Abstract
Background
IL-13, a helper T-cell type2 (Th2) cytokine transforms cultured airway epithelial cells to goblet cells and this is not inhibited by corticosteroids. IL-33 stimulates Th2 cytokines and is highly expressed in airways of persons with asthma. The effect of IL-33 on goblet cell differentiation and cytokine secretion has not been described.
Objective
We examined the effect of IL-33 on CXCL8/IL-8 secretion from goblet or normally differentiated human bronchial epithelial (NHBE) cells and signaling pathways associated with IL-33 activation in these cells.
Methods
NHBE cells were grown to goblet or normally differentiated ciliated cell phenotype at air-liquid interface in the presence or absence of IL-13. After 14 days, differentiated cells were exposed to IL-33 for 24 hours.
Results
CXCL8/IL-8 secretion into the apical (air) side of the goblet cells was greater than from normally differentiated cells (p<0.01) and IL-33 stimulated apical CXCL8/IL-8 release from goblet cells but not from normally differentiated cells (p<0.01). IL-33 increased ERK 1/2 phosphorylation in goblet cells (p<0.05) and PD98059, a MAPK/ERK kinase inhibitor attenuated IL-33 stimulated CXCL8/IL-8 secretion from goblet cells (p<0.001). IL-13 induced ST2 mRNA (p<0.02) and membrane bound ST2 protein expression on the apical side surface of goblet cells compared with normally differentiated cells, and neutralization with anti-ST2R antibody attenuated IL-33-induced apical CXCL8/IL-8 secretion from goblet cells (p<0.02).
Conclusions and Clinical Relevance
Goblet cells secrete CXCL8/IL-8 and this is increased by IL-33 through ST2R-ERK pathway suggesting a mechanism for enhanced airway inflammation in the asthmatic airway with goblet cell metaplasia.
Keywords: airway, asthma, goblet cell hyperplasia, IL-13, mucin, normal human bronchial epithelial cell, Th2 cytokine
Introduction
Asthma is characterized by structural and functional abnormalities of the bronchial epithelium, accumulation of inflammatory cells in the bronchial mucosa, and airway remodeling [1, 2]. The severity of mucus hypersecretion and airway goblet cell hyperplasia is associated with morbidity and mortality in asthma [3–5].
Allergen inhalation can trigger helper T-cell type2 (Th2)-type dominant inflammation in the lungs [6, 7]. The Th2 cytokines, IL-4, IL-9, and IL-13, can stimulate mucus production, bronchial hyperresponsiveness, and airway remodeling [2]. IL-13 in particular, has been shown to increase mucus production and goblet cell metaplasia in vivo and in vitro [2, 8–13].
IL-33 is a member of the IL-1 family that is a ligand for the orphan IL-1 family receptor ST2, and an inducer of Th2 immunity. IL-33 signals through a complex including the membrane bound ST2 (ST2L) protein [14]. ST2 is present on Th2 cells as well as mast cells, basophils, eosinophils, natural killer T cells [15], and bronchial epithelial cells [16]. The IL-33/ST2 axis triggers the release of proinflammatory chemokines and cytokines, and stimulates Th2 inflammation [14]. The IL-33/ST2 pathway also contributes to allergen-induced airway inflammation and hyperresponsiveness [17].
A likely source of IL-33 is the airway epithelium. IL-33 expression is increased in biopsies from the airways of subjects with asthma compared to healthy individuals and is greater still in subjects with severe asthma [18]. IL-33 expression is refractory to the effects of corticosteroids [18]. These data support a role for IL-33 in the pathogenesis of severe asthma.
Neutrophils are found in the airways of subjects with severe asthma and the extent of neutrophilia is related to the disease severity [19]. IL-8, now known as cysteine-X-cysteine chemokine 8 (CXCL8), is an important chemoattractant for neutrophil recruitment into airways of patients with severe asthma [20–22]. Normal human bronchial epithelial (NHBE) cells treated with IL-13 over 7 days have increased CXCL8/IL-8 secretion [23] and IL-13 exposure increases CXCL8/IL-8 in airway epithelial cells from healthy subjects and asthmatics [24].
IL-33 exposed mice have hypertrophy of the airway epithelium with large amounts of mucus and inflammatory infiltrates of neutrophils, mononuclear cells, and eosinophils in lung tissue and bronchoalveolar lavage fluid [14, 25].
We speculated that IL-33 would stimulate cultured NHBE cells toward goblet cell differentiation and stimulate CXCL8/IL-8 production in these goblet cells by activating the ST2 receptor (ST2R) pathway.
Materials and Methods
Reagents
Recombinant human IL-13 (rhIL-13), human ST2/IL-1 R4 antibody as well as anti-goat-IgG horseradish peroxidase (HRP) antibody were obtained from R&D Systems Inc. (Minneapolis, MN). Recombinant human IL-33 (rhIL-33) was obtained from Pepro Tech Inc. (Rocky Hill, NJ). Anti MUC5AC monoclonal antibody (45M1) was obtained from Lab Vision (Fremont, CA). PD98059 (2′-amino-3′-methoxyfl avone), an MAPK/ERK kinase (MEK) inhibitor (an upstream kinase of ERK1/2) was obtained from Calbiochem (La Jolla, CA). Phospho- and nonphospho-specific ERK1/2 and anti-rabbit-IgG HRP antibody were purchased from Cell Signaling Technology, Inc (Beverly, MA). Demethylsulfoxide (DMSO), anti-β-actin, anti-mouse-IgG-HRP antibody and all other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise indicated.
Culture and differentiation of NHBE cells
NHBE cells (Clonetics™ Lonza, Basel, Switzerland) were plated at 3,500 cells/cm2 in small airway epithelial cell growth medium (SAGM, Clonetcs™ Lonza) and cultured at 37 °C in a 5% CO2 incubator. Second passage NHBE cells were seeded at a density of 2.0×105/cm2 onto polyester inserts (6.5-mm diameter, 0.4-μm pore size and 10-μm thickness; Costar Transwell® Clear; Costar, Cambridge, MA) coated with type I rat tail collagen (Sigma, St. Louis, MO), and then cultured in serum-free DMEM/F12 medium containing Insulin-Transferrin-Selenium-Sodium Pyruvate (ITS-A) (1.0%; Invitrogen, Carlsbad, CA), triiodothyronine (10 ng/ml; ICN, Irvine, CA), epidermal growth factor (recombinant human EGF, 0.5 ng/mL; Invitrogen), all-trans retinoic acid (all-trans RA, 10−7 M; Sigma), hydrocortisone (0.5 μg/mL; ICN), bovine serum albumin (2.0 μg/mL; Sigma), bovine pituitary extract (30 μg/mL; Invitrogen) and antibiotic-antimycotic (1.0%; Invitrogen). Culture medium was added to both the apical and basolateral side of the inserts, and the cells were cultured submerged in medium. The culture medium was changed every 2 days. Confluence was reached within 5 days and the cells were then cultured at an air-liquid interface (ALI), feeding from the basolateral side only. The culture medium was changed every 2 days, and the cells were maintained in an incubator under a humidified 5% CO2 atmosphere at 37 °C [[8, 13, 26, 27].
IL-13 exposure
NHBE cells were grown from day 0 to day 14 with rhIL-13 at 2, 5 or 10 ng/mL, or vehicle (PBS). The culture medium with IL-13 or vehicle was changed every 48 h.
IL-33 exposure
NHBE cells were grown from day 0 to day 14 with rhIL-33 at 0.5, 5 or 10 ng/mL, or vehicle (PBS). The culture medium with IL-33 or vehicle was changed every 48 h.
IL-13 & IL-33 exposure
NHBE cells were grown with 5 ng/mL rhIL-13 or rhIL-13 vehicle (PBS) for 14 days. Cells were exposed from the basolateral side or the apical side to rhIL-33 (0, 0.5, or 5 ng/mL) for 24 hours. To collect samples, Hanks balanced salt solution was added to the apical (air) side of the inserts, and 24 hours-culture supernatants from the apical side and culture media from the basolateral side of inserts were harvested on day 14.
CXCL8/IL-8 secretion by ELISA
CXCL8/IL-8 protein in supernatants and culture media were measured using human CXCL8/IL-8 ELISA DuoSet (R&D Systems). The minimum detection level of CXCL8/IL-8 was 7.5 pg/mL. Optical density was measured at 450 nm using a microtiter plate reader (Spectra Max Plus; Molecular Devices, Sunnyvale, CA) with software (Soft Max Pro version 2.0; Molecular Devices). The concentration of each sample was obtained from standard curves and calculated as the mean of the results.
RT-PCR
To examine the effect of IL-13 on mRNA expression of CXCL8/IL-8, RNA was extracted from cells grown with rhIL-13 (0 or 5 ng/mL) for 14 days and exposed from the basolateral side or the apical side to rhIL-33 (0, 0.5, or 5 ng/mL) for 1 hour at day 14. To examine temporal mRNA expression of ST2, RNA was extracted from cells 5, 10, or 14 days after growing with rhIL-13 (0, 2, 5, 10 ng/mL). The preparation for RT-PCR was previously described [8, 13, 26, 27]. The apical side of the cells was washed three times with PBS, and total RNA from the cells was extracted using iScript RT-qPCR sample preparation reagent (Bio-Rad laboratories Inc., Hercules, CA, USA). The total RNA was then used to synthesize the first strand cDNA using the iScript cDNA synthesis kit (Bio-Rad). RT-PCR was performed on the C 1000™ thermal cycler equipped with CFX96™ real-time PCR system (Bio-Rad). For the relative quantification of ST2 mRNA expression, the mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control. EvaGreen was used as a DNA intercalator dye to monitor amplified DNA quantification, and real-time quantitative PCR curves were analyzed by the CFX Manager™ software (Bio-Rad) in order to obtain threshold cycle (Ct) values for each sample. mRNA expression level was calculated based on a generated standard curve.
The following primers were used [26–28]:
CXCL8/IL-8 forward: 5′-CTGCGCCAACACAGAAATTA-3′
CXCL8/IL-8 reverse: 5′-ACTTCTCCACAACCCTCTGC-3′
ST2L forward: 5′-CTGTCTGGCCCTGAATTTGC-3′
ST2L reverse: 5′-AGCAGAGTGGCCTCAATCCA-3′
soluble ST2 (sST2) forward: 5′-CTGTCTGGCCCTGAATTTGC-3′
sST2 reverse: 5′-TGGAACCACACTCCATTCTGC-3′
GAPDH forward: 5′-TGAACGGGAAGCTCACTGG-3′
GAPDH reverse: 5′-TCCACCACCCTGTTGCTGTA-3′.
Histochemical analysis
The cells on porous filters were fixed in 10% formalin neutral buffer, embedded in paraffin and cut into 8 μm slices. To examine morphology, haematoxylin-eosin (H&E) staining and periodic acid-Schiff (PAS) staining were performed [8, 13, 27]. Mucin production was confirmed using PAS staining, and goblet cells with secretory granules were evaluated with a light microscope (CKX41; Olympus, Tokyo, Japan) using a digital camera system (AxioCam ICc 1; Carl Zeiss, Thornwood, NY).
Immunohistochemical analysis
MUC5AC protein or ST2 expression was evaluated using immunohistochemistry. Sections were stained with anti-MUC5AC antibody or anti-ST2 antibody as 1st antibody. EnVisionTM+Dual Link System-HRP (DAKO, North America Inc., Carpinteria, CA) for MUC5AC or anti-goat IgG HRP for ST2 was added as the 2nd antibody. Antigen-antibody complexes were visualized using Liquid DAB+ Substrate Chromogen System (DAKO) [8, 13, 26, 27, 29].
Immunofluorescent confocal microscopy analysis
Acetylated α-tubulin (cilia protein) and MUC5AC (mucin) were identified in NHBE cells using immunofluorescence techniques and confocal microscopy [30, 31]. For the apical surface view, cultured NHBE cells membranes were cut from the insert using a scalpel and fixed in 3% formaldehyde. For the z-stack view, the cell membrane was embedded in Tissue-Tek OCT Compound (Sakura Finetechnical, Tokyo, Japan), frozen in liquid nitrogen and stored at −80 ° C. The frozen samples were cut into 8 μm slices using a cryostat at −20 ° C, mounted on slides and warmed to room temperature before fixation in 3% formaldehyde. The secondary antibodies were goat anti-mouse Alexa Fluor 488 (Invitrogen) for MUC5AC and goat anti rabbit Alexa Fluor 568 (Invitrogen) for acetylated α-tubulin. After incubation with the primary and secondary antibodies, the slides were rinsed with PBS three times, and nuclei were stained with DAPI (Invitrogen) for 1 min in the dark. The slides were again rinsed three times with PBS and immersed in VECTASHIELD® Mounting Medium for fluorescence (Vector Laboratories, Inc., Burlingame, CA). Coverslips were applied and the slides were stored at 4 ° C in the dark. Slides were examined within 24 h of staining using confocal laser scanning microscopy (LSM 700; Zeiss, Oberkochen, Germany). Immunostaining was visualized with a blue diode, argon ion, and helium neon laser using at 405, 488 and 568 nm laser lines, respectively.
The percentile ratios of MUC5AC (green) or acetylated α tubulin (red) stained area to total area were calculated using NIH Image J software (National Institutes of Health, Bethesda, MD). For the apical surface view, the stained areas were measured at a low magnification of x 20 in five random fields per specimen from 6 different inserts of cultured NHBE cells. For the z-stack view, the stained areas at apical surface of the cells were measured over 150 μm in five random sites per specimen from 6 different inserts of cultured NHBE cells.
Western blot analysis
For western analysis, cells were washed with cold PBS, and lysed on ice in a modified ratio immunoprecipitation buffer (1% Nonidet P-40, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris pH 7.5, 5 mM sodium pyrophosphate, 1 mM NaVO4, 5 mM NaF, 1μg/mL aprotinin, 1μg/mL leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride) for 15 minutes and scraped from the plates. DNA was collected by passing the lysate through a 27 gauge needle, and insoluble material was removed by centrifugation at 20,000 × g for 15 minutes at 4°C. The protein concentration of the supernatants was quantified using the Detergent Compatible (DC) Protein Assay (Bio-Rad). Equal amounts of protein extracts were loaded on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) mini gel and transferred to a nitrocellulose membrane (Bio-Rad) by Trans-Blot® Turbo™ Transfer system (Bio-Rad). Membranes were rinsed with distilled water, incubated overnight at 4°C in Tris-buffered saline (0.8% NaCl and 20 mM Tris pH 7.6) with 0.1% Tween 20 (TBS-T) with 5% nonfat dry milk to block nonspecific interactions, rinsed twice, and washed 3 times for 10 min with TBS-T. After washing, membranes were rinsed and incubated with the primary antibodies phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) or p44/42 MAPK (ERK1/2) rabbit polyclonal IgG (Cell Signaling Technology), or human ST2/IL-1 R4 goat polyclonal IgG for 2 hours in TBS-T. The membranes were then incubated for 2 hours with the anti-rabbit IgG HRP secondary antibody or the anti-goat IgG HRP secondary antibody. After washing, antibody binding was detected using LumiGLO chemiluminescent substrate peroxide (Cell Signaling Technology). Membranes were stripped with a stripping buffer (100mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris/HCL pH 6.7) for 20 minutes. Western blot images were scanned and analyzed using NIH Image J software (National Institutes of Health).
Statistical analysis
Data are expressed as mean values ± SEM. Comparisons between two groups were made by unpaired two tailed Student t test. Multiple comparisons were made by one-way analysis variance (ANOVA) except for ST2 mRNA expression over time, which was analyzed by two-way repeated measure ANOVA. A post hoc analysis for multiplicity was performed by using the Bonferroni’s method. Conventionally, p<0.05 was considered significant.
Results
IL-13-induced goblet cell differentiation
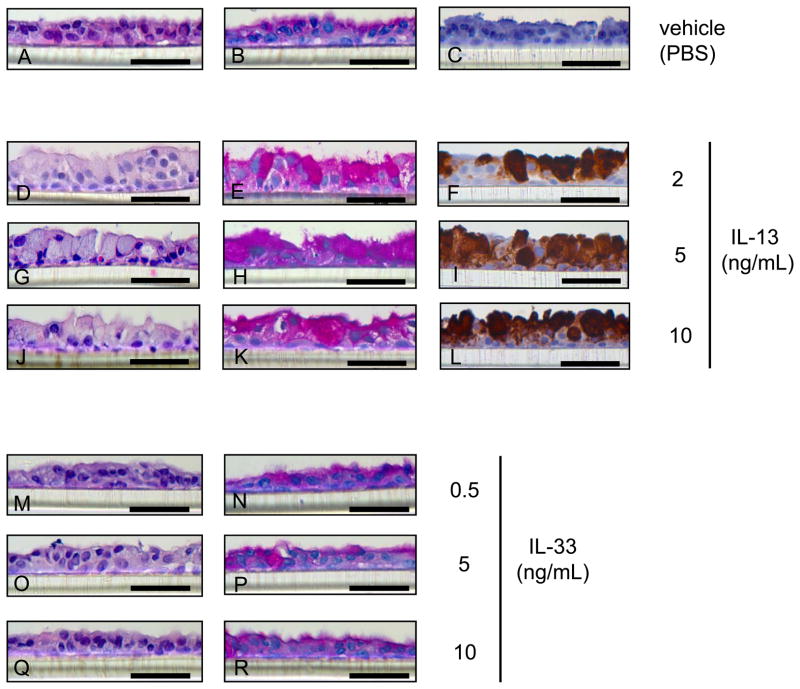
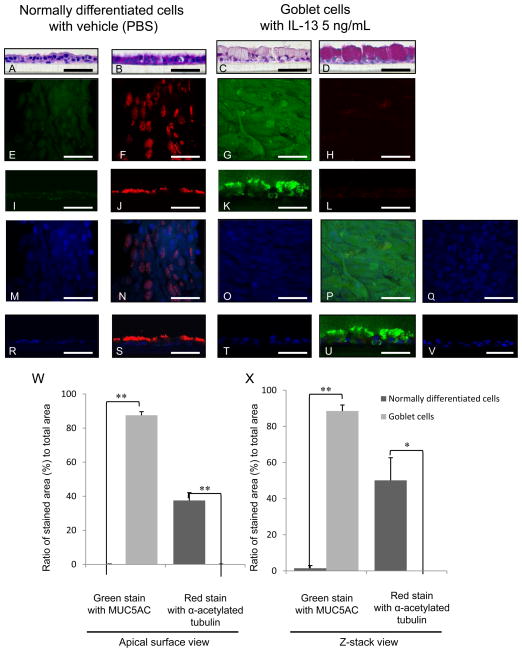
NHBE cells cultured at ALI demonstrated a well-differentiated morphology with ciliated cells at the surface of epithelial layers (referred to normally differentiated cells for convenience) (Figs. 1A–C, 2A, B, E, F, I, J, M, N, R, S) and weakly stained with PAS (Figs. 1B, 2B) and MUC5AC (Figs 1C, 2E, I), and strongly stained with acetylated α tubulin (Figs. 2F, J, N, S). In the presence of IL-13 at 2 (Figs. 1D–F), 5 (Figs. 1G–I, 2C, D, G, H, K, L, O, P, T, U) or 10 (Fig. 1J–L) ng/mL, NHBE cells differentiated into goblet cells with secretory granules (goblet phenotype) (Figs. 1D, G, J, 2C) strongly stained with PAS (Figs. 1 E, H, K, 2D) and MUC5AC (Figs. 1F, I, L, 2G, K, P, U), but not with acetylated α tubulin (Figs. 2H, L, P, U). The apical surface of normally differentiated cells is shown in red immunofluorescence using acetylated α-tubulin (Figs. 2F, J, N, S) and goblet cells in green using MUC5AC (Figs. 2G, K, P, U). Images (Figs. 2C, D, G, H, K, L, O, P, T, U) show predominantly goblet cells with few or no ciliated cells after growth 5 ng/mL IL-13. The ratio of green stained area with MUC5AC to total area increased to 87.53 ± 2.13 % at apical surface view (Fig. 2W) and 88.52 ± 3.34 % at z-stack view (Fig. 2X) in goblet cells compared to 0.19 ± 0.084 % at apical (Fig. 2W) and 1.48 ± 1.48 % at z-stack (Fig. 2X) in normally differentiated cells (p< 0.001). In contrast, the red stained area with acetylated α tubulin decreased to 0.18 ± 0.046 % at apical (Fig. 2W) and was undetectable at z-stack (Fig. 2X) in goblet cells compared to 37.53 ± 4.59 % at apical (Fig. 2W) and 50.13 ± 12.54 % at z-stack (Fig. 2X) in normally differentiated cells (p< 0.01).
Figure 1.
Haematoxylin and eosin (HE) staining (A, D, G, J, M, O, Q), Periodic acid-Schiff (PAS) staining (B, E, H, K, N, P R), and MUC5AC immunohistochemical staining (C, F, I, L) of normal human bronchial epithelial (NHBE) cells grown for 14 days at an air-liquid interface (ALI) with IL-13 at 2 (D-FC), 5 (G–I) or 10 (J–L) ng/ml, IL-33 at 0.5 (M, N), 5 (O, P) or 10 (Q, R) ng/ml, or vehicle (PBS) (A–C). Bar = 50 μm.
Figure 2.
HE staining (A, C), PAS staining (B, D), and immunofluorescence with laser confocal microscopy (E–V) of NHBE cells grown at an ALI with IL-13 vehicle (PBS) (A, B, E, F, I, J, M, N, R, S) or 5 ng/mL IL-13 (C, D, G, H, K, L, O, P, Q, T, U, V) for 14 days. Representative images of apical surface (E–H, M–Q) and z-stack (I–L, R–V). Immunofluorescence shows MUC5AC (Alexa Fluor 488, green; E, I, G, K), acetylated α-tubulin (Alexa Fluor 568, red; F, J, H, L), nuclei (DAPI, blue; M, R, Q, T), and merged image of the double staining and DAPI (N, S, P, U). The negative control of NHBE cells with 5 ng/mL IL-13 without the primary antibodies (Q, V). Bar = 50 μm. Ratio of green stained area with MUC5AC or red stained area with α acetylated tubulin to total area at (W) apical surface view or (X) z-stack view in immunofluorescence of NHBE cells grown with IL-13 vehicle (PBS) (normally differentiated cells) or 5 ng/mL IL-13 (goblet cells). The results are the means ± SEM from 6 samples. Significant differences are indicated by *p<0.01, **p<0.001.
IL-33-did not affect NHBE cells morphology
IL-33 at 0.5 (Fig. 1M, 1N), 5 (Fig. 1O, 1P) or 10 (Fig. 1Q, 1R) ng/mL did not affect NHBE cells differentiation (Fig. 1M–R) and were morphologically indistinguishable from cells grown without exposure to IL-33 (Fig. 1A, B)
IL-33 stimulated CXCL8/IL-8 protein secretion and mRNA expression in goblet cells
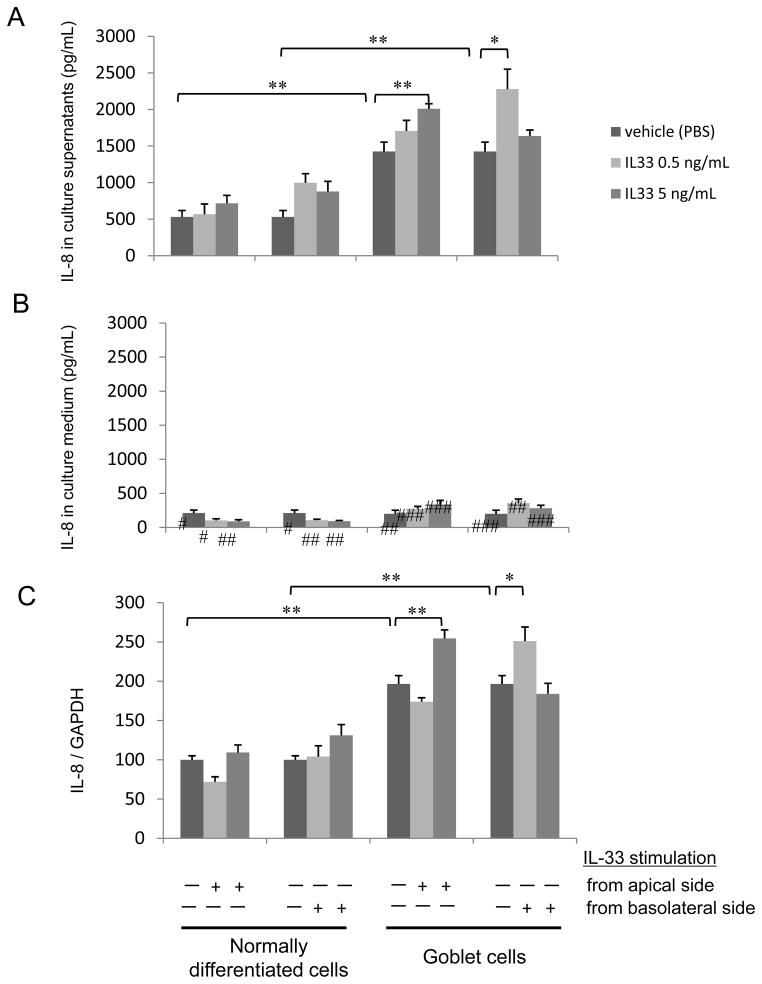
On day 14 after 24 hours after exposure to IL-33 (0, 0.5 or 5 ng/mL), CXCL8/IL-8 concentration was greater in the apical supernatants than the culture medium (Fig. 3A, B; p<0.01). IL-13 at 5 ng/mL further increased apical CXCL8/IL-8 secretion (Fig. 3A; IL-8 1426 ± 129 pg/mL with 5 ng/mL of IL-13 compared with no IL-13 p<0.01) but not in the basal culture medium (p=0.86). IL-13 at 5 ng/mL increased CXCL8/IL-8 mRNA expression (Fig. 3C; compared with no IL-13 p<0.01). The IL-13 exposed cells had >2-fold increase in the CXCL8/IL-8 mRNA/GAPDH ratio (Fig. 3C) compared to no IL-13 exposed culture. Apical exposure to 5 ng/mL IL-33 (p<0.01), or basolateral exposure (p<0.02) increased CXCL8/IL-8 concentration in the supernatants of goblet cells but not normally differentiated cells (Fig. 3A). Exposure to IL-33 did not affect CXCL8/IL-8 concentration in the basolateral side culture medium (Fig. 3B). Apical exposure to 5 ng/mL IL-33 (p<0.01), or basolateral exposure (p<0.05) increased CXCL8/IL-8 mRNA expression in goblet cells but not normally differentiated cells (Fig. 3C).
Figure 3.
Apical or basolateral IL-33 stimulated CXCL8/IL-8 secretion in (A) apical culture supernatants or (B) basolateral culture medium from normally differentiated cells or goblet cells. IL-33 (0. 0.5, or 5 ng/mL) was introduced at the apical or basolateral side of cells for 24 hours on day 14 of growth. The results are the means ± SEM from 6 samples. Significant differences are indicated by *p<0.02, **p<0.01. Significant differences from culture supernatant at each condition are indicated by #p<0.01, ##p<0.001, ###p<0.0001. (C) Apical or basolateral IL-33 stimulated CXCL8/IL-8 mRNA expression in normally differentiated cells or goblet cells. IL-33 (0. 0.5, or 5 ng/mL) was introduced at the apical or basolateral side of cells for 1 hour on day 14 of growth. The results are the means ± SEM from 4 samples. Significant differences are indicated by *p<0.05, **p<0.01.
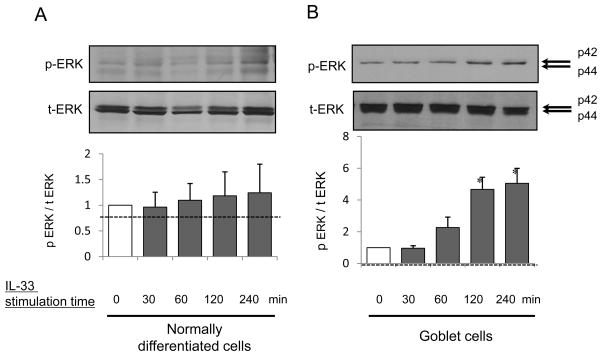
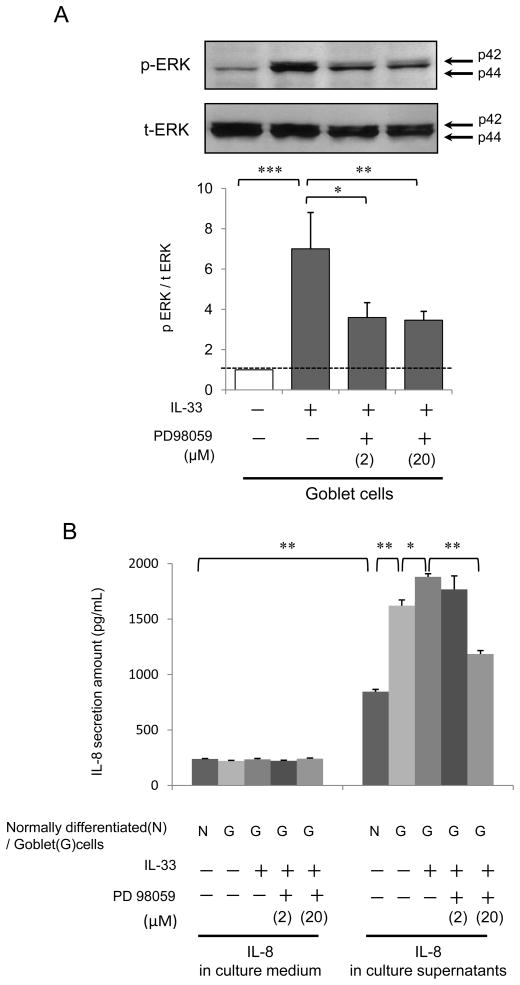
Western blot analysis of IL-33 stimulated phosphorylation of p44/42 MAPK (ERK1/2)
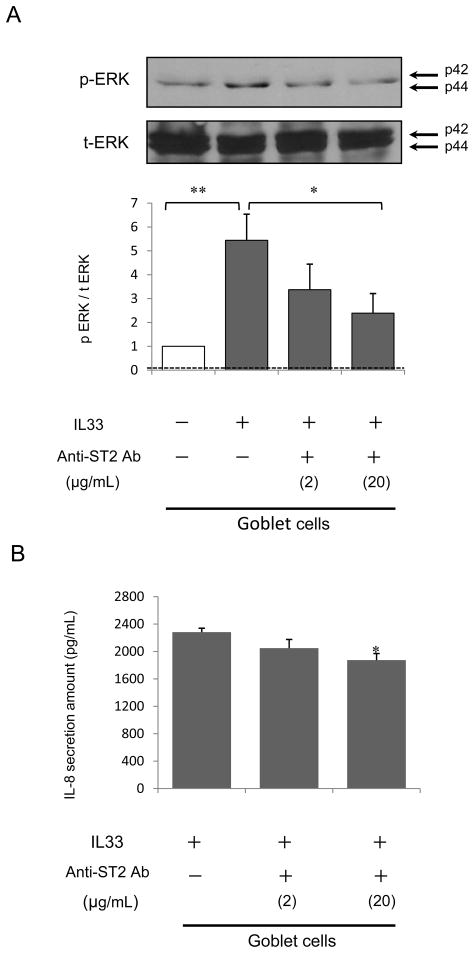
IL-33 phosphorylated ERK at 30, 60, 120 and 240 minutes in both normally differentiated cells (Fig. 4A) and goblet cells (Fig. 4B). Western blot analysis of cell lysates showed that IL-33 significantly increased p-ERK expression at 120 or 240 minutes in goblet cells (p<0.05) (Fig. 4B) but not normally differentiated cells (Fig. 4A). This was suppressed by PD98059 (2 or 20 μM) (Fig. 5A) or anti-ST2 R antibody (20 μg/mL) at the apical side of goblet cells (Fig. 7A). IL-33 alone increased the p-ERK/t-ERK ratio (p<0.01), and this was attenuated by PD98059 at 2 or 20 μM (2 μM; p<0.03, 20 μM; p<0.05) (Fig. 5A), or anti-ST2R antibody (20 μg/mL; p< 0.05) (Fig. 7A).
Figure 4.
NHBE cells were grown to normally differentiated ciliated cells or goblet cells with IL-13 vehicle (PBS) or 5 ng/mL IL-13 at an ALI. The cells were then grown without IL-13 and supplements for 48 hours before IL-33 stimulation to avoid influence of IL-13 and growth factors on cell signaling. Cell lysates with (A) normally differentiated cells or (B) goblet cells were harvested on day 16 after 0, 30, 60, 120 or 240 min apical exposure to 5 ng/mL IL-33. Western blot of phosphorylated p44/42 MAPK (ERK1/2) (p-ERK), p44/42 MAPK (ERK1/2) (t-ERK) expressed as the ratio of p-ERK/t-ERK. The results are the means ± SEM from 3 samples. Significant differences from 0 min are indicated by *p<0.05.
Figure 5.
(A) NHBE cells were differentiated to goblet cells with 5 ng/mL IL-13 at an ALI. The cells were then grown without IL-13 and supplements for 48 hours before IL-33 stimulation to avoid influence of IL-13 and growth factors on cell signaling. Cell lysates were harvested on day 16 after 120 min apical exposure to 5 ng/mL IL-33 or PBS after 2 hour pretreatment with PD98059 (0, 2 or 20 μM). Western blot data are expressed as the ratio of p-ERK/t-ERK. The results are the means ± SEM from 4 samples. Significant differences are indicated by *p<0.05, **p<0.03, ***p<0.01. (B) Apical IL-33 stimulated CXCL8/IL-8 secretion from goblet cells and this was attenuated by the MEK inhibitor, PD 98059. On day 14, cells were exposed to IL-33 after 2 hours of treatment with PD98059 at 0, 2 or 20 μM. The results are the means ± SEM from 6 samples. Significant differences are indicated by *p<0.01, **p<0.001.
Figure 7.
(A) Goblet cells were differentiated with 5 ng/mL IL-13 for 14 days at an ALI. The cells were then grown without IL-13 and supplements for 48 hours before IL-33 stimulation to avoid influence of IL-13 and growth factors on cell signaling. Cell lysates were harvested on day 16 after 120 min apical exposure to 5 ng/mL IL-33 or PBS after 2 hour pretreatment with anti-ST2R antibody (0, 2, or 20 μg/mL). Western blot data are expressed as the ratio of p-ERK/t-ERK. The results are the means ± SEM from 4 samples. Significant differences are indicated by *p<0.05, **p<0.01. (B) Goblet cells in culture were apically pretreated with anti-ST2R antibody (0, 2, or 20 μg/mL) before apical exposure to 5 ng/mL IL-33 for 24 hours on day 14. The results are the mean ± SEM from 5 samples. Significant differences from anti-ST2 antibody vehicle (PBS) are indicated by *p<0.02.
Effect of PD98059 on IL-33 stimulated CXCL8/IL-8 secretion from goblet cells
NHBE cells were grown with 0 or 5 ng/mL IL-13 at ALI and on day 14 PD98059 at 0, 2, or 20 μM was added to the basal culture medium. Apical supernatants and basal medium were collected after an additional 24 hours exposure to 0 or 5 ng/mL IL-33. This pretreatment with PD98059 at 20 μM attenuated IL-33 stimulated apical CXCL8/IL-8 secretion from the goblet cells (Fig. 5B; p<0.001).
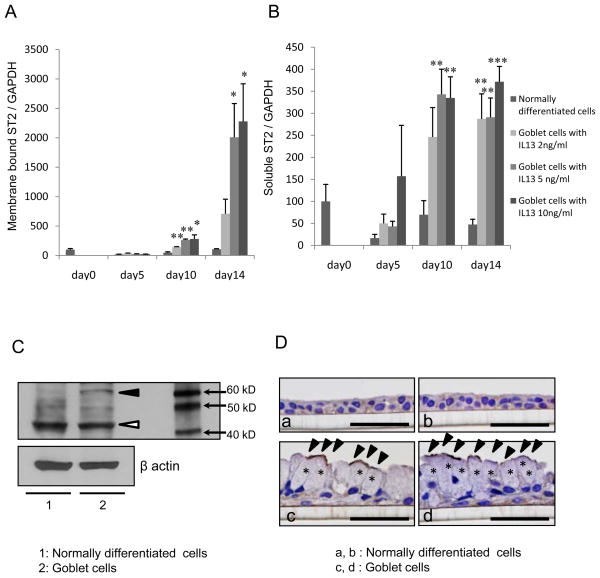
ST2 mRNA expression in goblet cells
IL-13 increased membrane bound ST2 mRNA expression (Fig. 6A; p<0.02 to PBS). The soluble ST2 mRNA expression also increased with exposure to IL-13 at 2, 5 or 10 ng/mL as shown in Fig. 6B (p<0.01at 2 or 5 ng/mL, p<0.001 at 10 ng/mL of IL-13 when compared with normally differentiated cells at each time point).
Figure 6.
The (A) membrane bound ST2 (ST2L) and (B) soluble ST2 (sST2) mRNA expression were increased in goblet cells with IL-13. (A) ST2L or (B) sST2 mRNA expression in cell lysates grown with basolateral exposure to IL-13 (0, 2, 5 or 10 ng/mL), for 0, 5, 10 or 14 days. The results are the mean ± SEM from 4 samples. Significant differences from PBS vehicle at each date are indicated by *p<0.02, **p<0.01, ***p<0.001.(C) ST2 was increased in goblet cells. NHBE cells were differentiated to goblet cells IL-13 at 5 ng/mL and the cell lysates were harvested on day 14. Representative Western blots of 3 separate experiments for ST2. β-actin was used as an internal housekeeping control. The white arrowhead identifies the sST2 and the black arrowhead, the ST2L. (D) Representative figures with ST2 immunohistochemical staining of (a, b) normally differentiated cells with IL-13 vehicle (PBS) or (c, d) goblet cells with 5 ng/mL IL-13 at an ALI. The asterisks indicated mucous granules and the black arrowheads identified strong staining with anti-ST2 antibody, suggesting the strong expression with ST2L on the apical side surface of goblet cells. Bar = 50 μm.
ST2 protein expression
Western blot analysis of cell lysates showed that the ST2L was expressed more in goblet cells than normally differentiated cells while the sST2 was similar in these cell types (Fig. 6C). Immunostaining showed that the apical side surface of goblet cells strongly stained with ST2 compared to normally differentiated cells (Fig. 6D).
Effects of anti-ST2/IL-1 R4 antibody on IL-33stimulated CXCL8/IL-8secretion
Pretreatment with anti-ST2R antibody (20 μg/mL) at the apical side of goblet cells attenuated IL-33 stimulated CXCL8/IL-8 secretion compared with PBS (Fig. 7B; p<0.02). Pretreatment with a normal goat polyclonal IgG control antibody at the apical side did not affect CXCL8/IL-8 secretion from goblet cells (data not shown). The 50% neutralization dose (ND50) with the anti ST2/IL-1 R antibody is typically 3–10 μg/mL.
Discussion
Because CXCL8/IL-8 is increased in more severe asthma, we hypothesized that, similar to the IL-13 data, IL-33 would drive cultured NHBE cells toward goblet cell differentiation and stimulate CXCL8/IL-8 production in these goblet cells by activating the ST2R pathway. IL-13 exposed cells were transformed to highly enriched goblet cells with MUC5AC protein covering the apical surface but cilia were not seen and that cilia, but not mucin was seen in the cells without exposure to IL-13. Although NHBE cells in the presence of IL-33 did not differentiate into goblet cells, exposure to IL-33 significantly increased CXCL8/IL-8 mRNA expression and apical CXCL8/IL-8 release from goblet cells but not from normally differentiated ciliated cells.
IL-33 is involved in the pathogenesis of allergic diseases, such as asthma [32], allergy [33] and atopic dermatitis [34]. IL-33 increases production of Th2 cytokines from hematopoietic cells that express ST2 [14, 35–39]. It has been reported that ST2 is preferentially expressed in NHBE cells and IL-33 induces CXCL8/IL-8 production via ST2 [28]. There is increased expression of IL-33 and ST2 in the nasal epithelial cells of patients with allergic rhinitis and IL-33 promoted production of CXCL8/IL-8 and GM-CSF via ST2 [40]. A genome-wide association study suggested that polymorphic variation in ST2 is associated with asthma severity [41]. We show here that IL-13 induced ST2 mRNA and ST2L protein expression on the apical side surface of goblet cells, and that anti-ST2R antibody attenuated IL-33-induced apical CXCL8/IL-8 secretion from these goblet cells. These findings further support the relevance of the IL-33/ST2 pathway in the asthmatic airway.
ST2 is produced as two isoforms. The membrane-bound isoform (ST2L) induces an immune response when bound to its ligand, IL-33. The other isoform is a soluble protein (sST2) that is thought to be a decoy receptor for IL-33 signaling [42]. We surmise that sST2 can be a negative feedback system in IL-33 induced neutrophil inflammation on asthmatic airway.
Binding of IL-33 to ST2 activates NF-κB and MAPKs, including ERK1/2, p38 MAPK and JNK, and induces Th2 cytokine expression [14, 34]. It was reported that IL-33 increases ERK phosphorylation in NHBE cells [28] and human nasal epithelial cells [40]. In contrast with these reports, we show that IL-33 mediated production of CXCL8/IL-8 from goblet cells but not normally differentiated ciliated cells and this is significantly attenuated by an ERK inhibitor or ST2 neutralization.
The exogenous administration of recombinant IL-33 to mice [14, 25, 32, 43] or the transgenic overexpression of IL-33 in mice [44] suggests that increased expression of IL-33 in vivo can increase the number of inflammatory cells in the airway through the release of endogenous Th2 cytokines and chemokines and administering anti IL-33 attenuates cytokine secretion and eosinophilic recruitment [45]. IL-33-deficient mice are resistant to allergen-induced bronchial hyperresponsiveness [46]. Both administration [25] and transgenic overexpression [44] of IL-33 in mice increases the number of airway neutrophils and eosinophils but neutrophils are not directly IL-33 responsive because they have few ST2 receptors [28].
Goblet cell metaplasia and mucus plugging are common in patients with severe asthma. These patients also show neutrophil recruitment into the airways [47–49]. Our results suggest that basolateral secreted CXCL8/IL-8 from bronchial cells may induce neutrophil migration to subepithelial connective tissue from submucosal blood vessels and these neutrophils may then migrate to the airway surface along a CXCL8/IL-8 concentration gradient from the basolateral side to apical side of bronchial epithelium. We surmise that IL-33 can promote neutrophil infiltration into the airway through IL-33 induced release of neutrophil chemoattractants, including CXCL8/IL-8 primarily from goblet cells. This may be one mechanism that prolongs neutrophil inflammation in the asthmatic airway.
Glucocorticosteroids are highly effective in controlling mild to moderate asthma [50]. Inhaled corticosteroids decrease IL-13 mRNA expression in steroid-sensitive asthmatics but not in steroid-resistant asthmatics [51]. Corticosteroids are less effective in suppressing IL-13-induced goblet cell metaplasia in animal models of asthma [52]. We have shown that dexamethasone does not inhibit goblet cell differentiation by IL-13, suggesting a possible mechanism of steroid-resistant in severe asthma [13]. It has been suggested that because IL-33 mediated inflammation is also corticosteroid resistant, this may be another mechanism for steroid resistance in severe asthma [28]. Dexamethasone does not affect TNF-α-induced IL-33 produced by cultures human airway smooth muscle cells [18], further supporting that IL-33-mediated inflammation is important in the pathogenesis of steroid-resistant asthma.
IL-33 and sST2 levels in induced sputum and serum are significantly higher in asthma compared with healthy subjects [53]. IL-33 promotes airway remodeling in asthma airways that is resistant to high-dose steroid therapy and IL-33 is a less steroid-sensitive than IL-13 in subjects with severe asthma [54].
There are several possible explanations for why the culture system we use creates a more enriched goblet cell phenotype compared to other studies [30, 55–57]. First, while other studies calculate the number of goblet cell nuclei to total cell nuclei [30, 56, 57], we calculated the MUC5AC positive staining area to the total area from confocal images. As well, the NHBE cell culture system with IL-13 that we developed differs in several ways including our use of different culture media and supplements [8, 12, 13]. For example, we use higher concentration of retinoic acid (RA) and epidermal growth factor (EGF) than used in studies [30, 56] where the goblet cell number was in the range of 20–40%. Other studies [55, 57] used 2 % UltroSer G serum substitute (Pall Bio Sepra, Cergy Saint Cristophe, France) instead of RA, EGF and growth factors. The concentration of EGF can affect MUC5AC expression [58] and the concentration of RA affects differentiation of NHBE cells under stimulation by IL-13 [59]. Finally others performed cultures using IL-13 at 10 [57], 20 [30] or 25 ng/mL [56] and for 48 hours [56], 5 days [55], 7 days [57] or 28 days [30] while we have demonstrated that in our cultures, the greatest goblet cell enrichment occurs at 14 days and with 5 ng/mL IL-13. None of these differences stands out as an explanation for why our culture conditions lead to such a highly enriched goblet cell phenotype but the results are consistent in our lab for over 5 years [8, 12, 13].
This study showed that IL-33 acts directly on goblet cells to stimulate CXCL8/IL-8 production. This may be a mechanism contributing to the mucus hypersecretion and neutrophil inflammation in the airways of persons with severe asthma [60, 61].
Acknowledgments
We thank the Department of Allergy and Immunology, National Research Institute for Child Health and Development, Akio Matsuda, PhD, for advise regarding design with PCR primers and the Anatomic Pathology Research Services at Virginia Commonwealth University (VCU) Medical Center, Jorge A. Almenara, PhD and histotechnologists for technical assistance in tissue processing, sectioning, and staining. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from the NIH-NINDS Center core grant (5P30NS047463). Also, we thank Melissa A. Yopp, Jennifer L. Bradley and Frances K.A. White for technical assistance, and Erika Tokita, MD, PhD, for helpful discussions.
This research was funded in part, by a grant from the Denny Hamlin Foundation.
Abbreviations
- ALI
air-liquid interface
- CXCL8
cysteine-X-cysteine chemokine 8
- DC
detergent compatible
- DMSO
demethylsulfoxide
- EGF
epidermal growth factor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
haematoxylin-eosin
- HRP
horseradish peroxidase
- ITS-A
Insulin-Transferrin-Selenium-Sodium Pyruvate
- NHBE
normal human bronchial epithelial
- PAS
periodic acid-Schiff’s
- RA
retinoic acid
- rhIL-13
recombinant human IL-13
- rhIL-33
recombinant human IL-33
- RT-PCR
real-time quantitative polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- sST2
soluble ST2
- ST2L
membrane bound ST2
- ST2R
ST2 receptor
- TBS-T
Tris-buffered saline with Tween 20
- Th2
helper T-cell type2
Footnotes
Author contributions:
Dr. Tanabe: is the principal investigator and guarantor of this manuscript. He contributed to the study design, conducting the experiments, data analysis, and preparing the draft of the manuscript and figures.
Dr. Shimokawaji: contributed to the data analysis and the performance of the study.
Dr. Kanoh: contributed to data collection, and revising of the manuscript.
Dr. Rubin: contributed to the study concept and design, analysis of the results, and editing of the manuscript for publication.
The authors report no conflicts or apparent conflicts of interest.
References
- 1.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 2.Izuhara K, Ohta S, Shiraishi H, Suzuki S, Taniguchi K, Toda S, Tanabe T, Yasuo M, Kubo K, Hoshino T, Aizawa H. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009;16:2867–75. doi: 10.2174/092986709788803196. [DOI] [PubMed] [Google Scholar]
- 3.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–50. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Sidebotham HJ, Roche WR. Asthma deaths; persistent and preventable mortality. Histopathology. 2003;43:105–17. doi: 10.1046/j.1365-2559.2003.01664.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 6.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 7.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107:9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol. 2011;45:1075–83. doi: 10.1165/rcmb.2010-0327OC. [DOI] [PubMed] [Google Scholar]
- 9.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 10.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–23. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 11.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L730–9. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K, Ise H, Yamaya M. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. Clin Exp Allergy. 2008;38:122–34. doi: 10.1111/j.1365-2222.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011;41:1747–56. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 16.Fujita J, Kawaguchi M, Kokubu F, Ohara G, Ota K, Huang SK, Morishima Y, Ishii Y, Satoh H, Sakamoto T, Hizawa N. Interleukin-33 induces interleukin-17F in bronchial epithelial cells. Allergy. 2012;67:744–50. doi: 10.1111/j.1398-9995.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- 17.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–81. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 19.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 20.Richman-Eisenstat JB, Jorens PG, Hebert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993;264:L413–8. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- 21.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–90. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 22.Gagliardo R, Chanez P, Vignola AM, Bousquet J, Vachier I, Godard P, Bonsignore G, Demoly P, Mathieu M. Glucocorticoid receptor alpha and beta in glucocorticoid dependent asthma. Am J Respir Crit Care Med. 2000;162:7–13. doi: 10.1164/ajrccm.162.1.9911032. [DOI] [PubMed] [Google Scholar]
- 23.Therien AG, Bernier V, Weicker S, Tawa P, Falgueyret JP, Mathieu MC, Honsberger J, Pomerleau V, Robichaud A, Stocco R, Dufresne L, Houshyar H, Lafleur J, Ramachandran C, O’Neill GP, Slipetz D, Tan CM. Adenovirus IL-13-induced airway disease in mice: a corticosteroid-resistant model of severe asthma. Am J Respir Cell Mol Biol. 2008;39:26–35. doi: 10.1165/rcmb.2007-0240OC. [DOI] [PubMed] [Google Scholar]
- 24.Lordan JL, Bucchieri F, Richter A, Konstantinidis A, Holloway JW, Thornber M, Puddicombe SM, Buchanan D, Wilson SJ, Djukanovic R, Holgate ST, Davies DE. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol. 2002;169:407–14. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]
- 25.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–77. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 26.Kanoh S, Tanabe T, Rubin BK. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest. 2011;140:980–90. doi: 10.1378/chest.10-2908. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe T, Kanoh S, Moskowitz WB, Rubin BK. Cardiac asthma: TGF-beta from the failing heart leads to squamous metaplasia in human airway cells and in the murine lung. Chest. 2012;142:1274–83. doi: 10.1378/chest.11-1710. [DOI] [PubMed] [Google Scholar]
- 28.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–50. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 29.Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, Oh HJ, Heo YJ, Park SH, Kim HY, Min JK. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J Korean Med Sci. 2011;26:1132–9. doi: 10.3346/jkms.2011.26.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG, Shields MD. Effects of IL-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Pediatr Res. 2011;69:95–100. doi: 10.1203/PDR.0b013e318204edb5. [DOI] [PubMed] [Google Scholar]
- 31.Parker J, Sarlang S, Thavagnanam S, Williamson G, O’Donoghue D, Villenave R, Power U, Shields M, Heaney L, Skibinski G. A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates unstimulated morphological differences between asthmatic and nonasthmatic cells. Pediatr Res. 2010;67:17–22. doi: 10.1203/PDR.0b013e3181c0b200. [DOI] [PubMed] [Google Scholar]
- 32.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 33.Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, Fujieda S, Nakamura Y, Yasuda K, Nakanishi K, Tamari M. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–81. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 34.Pushparaj PN, Tay HK, H’Ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–8. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–4. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 36.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–90. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 37.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–34. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 39.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Kamekura R, Kojima T, Takano K, Go M, Sawada N, Himi T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin Exp Allergy. 2012;42:218–28. doi: 10.1111/j.1365-2222.2011.03867.x. [DOI] [PubMed] [Google Scholar]
- 41.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 42.Ho JE, Chen WY, Chen MH, Larson MG, McCabe EL, Cheng S, Ghorbani A, Coglianese E, Emilsson V, Johnson AD, Walter S, Franceschini N, O’Donnell CJ, Consortium CA, Group CIW, Dehghan A, Lu C, Levy D, Newton-Cheh C, Group CHFW, Lin H, Felix JF, Schreiter ER, Vasan RS, Januzzi JL, Lee RT, Wang TJ, Assimes TL, Deloukas P, Erdmann J, Holm H, Kathiresan S, Konig IR, McPherson R, Reilly MP, Roberts R, Samani NJ, Schunkert H, Stewart AF. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J Clin Invest. 2013;123:4208–18. doi: 10.1172/JCI67119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–90. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 44.Zhiguang X, Wei C, Steven R, Wei D, Wei Z, Rong M, Zhanguo L, Lianfeng Z. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett. 2010;131:159–65. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–5. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis. 1991;143:138–43. doi: 10.1164/ajrccm/143.1.138. [DOI] [PubMed] [Google Scholar]
- 48.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–21. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 49.Nadel JA, Takeyama K, Agusti C. Role of neutrophil elastase in hypersecretion in asthma. Eur Respir J. 1999;13:190–6. doi: 10.1034/j.1399-3003.1999.13a35.x. [DOI] [PubMed] [Google Scholar]
- 50.Stoloff SW, Kelly HW. Updates on the use of inhaled corticosteroids in asthma. Curr Opin Allergy Clin Immunol. 2011;11:337–44. doi: 10.1097/ACI.0b013e328348a813. [DOI] [PubMed] [Google Scholar]
- 51.Naseer T, Minshall EM, Leung DY, Laberge S, Ernst P, Martin RJ, Hamid Q. Expression of IL-12 and IL-13 mRNA in asthma and their modulation in response to steroid therapy. Am J Respir Crit Care Med. 1997;155:845–51. doi: 10.1164/ajrccm.155.3.9117015. [DOI] [PubMed] [Google Scholar]
- 52.Kibe A, Inoue H, Fukuyama S, Machida K, Matsumoto K, Koto H, Ikegami T, Aizawa H, Hara N. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am J Respir Crit Care Med. 2003;167:50–6. doi: 10.1164/rccm.2110084. [DOI] [PubMed] [Google Scholar]
- 53.Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013;50:803–9. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 54.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, Denney L, Bossley CJ, Oates T, Walker SA, Bush A, Lloyd CM. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. 2013;132:676–85.e13. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue D, Numasaki M, Watanabe M, Kubo H, Sasaki T, Yasuda H, Yamaya M, Sasaki H. IL-17A promotes the growth of airway epithelial cells through ERK-dependent signaling pathway. Biochem Biophys Res Commun. 2006;347:852–8. doi: 10.1016/j.bbrc.2006.06.137. [DOI] [PubMed] [Google Scholar]
- 56.Yadav UC, Aguilera-Aguirre L, Ramana KV, Boldogh I, Srivastava SK. Aldose reductase inhibition prevents metaplasia of airway epithelial cells. PLoS One. 2010;5:e14440. doi: 10.1371/journal.pone.0014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010;43:652–61. doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mata M, Sarria B, Buenestado A, Cortijo J, Cerda M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005;60:144–52. doi: 10.1136/thx.2004.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Wetering S, Zuyderduyn S, Ninaber DK, van Sterkenburg MA, Rabe KF, Hiemstra PS. Epithelial differentiation is a determinant in the production of eotaxin-2 and -3 by bronchial epithelial cells in response to IL-4 and IL-13. Mol Immunol. 2007;44:803–11. doi: 10.1016/j.molimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Rubin BK, Tomkiewicz R, Fahy JV, Green FH. Histopathology of fatal asthma: drowning in mucus. Pediatr Pulmonol. 2001;(Suppl 23):88–9. doi: 10.1002/ppul.1950230851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carroll N, Carello S, Cooke C, James A. Airway structure and inflammatory cells in fatal attacks of asthma. Eur Respir J. 1996;9:709–15. doi: 10.1183/09031936.96.09040709. [DOI] [PubMed] [Google Scholar]