.
In the case of investigating the effects of post-acquisition treatment (Cases 3 or 4, see EXPERIMENTAL DESIGN), perform the scheduled experimental treatment now, counterbalancing control and experimental groups according to acquisition speed (number of trials to reach criterion) and performance (percentage correct on the final phase of acquisition). In the case of e.g., microinfusion studies (Case 4), animals should be rebaselined after surgery, until a stable performance level is reached. Subjecting animals to the relevant probes should also occur at this point. Before commencing subsequent vehicle and drug infusion, a mock infusion involving the insertion of the infusion cannula only should be performed, followed by a vehicle infusion to assess non-specific effects on performance.
Post-training manipulations – probe sessions
-
v.
Expose animals to probe sessions, the nature of which may vary depending on the experimental hypothesis (for suggestions, see EXPERIMENTAL DESIGN, TUNL). Post-acquisition within-subject treatments (Case 4) may be performed at this stage in an appropriately controlled way, e.g., Latin square or cross-over design.
Critical step Probe sessions are usually similar to acquisition sessions with respect to the number of trials and maximum session time (1 h). Ensure that on each probe session, animals are exposed to the experimental conditions (i.e. trials with certain separation and/or delays) in a counterbalanced fashion, depending on the probe design.
Critical step In order to minimize the development of mediating strategies, alternating different probe sessions is recommended, in particular when using probe sessions of a fixed, maximum separation. We recommend a maximum of ~3 consecutive sessions.
Critical step The order of exposure to probes should be counterbalanced between groups. Regular analysis of performance is recommended to assess whether sufficient data on all permutations of delays and separations has been collected.
Data analysis
-
vi. Record the following behavioral variables for each subject:
- Number of sessions required to complete pretraining (Steps 5-9), and/or individual pretraining stages.
- Accuracy, expressed as the percentage correct, should be calculated as (number of correct responses)/(number of correct responses + number of incorrect responses) * 100. Note that this does not include data from correction trials. Overall accuracy, and accuracy on particular separation and delay conditions, should be calculated for each session. This is particularly important during acquisition in order to determine whether animals have reached criterion.
- Number of correction trials per session.
- Number of incorrect correction trials per session, to assess perseverative responding to certain locations on the screen.
- Correct response latency (ms), which is the time between exiting the magazine at initiation and making a correct response.
- Incorrect response latency (ms), which is the time between exiting the magazine at initiation and making an incorrect response.
- Reward collection latency (ms), which is the time between making a correct response and entering the magazine to collect the reward.
- Number of screen touches during the ITI and time out.
- Depending on the results, it may be advisable to perform analysis of the above variables on trials only during which the actual (self-imposed) delay does not exceed a certain value.
- For analysis, compare variables between experimental groups using the appropriate statistical tests. Exact choice of parameters and tests will depend on the nature of the experiment (e.g., as described in the different Cases in EXPERIMENTAL DESIGN), and number of experimental groups. For Cases 1 and 2, we often compare acquisition curves (plotted as percentage correct on daily sessions) using a repeated-measures analysis of variance. Alternatively (especially when animals have been “rested” after reaching criterion), the number of trials or sessions to criterion can be compared, using a t-test or ANOVA for independent samples. For Cases 3 and 4 a within-subject comparison can be performed, comparing subjects at asymptotic performance level or on specific probe sessions, pre- and post-experimental manipulation. In all instances, response latencies and reward collection latencies should be analyzed to assess whether results may be explained by non-specific effects on performance such as changes in motivation. In addition, statistical tests should be applied after checking for the appropriate assumptions for the test in question, e.g., the normality of the data.
B. LD
Location discrimination intermediate training phase
-
i.
Assign subjects (in each experimental condition, if pre-experimental or pre-acquisition manipulations have been conducted) to two groups counterbalanced according to the number of sessions required to complete pretraining, and mean performance on the last two days of pretraining. For one group, the stimulus in the left window (location 2) will be correct in the first intermediate training session, and for the other, the right window (location 5 for the mouse-mask; location 6 for the rat-mask, see MATERIALS, EQUIPMENT SETUP).
-
ii.
Begin training on once-daily sessions of LD intermediate-separation training, 5-7 days per week. Provide a single free reward (if your program does not do this automatically). Set up the apparatus as detailed for this task in MATERIALS and the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN, with reward contingency as appropriate for each subject. Place each subject in its assigned chamber, and start the session. The correct location for a particular animal at the end of an intermediate training session is used as the correct location for that animal at the beginning of the next session. Sessions terminate after 60 trials (mice) or 100 trials (rats), or 60 minutes, whichever comes first.
Critical step Ensure that animals in experimental and control groups complete a comparable numbers of trials per session throughout task acquisition (particularly important in Cases 1 and 2, see EXPERIMENTAL DESIGN). This can be done by only giving subjects 30 (mice) or 50 (rats) trials per session on the first few sessions. Continue with these reduced sessions until subjects complete them in 30 min. Ensure that you give an even number of such sessions, so that they can be combined for data analysis. If a subject completes fewer trials than required, the missed trials may be added on to the trials required in the next session, by increasing the maximum number of trials to be completed (only if less than ~10), or must be given in an additional session to ensure that exposure to the task is equal between subjects. If the problem persists, consider further capping the number of trials given per session to accommodate the lowest responders.
Critical step At the end of each session, record critical data for each subject (e.g., number of correct responses, number of trials completed, number of within-session reversals). However, most software programs will log many other measures (see Data analysis).
-
iii.
When all animals have reached the overall criterion, proceed to the next step. The overall criterion for the intermediate training phase is achieving acquisition and re-acquisition of the reversed contingencies in 3 out of 4 consecutive sessions. Regardless of the experimental manipulation (Cases 1-4), we suggest “resting” animals when they reach criterion, with “reminder” sessions once or twice per week (and continued food restriction), until the entire group has achieved criterion, see EXPERIMENTAL DESIGN. If an animal’s performance falls below criterion in a reminder session, that animal is trained daily until criterion is re-attained. When all animals have reached the overall criterion once, re-baseline the group (i.e. test all animals daily) before beginning probe sessions.
Critical step Refer to the TROUBLESHOOTING section for solutions in the case of incompletion of sessions, or when animals are unable to reach criterion.
-
iv.
If subjects are scheduled to receive experimental treatments after intermediate training but before probe sessions (i.e. post-acquisition between-subject manipulations; Case 3), perform these when all animals have achieved overall criterion once, counterbalancing control and experimental groups according to acquisition performance, and then re-baseline all animals. Progress to Step 11B-v when performance of all subjects has been stable at criterion, for at least two out of three consecutive days.
Critical step In case of investigating the effects of a post-acquisition treatments (Case 3 or 4) on behavioral challenges (i.e. probe sessions), animals should be exposed to these conditions before treatment to avoid confounds due to novelty or contextual change, and to allow for a within-subject pre- and post treatment comparison of performance level. In the case of microinfusion studies, animals should be rebaselined until stable prior to a mock infusion involving the insertion of the infusion cannula only, followed by a vehicle infusion to assess non-specific effects on performance.
Location discrimination performance probe sessions
-
v.
Assign subjects (of each experimental condition, if manipulations have been performed) into four groups, counterbalanced according to the number of days to criterion and the number of reversals to criterion in the intermediate training phase. In the case of testing LD in mice, using a mask with 6 locations, one group will receive the small separation condition first with the stimulus in the left window correct in the first probe session (location 3), another will receive the small separation condition with right correct (location 4). The third group will receive the large separation condition with left correct (location 1), and the final group the large separation condition with right correct (location 6). In the case of using a mask with 7 windows, as used in LD in rats, the middle location (location 4) remains inactive. The small separation is represented by locations 3 and 5, whereas the large separation condition is represented by the locations 1 and 7.
-
vi.
Proceed as in Step 11B-ii, but with a modified software program (see EXPERIMENTAL DESIGN). Post-acquisition within-subject treatments (Case 4) may be performed at this stage in an appropriately controlled way, e.g., Latin square or cross-over design. Train subjects in daily sessions, 6-7 days per week if possible. There is no trial limit and the session will terminate after 60 minutes or when the animal is able to acquire the initial location-reward contingency and the subsequent reversal (whichever occurs first). Give animals two consecutive sessions of each probe type (large, small) for multiple days (e.g., 20). As before, the final correct location of a session is used as the starting correct location in the next session, except when an animal is switched between probe session types, in which case the correct location is designated based on the initial counterbalancing and not the previous session.
Data analysis
-
vii. Record or calculate the following behavioral output measures per animal:
- Number of sessions required to complete pretraining (Steps 5-9), and/or individual pretraining stages
- Sessions/trials to criterion in the intermediate training phase.
- Average number of trials required to attain acquisition criterion for each probe separation (small, large) separately. This measure may be highly variable, and data may be absent for some sessions if the animal did not attain criterion, so for analysis, we suggest taking the average of this measure from several sessions, e.g., all sessions of a given separation. Include experimental group and separation as factors in the analysis.
- Average number of trials required to attain reversal (re-acquisition) criterion for each probe separation.
- Correct (or incorrect) reaction time, defined as the average latency to respond to the correct (or incorrect) stimulus, following the presentation of stimuli, averaged over all probe sessions.
- Magazine latency, defined as the latency to enter the magazine to collect reward following a correct response, averaged over all probe sessions.
- Number of screen touches during the ITI and time out.
- For analysis, compare variables described above between experimental groups using the appropriate statistical tests. Exact choice of parameters and tests will depend on the nature of the experiment (e.g., as described in the different cases in EXPERIMENTAL DESIGN), and number of experimental groups. Number of trials or sessions to overall criterion during the intermediate separation training phase can be compared between experimental groups, e.g., using a t-test for independent samples. To analyze the average number of trials taken to acquire (or re-acquire) the within-session criterion on the separation probes, we typically subject data to a repeated measures ANOVA with experimental group as a between-subject factor, and separation (small, large) as a within-subject factor. In all instances, response latencies and reward collection latencies should also be analyzed to assess whether results may be explained by non-specific effects on performance such as changes in motivation. In addition, statistical tests should be applied after checking for the appropriate assumptions for data distribution.
TROUBLESHOOTING
For general troubleshooting advice on the touchscreen operant chamber testing method, including technical issues, see Table 1.
Table 1.
Problem solving.
| Problem | Possible Reason | Solution |
|---|---|---|
| Incomplete consumption of reward | Animal insufficiently food restricted | Decrease weight as regulations permit |
| Animal insufficiently habituated to reward | Provide reward in home cage for additional days | |
| Unstable or poor performance | Low or excessive motivation | Closer attention to weight control; consider temporary feeding separation, according to rate of responding |
| Aversion to mask or touchscreen | Increase exploration of the mask and screen by applying food reward on the mask (e.g. peanut butter, pellets or other) | |
| Excessive fighting in home cage | Monitor home cage and general health of animal, separate if necessary | |
| Stressors in housing room (e.g. noise) | Make frequent observations of room and cage, move if necessary | |
| Poor learning ability | Exclusion may be necessary | |
| Abrupt decline in performance and/or trial completion | Touchscreen error (e.g. non-responsiveness, not displaying images) | Check physical connections, clean, run test program (if available), recalibrate, reboot the system |
| Reward delivery ceased or inconsistent | Check for physical blockage/disconnection, check for interface error, replace reward dispenser | |
| Initiation not detected | Clean magazine photobeam, check physical connections, replace if faulty | |
| Controlling system error (software or hardware) | Check physical connections, reboot the system, change hardware if necessary | |
| Animal appears to make unusually low/high number of beam crosses (Campden only) | Infrared beam failure | Clean infrared beam pathway, check position of infrared switch, replace faulty beams |
TUNL task specific troubleshooting
In TUNL, individual animals may fail to progress through a session. This is usually due to the fact that rats show perseverative responding to certain locations on the screen (location bias), as occasionally found in trials where both stimuli appear in one column (rats may prefer responding to the lower stimulus). In our experience, the problem is likely to reduce with further training. If not, the number of sequential correction trials can be capped. In addition to this, individual animals may have difficulties reaching criterion (especially when a different strain or disease model is used), even though recent changes such as increased stimulus size have produced substantial improvement in acquisition rate of TUNL (e.g., ANTICIPATED RESULTS). We suggest the following solutions in the case of slow learning rate. Firstly, when animals have acquired the non-matching rule, but are not attaining the (relatively stringent) criterion, lowering this criterion may be preferred to excluding individuals, so that group performance can be compared on subsequent probe sessions. It should be noted that lowering criterion will likely result in a more variable performance level during further testing (e.g., during probes). This is not desirable in, for example, pharmacological studies (e.g., Case 4) where treatments are often implemented within a single session. As an alternative to lowering the criterion, task parameters can be adjusted to facilitate learning (e.g., omitting smaller separations). However, in this scenario care should be taken to avoid the use of mediating strategies and we advise alternating “easier” sessions with normal sessions in which all separations are presented. Finally, certain animals may have to be excluded from the experiment when they are unable to reach criterion.
LD task specific troubleshooting
In LD, if the subject does not achieve acquisition and/or reversal (re-acquisition) criterion in a given session, data (“trials to criterion”) will not be generated. If there are several such instances of missing data for a given subject, it may be necessary to exclude the subject from analysis. For example, one might require that data is available from at least one session, from each session pair, or that at least 5 sessions of data are available from a full 10 sessions of each separation. If a large amount of data are missing, the number of mice failing to achieve acquisition and/or reversal (re-acquisition) criterion may be analyzed and compared between groups. This problem is likely to be more prevalent with mice, which may demonstrate a more variable performance and complete fewer trials per session. Reversal data are more likely to be incomplete than acquisition, because acquisition precedes reversal in each session. Note that acquisition data may still be analyzed even if reversal data are incomplete, although this may increase variability of acquisition performance.
TIMING
Approximate timing for each step below is indicated in number of sessions (i.e. days). As a rule, allow up to ~80 min per day per animal per testing session from Step 5 onwards. These 80 minutes include 60 min testing time, plus an additional 20 minutes for transporting animals from home- to testing room, setting up software, etc. Cumulative time taken to test all animals on a daily basis, depends on the capacity to load multiple animals per test-run (i.e. number of chambers). Subsequent values for the number of days (sessions) it takes to execute these experiments, reflect the approximate time it takes to test an average cohort of animals on each stage of the task and are estimates based on our experience.
Steps 1-4, Preparation for pretraining: ~3 to 10 (3+7) days. Timing depends on whether animals are acquired from an external source, in which case a 7 day acclimatization period is required, before onset of food restriction. After acclimatization, allow for approximately 3 days of initial food restriction before starting Stage 1 pretraining. Regular handling and weighing of animals can be started ~2 days after arrival. Reserve an average time per animal per day of ~5 min.
Steps 5-9, Pretraining: ~10-30 sessions. For TUNL pretraining, up to 15 sessions may be needed due to the extended Stage 3 (i.e. repeating step 7B). For LD in mice, occasionally up to 30 sessions may be expectec. Full pretraining is only necessary prior to the first instrumental task that an animal is tested on, otherwise expose animals to Step 9 only, using the box setup appropriate for the planned task (see MATERIALS).
Step 10, Experimental treatment (e.g., surgery): timing of this depends on the procedure, but allow for at least 7 days recovery in the case of surgery. Please note that this step is optional and can be implemented in the case of manipulations that are planned after pretraining, but prior to task acquisition (i.e. Case 2, see Experimental design). Please note that for TUNL and LD, we often apply experimental treatment after task acquisition, but prior to probe sessions. This has been earlier described as hypothetical options in Experimental design (Cases 3 and 4) and are described in the procedure as part of Step 11A-iv (TUNL) or Step 11B-iii (LD).
Step 11 (A, B), Training on TUNL and LD: In general, the duration of training of (any) touchscreen task, with respect to the number of sessions (i.e. days), will depend on the experimental design such as choice of animals, experimental manipulation (e.g., surgery) and research question (e.g., use of probes). Here we provide approximate timing with respect to the number of sessions (i.e. days) of several stages.
Step 11 Ai-ii: for TUNL acquisition, approximately 30-35 sessions are required for animals to reach criterion (using the recommended 3×5 mask on the lower half of the screen) for details see ANTICIPATED RESULTS.
Step 11 Aiii, v: The number of additional probe sessions required, depends on experimental design and variability in performance. In our experience, 4-10 sessions per probe condition may be needed.
Step 11 Bii-iii: for LD acquisition on the intermediate training phase, approximately 20-25 (rats) or 20-40 (mice) sessions may be expected.
Step 11 Bvi: Probing in LD on small versus large separation conditions may require an additional 20 sessions (rats and mice), again depending on experimental setup and variability.
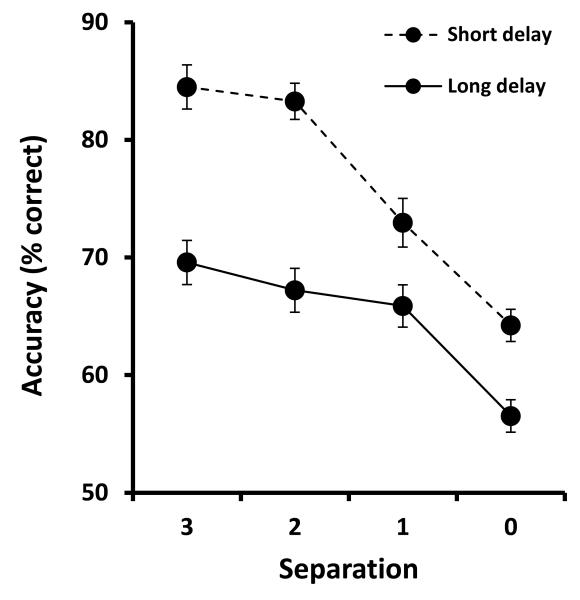
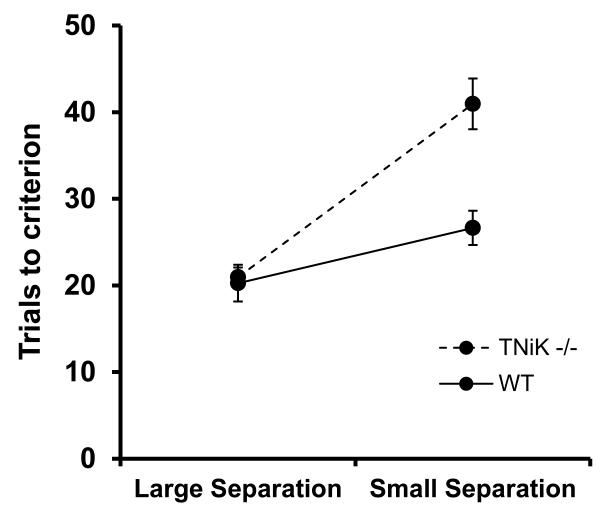
ANTICIPATED RESULTS
TUNL
TUNL is particularly interesting for assessing differences in delay-dependent and separation-dependent performance between groups, such as measured during post-acquisition probes. The example data described in this section were collected in healthy 10-month old, male Lister hooded rats (n=12) and averages are presented ± s.e.m. The mask dimensions specified in this protocol were used (see MATERIALS, 3×5 design, positioned on the lower half of the screen) and in this particular experiment the largest separation was omitted during initial acquisition (i.e. trials on which the far left and far right column are lit were not used, please note that we currently recommend including the largest separation during acquisition). Throughout task acquisition, a relatively short delay (2 s) was used. Animals were subjected to task acquisition until criterion was reached, after which individual rats were “rested” and rebaselined twice a week (see EXPERIMENTAL DESIGN). The task was acquired by all 12 rats used in this study, in an average of 1939 ± 79 trials or 34 ± 1 daily sessions (C.A.O., unpublished data). This is based on the criterion (as described in PROCEDURE, Step 11A-ii) of 80% correct, on 2 consecutive days on the largest separation in this task setup (i.e. trial types where lit response locations are separated -horizontally- by 2 blank squares). Overall performance level during rebaseline sessions after all rats had reached criterion, was 72.7 ± 1.1%.
In order to measure delay-dependent and separation-dependent performance, ten probe sessions with different delay and separation conditions (within session) were implemented. During probe sessions, all possible combinations of sample and choice location were presented, including the largest possible separation (i.e. 3 inactive response locations between the sample and choice location). Average performance on these probe sessions was calculated for different trial types with respect to delay and separation. Trial types were collapsed into four separation categories ranging from maximum [3 squares] to adjacent [0 squares] separation (i.e. separation is the number of response windows between the two active locations, horizontally). In this experiment, the mean correct response latency was 3.28 ± 0.31 s and the incorrect response latency was 3.96 ± 0.38 s. Average time to collect reward after a correct response was 1.22 ± 0.15 s. As can be seen from the graph (Fig. 3), performance depends on both separation and delay, as there is a significant within-subject effect of separation (p<0.001), delay (p<0.001) and an interaction (p=0.005) between the two. As for expected results on separation and delay in animal models of, for example, prefrontal cortex- or hippocampus pathology, those remain to be determined. It has been shown that the prefrontal cortex is necessary for delay-dependent performance in TUNL60, and a working memory deficit of up to ~15%, may be expected on longer delays (6 s), without changes in performance during no-delay conditions or when using similar separations60. In addition, work in animals with hippocampal lesions showed a similar drop in performance (up to ~20%) on long (but not short) delay conditions (6 s), and animals were impaired on small, but not large separations (impairment of ~10%)3.
Figure 3.
Average performance (error bars s.e.m.) of male Lister hooded rats (n=12) on TUNL probe-sessions for different delays and separations. Performance during short delay (0.5 s, dashed line) is higher than during long delay conditions (9 s, solid line). In addition, within each delay condition, accuracy is dependent on the spatial separation, which is expressed as the distance (i.e. number of blank windows) between response locations. A significant effect of separation (p<0.001), delay (p<0.001) and a significant interaction (p=0.005) was found.
Location Discrimination
As an example of LD task performance the effect of global TNiK (Traf2 and NcK interacting kinase) gene knock-out in male C57BL/6 × 129S5 mice72 is shown (see Fig. 4) compared to control mice of the same background. Task acquisition in the intermediate training phase was not different between groups (wt: 458.1 ± 45.8; TNiK-/- 576.3 ± 74.2 trials to criterion, p= 0.248). However, this genetic manipulation impairs task performance in a separation-dependent manner on probe sessions; performance is only impaired in the probe session with minimum distance between stimulus locations (small separation). There were no effects of genotype on response latencies (large separation: wt 5.0 ± 1.1 s; TNiK-/- 5.2 ± 0.8 s; small separation: wt 4.9 ± 0.7 s; TNiK-/- 5.0 ± 0.9 s.). For this analysis, the average number of trials needed for animals to reach criterion (seven correct touches out of eight responses for acquisition and reversal) was calculated for each separation condition. Differences between groups were tested using an independent samples t test or a repeated-measures ANOVA (genotype as between subject factor; separation as within-subject factor). A paired samples t test was used for post hoc analysis of significant interaction effects. Manipulations impairing hippocampal function, or specifically reducing hippocampal neurogenesis, have a similar effect28. A similar pattern of results is anticipated for rats, in light of the separation-dependent impairment of hippocampus-lesioned animals, found using a previous version of this protocol1. Conversely, manipulations increasing neurogenesis may selectively improve performance71.
Figure 4.
Average performance of TNiK-/- (n=12 , dashed line) and wild-type mice of the same background (C57BL/6 × 129S5, n=8, solid line) on the LD task in both small and large separation probe conditions (error bars s.e.m.). Genotype × separation interaction p<0.01; small separation p<0.05. Between-group differences are selectively observed in the small separation condition, with the TNiK-/- group requiring significantly more trials to reach the performance criterion, redrawn from72.
ACKNOWLEDGEMENTS
The protocols described here are those used in our laboratory at present, and were written by current members of the group. However many researchers have contributed to the development of touchscreen tasks and we would like to gratefully acknowledge their contribution. They include Susan Bartko, Jonathan Brigman, Suzanna Forwood, Carolyn Graybeal, Alicia Izquierdo, Louisa Lyon, Anelise Marti, Katie McAllister, Stephanie McTighe, Jess Nithianantharajah, Carola Romberg, John Talpos and Boyer Winters.
The research leading to these results has received support from the Innovative Medicine Initiative Joint Undertaking under grant agreement n° 115008 of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme [FP7/2007-2013]; the Wellcome Trust/MRC [089703/Z/09/Z] and Alzheimer’s Research UK [ART/PG2006/5]. A.E.H. receives funding from the European Union Seventh Framework Programme under grant agreements No. 241995 (Project “GENCODYS”) and No. 242167 (Project “SYNSYS”).
Footnotes
COMPETING FINANCIAL INTERESTS L.M.S. and T.J.B. consult for Campden Instruments Ltd. A.E.H. is an employee of Synome Ltd.
REFERENCES
- 1.McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- 2.Talpos JC, Dias R, Bussey TJ, Saksida LM. Hippocampal lesions in rats impair learning and memory for locations on a touch-sensitive computer screen: the “ASAT” task. Behav Brain Res. 2008;192:216–225. doi: 10.1016/j.bbr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ. A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology (Berl) 2009;205:157–168. doi: 10.1007/s00213-009-1526-3. [DOI] [PubMed] [Google Scholar]
- 5.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 6.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 7.Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiol Behav. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- 8.Olton DS. Mazes, maps, and memory. The American psychologist. 1979;34:583–596. doi: 10.1037//0003-066x.34.7.583. [DOI] [PubMed] [Google Scholar]
- 9.Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology (Berl) 1985;87:357–363. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- 10.Bierley RA, Kesner RP. Short-term memory: the role of the midbrain reticular formation. J Comp Physiol Psychol. 1980;94:519–529. doi: 10.1037/h0077684. [DOI] [PubMed] [Google Scholar]
- 11.Aggleton JP, Keith AB, Rawlins JN, Hunt PR, Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behav Brain Res. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- 12.Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampson RE, Deadwyler SA. Ensemble codes involving hippocampal neurons are at risk during delayed performance tests. Proc Natl Acad Sci U S A. 1996;93:13487–13493. doi: 10.1073/pnas.93.24.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussey TJ, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussey TJ, et al. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horner AE, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–1984. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nithianantharajah J, et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci. 2013;16:16–24. doi: 10.1038/nn.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mar A, et al. The touchscreen operant platform for testing executive function in rats and mice. Nat Protoc. 2013 doi: 10.1038/nprot.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 20.Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- 21.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 22.Treves A, Tashiro A, Witter MP, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 23.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 25.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 28.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisch AJ, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 32.Lucassen PJ, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 34.Toro CT, Deakin JF. Adult neurogenesis and schizophrenia: a window on abnormal early brain development? Schizophr Res. 2007;90:1–14. doi: 10.1016/j.schres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Jin K, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan MH, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 37.Wen PH, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav. 2008;7(Suppl 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 39.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 40.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 41.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 42.Oomen CA, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 50.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 51.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Seminars in cell & developmental biology. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Dunnett SB, Wareham AT, Torres EM. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1:61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- 55.Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 56.Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- 57.Dudchenko P, Sarter M. Behavioral microanalysis of spatial delayed alternation performance: rehearsal through overt behavior, and effects of scopolamine and chlordiazepoxide. Psychopharmacology (Berl) 1992;107:263–270. doi: 10.1007/BF02245146. [DOI] [PubMed] [Google Scholar]
- 58.Hearst E. Delayed alternation in the pigeon. Journal of the experimental analysis of behavior. 1962;5:225–228. doi: 10.1901/jeab.1962.5-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- 60.McAllister KAL, Saksida LM, Bussey TJ. Dissociation between memory retention across a delay and pattern separation following medial prefrontal cortex lesions in the touchscreen TUNL task. Neurobiology of Learning and Memory. 2013;101:120–126. doi: 10.1016/j.nlm.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Postman L, Underwood B. Critical Issues in Interference Theory. Memory & Cognition. 1973;1:19–40. doi: 10.3758/BF03198064. [DOI] [PubMed] [Google Scholar]
- 62.Dale RHI, Roberts WA. Variations in Radial Maze Performance under Different Levels of Food and Water-Deprivation. Animal Learning & Behavior. 1986;14:60–64. [Google Scholar]
- 63.Roberts WA, Dale RHI. Remembrance of Places Lasts - Proactive-Inhibition and Patterns of Choice in Rat Spatial Memory. Learning and Motivation. 1981;12:261–281. [Google Scholar]
- 64.Grant DS. Intertrial interference in rat short-term memory. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:217–227. [Google Scholar]
- 65.Nuechterlein KH, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 66.Barch DM, et al. CNTRICS final task selection: working memory. Schizophrenia bulletin. 2009;35:136–152. doi: 10.1093/schbul/sbn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 68.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 69.Meyer-Lindenberg AS, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 70.Carter CS, et al. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 71.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coba MP, et al. TNiK is required for postsynaptic and nuclear signaling pathways and cognitive function. J Neurosci. 2012;32:13987–13999. doi: 10.1523/JNEUROSCI.2433-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- 74.Beeler JA, Prendergast B, Zhuang X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol Behav. 2006;87:870–880. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 75.Roedel A, Storch C, Holsboer F, Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab Anim. 2006;40:371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- 76.Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Temperature rhythm reentrains faster than locomotor rhythm after a light phase shift. Physiol Behav. 2006;88:404–410. doi: 10.1016/j.physbeh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 77.Bussey TJ, Muir JL, Robbins TW. A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neurosci Res Commun. 1994;15:103–110. [Google Scholar]
- 78.Cardinal RN, Aitken MR. Whisker: a client-server high-performance multimedia research control system. Behavior research methods. 2010;42:1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]