Abstract
Smoking during pregnancy is associated with a variety of untoward effects on the offspring. However, recent epidemiological studies have brought into question whether the association between neurobehavioral deficits and maternal smoking is causal. We utilized an animal model of maternal smoking to determine the effects of prenatal cigarette smoke (CS) exposure on neurobehavioral development. Pregnant mice were exposed to either filtered air or mainstream CS from gestation day (GD) 4 to parturition for 4 hr/d and 5 d/wk, with each exposure producing maternal plasma concentration of cotinine equivalent to smoking <1 pack of cigarettes per day (25 ng/ml plasma cotinine level). Pups were weaned at postnatal day (PND) 21 and behavior assessed on at 4 weeks of age and again at 4–6 months of age. Male, but not female, offspring of CS-exposed dams demonstrated a significant increase in locomotor activity during adolescence and adulthood that was ameliorated by methylphenidate treatment. Additionally, male offspring exhibited increased aggression, as evidenced by decreased latency to attack and number of attacks in a resident intruder task. These behavioral abnormalities were accompanied by a significant decrease in striatal and cortical dopamine and serotonin and a significant reduction in brain-derived neurotrophic factor (BDNF) mRNA and protein. Taken in concert, these data demonstrate that prenatal exposure to CS produces behavioral alterations in mice that are similar to those observed in epidemiological studies linking maternal smoking to neurodevelopmental disorders and suggest a role for monoaminergic and BDNF alterations in these effects.
Keywords: ADHD, maternal smoking, BDNF, dopamine, hyperactivity, aggression, nicotine
Introduction
Despite many public health programs promoting the risks of smoking while pregnant, only 25% of women who smoke quit smoking cigarettes during pregnancy. Several reports established that maternal smoking during pregnancy adversely affects pre- and postnatal growth and increases the risk of fetal mortality, pre-term birth, low birth weight, and altered cognitive development (Wigle et al., 2008). Importantly, the adverse effects of maternal smoking on neurodevelopment persist through at least the adolescent period and may extend into adulthood (Keyes et al., 2011).
Although a large number of epidemiological studies reporting increased neurobehavioral deficits following maternal smoking exist, recent reports questioned their validity. The most notable regards the association between maternal smoking and attention-deficit hyperactivity disorder (ADHD). Several groups linked maternal smoking to an increased risk of attention-deficit hyperactivity disorder (Banerjee et al., 2007; Langley et al., 2005; Linnet et al., 2003). In one of the first studies to directly assess the relationship between maternal smoking and ADHD, Milberger and co-workers (Milberger et al., 1998) found a 2.7-fold increased risk for ADHD associated with maternal smoking, a finding that has been replicated by several groups in a variety of cohort studies (Leech et al., 1999; Linnet et al., 2005; Obel et al., 2009; Thapar et al., 2003; Weissman et al., 1999). Using a cross-sectional study design from the National Health and Nutrition Examination Survey, Braun and co-workers found that pre- but not postnatal exposure to tobacco smoke led to an increased risk of ADHD diagnosis and the authors calculated that maternal smoking led to 270,000 excess cases of ADHD (Braun et al., 2006). This finding was confirmed even when more stringent criteria were applied (Froehlich et al., 2009).
However, several recent papers concluded that there is no causal relationship between maternal smoking and behavioral dysfunction, or that the effect sizes in previous studies were over-estimated (Ball et al., 2010; Langley et al., 2012; Lindblad and Hjern, 2010; Obel et al., 2011; Thapar et al., 2009). Rather, these studies argued that the association between maternal smoking and these effects is likely the result of a variety of confounders including, genetic factors, other environmental factors, and/or existing maternal psychopathology that were not satisfactorily adjusted for in the previous studies.
To address this controversy and to gain insight into the mechanism(s) responsible for potential neurobehavioral effects of maternal smoking, we employed a pregnant mouse model of maternal smoking. Data demonstrate that in utero exposure to cigarette smoke (CS; at a concentration reflective of smoking <1 pack of cigarettes/d) produces behavioral alterations in the offspring (specifically, the males) that are similar to those observed in epidemiological studies of maternal smoking, including hyperactivity and increased aggression. Mechanistically, these behavioral deficits are associated with decreased monoamine levels and brain-derived neurotrophic factor mRNA and protein. These data provide mechanistic support for the reported link between in utero CS exposure and behavioral deficits in the epidemiological literature.
Materials and methods
Animals
B6C3F1 male and female mice were purchased from Jackson Laboratory (Bar Harbor, ME). These mice were chosen based on previous experiments characterizing developmental outcomes of CS exposure (Ng et al., 2006). Mice were housed in pairs (females) or individually (males) in polycarbonate cages (with corncob bedding) in temperature-controlled (20°–23°C) and humidity-controlled (~55% RH) rooms. Food (purified AIN-98) and tap water were available ad libitum. The light/dark cycle was maintained on 12 h intervals. Mice were acclimated for at least 1 week prior to use. For breeding, a single male mouse was paired with two females for four days, with the 4th day of coupling designated gestation day (GD) 4. All animal procedures were conducted under an animal protocol approved by New York University and Robert Wood Johnson Medical School’s (RWJMS) Institutional Animal Care and Use Committee (IACUC).
Smoke Generation
Mainstream cigarette smoke was generated from the burning of filtered 1R3F cigarettes (Kentucky Tobacco Research & Development Center, Lexington, KY) using an automated cigarette generation system (Baumgartner-Jaeger CSM 2070, CH Technologies [USA] Inc., Westwood, NJ). Reference cigarettes were stored long-term at 4°C–7°C (55% RH); 24h prior to use, cigarettes were relocated to a humidor and stored at 20°–23°C (55% RH). The continuous smoking machine was adjusted to load and light 4–5 cigarettes simultaneously, each of which produced 2-s puffs of 35ml volume/puff under the control of an automatically regulated piston pump that cycled once per minute (Ng et al., 2006). Smoke was diluted 90% prior to introduction into the exposure chamber. Filtered dilution air entered the bottom of the generation chamber, and the output was introduced into the top of the chamber.
Cigarette Smoke Exposure
Males were removed and 2 females per cage were exposed whole-body to either mainstream cigarette smoke (CS) or filtered air in poly-carbonate cages with wire mesh tops. Cages were rotated among three racks in the chamber to assure even smoke distribution to all animals. Chamber levels of carbon monoxide (CO) and total suspended particulates (TSP) were monitored throughout the exposure. Particle samples were collected from the exposure chambers every hour (for the entire duration of exposure) on Pallflex EMfab filters (Pall Corporation, East Hills, NY); mean TSP levels were determined gravimetrically from filters weighed before and after sampling. Chamber CO levels were measured continually over the entire 4-hr exposure period suing a 48C CO analyzer (Thermo Environmental Instruments, Inc., Franklin, MA). This exposure paradigm produces plasma cotinine levels in the dams of approximately 25–28 ng/ml (Ng et al., 2006), equivalent to smoking 1–7 cigarettes per day, or less than 1 pack (Peacock et al., 1998).
On GD18, dams were separated and housed individually and each mother/offspring set was maintained in clean filtered air following parturition. Pups were weaned at 3 weeks of age and group housed based on sex with no more than 5 mice per cage. Early behavioral parameters (social interaction, play, and initial motor activity determinations) determined at 4 weeks of age, as described below. Following these determinations, mice were shipped to RWJMS and allowed to acclimate for 2 months before additional behavioral testing was conducted. All subsequent behavioral analysis and sacrifices for neurochemical determinations were conducted during the light phase.
Social Interaction and Play Behaviors
Pups were individually housed 4–5 days prior to the social behavioral test session. Pairs of non-sibling, same-sex and same-treatment condition pups were placed in a standard large cage crossed by infrared beams and observed for social interactions at 4-weeks of age for one 30 minute session and scored by two trained observers for the number of times that a member of the pair engaged in a behavior. During testing, the behaviors observed were: ano-genital sniffs, face sniffs, crawl-under/ over behaviors, self-grooming, fighting behaviors, paired motor activity, and allogrooming. Allogroom behaviors are defined as one mouse rising up on its hind legs to touch paws and snout to the other mouse to perform grooming motions. For more extensive information on testing conditions please refer to Yochum and co-workers (Yochum et al., 2008).
Locomotor Activity
For studies in 4-week old mice, the activity chamber consisted of a standard large cage crossed by infrared beams (Opto-Varimex, Columbus OH), as described previously (Sheleg et al., 2013). The number of horizontal motor movements was calculated by infrared beam breaks for a 30 minute session. For studies in adults, 4- month old mice were placed in sound-attenuated boxes equipped with photobeams (Med Associates, St. Albans, VT). Locomotor activity was quantified over 1–2 hours, with the first 30 min considered a habituation period, and calculated over 5 min blocks and summed across the session. For experiments with methylphenidate, mice were habituated to the open field by 4 daily 60 min sessions. On the fifth day, mice were gavaged with saline after a 30 min habituation period in the locomotor box, and locomotor activity monitored for an additional 60 min. On the sixth day, mice were gavaged with methylphenidate (1 mg/kg) after the 30 min habituation period and locomotor activity monitored for an additional 60 min. Locomotor activity was calculated as total distance traveled in cm.
Resident Intruder Task
Residents were adult male mice (4 months old) that were individually housed two weeks prior to testing. Intruders were age-matched adult males of the same treatment as the resident. An intruder was introduced into the home cage of the resident for a ten min session. The latency for the first attack, the number of attacks and which mouse attacked (either the resident or intruder) were recorded.
HPLC Analysis
HPLC analysis was performed as described previously (Bradner et al., 2013; Schuh et al., 2009). Briefly, samples were sonicated in 500 µL of 0.1 N perchloric acid and centrifuged at 14,000 rpm for 20 min at 4°C. The pellets were kept for protein assay and the supernatants were filtered and an aliquot of 5 µl supernatant was injected into HPLC with electrochemical detection (Waters, Milford, MA, USA) for neurochemical analysis of norepinephrine (NE), dopamine (DA) and its metabolites, 3,4- dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) as well as serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). These components were separated on a cation exchange column (MD-150 × 3.2 column, ESA Biosciences Inc.) using isocratic mobile phase (MD-TM mobile phase, ESA Biosciences Inc.) containing 2.2 mM NaCl pumped at constant flow rate of 0.5 mL/min. The compounds were quantified by electrochemical detection using a glassy carbon working electrode (2 mm diameter) with an in situ silver reference electrode (flow cell, 2 mm GC WE, ISAC, Waters, Milford, MA, USA).
Quantitative Real-Time PCR (qPCR)
qPCR was performed as described previously (Fortin et al., 2013). RNA from the striatum of the offspring was isolated using the Qiagen RNEasy Lipid Tissue Mini Kit (Valencia, CA) according to instructions by the manufacturer. RNA concentration was determined by standard spectrophotmetric analysis and 1 µg of total RNA was used for cDNA synthesis with the Applied Biosystems High Capacity cDNA Archive kit (Bedford, MA) according to the manufacturer’s protocol. qPCR was performed using an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Bedford, MA). Reactions were performed in a total volume of 25 µl using SYBR Green Master Mix (Life Techologies, Grand Island, NY). To normalize the amount of total mRNA present in each reaction, levels of actin were monitored in parallel samples. The amount of target (treated sample), normalized to an endogenous reference (actin) and relative to the calibrator (control sample), was defined by the Ct method. All primer sets yielded a single PCR product of expected size by agarose gel electrophoresis. Specificity was routinely monitored by checking product melting curves (dissociation curves) in each reaction well. The forward and reverse primer sequences for Bdnf (NM_007540.4) were ATGTCTATGAGGGTTCGGCG and CAGTTGGCCTTTGGATACCG, respectively. Actin sequences were described previously (Fortin et al., 2013).
Western Blot
Western blot analysis was used to quantify the amount of tyrosine hydroxylase (TH) and brain derived neurotrophic factor (BDNF) in samples of striatal tissue from CSexposed and control mice as described previously (Richardson et al., 2008). Briefly, samples (5–10 µg protein) were subjected to polyacrylamide gel electrophoresis on 4–20% precast NuPage gels (InVitrogen, Carlsbad, CA) and transferred to a PVDF membrane. Membranes were then incubated overnight with anti-TH (EMD Millipore, Billerica, MA). TH antibody binding was detected using a goat anti-rabbit horseradish peroxidase secondary antibody and enhanced chemiluminescence (Pierce Super Signal Dura West). The luminescence signal was captured on an Alpha Innotech FluorChem E imaging system (Protein Simple, San Jose, CA) and stored as a digital image for quantification by densitometry. Membranes were stripped for 15 minutes at room temperature with Pierce Stripping Buffer and re-probed with anti-BDNF (sc-33904, Santa Cruz Biotechnology, Santa Cruz, CA), then stripped and re-probed with α-tubulin (Sigma-Aldrich, St. Louis, MO) to ensure equal protein loading across samples. Data were calculated based on ratio of the protein of interest to tubulin based on individual densitometric values.
Statistical Analysis
The litter was considered the smallest unit of analysis, with each litter representing an independent replication (n = 6–10 litters per treatment group). No more than one pup per litter was used for any analysis. Statistics were calculated using SPSS (Chicago, IL) or GraphPad Prism 5.0 (La Jolla, CA). Body weight gain for dams and pups were analyzed by repeated measures ANOVA. All other neurochemical and behavioral data were analyzed using ANOVA or Student’s t-test where appropriate. When a significant F was determined, post hoc comparisons were performed using Bonferroni-corrected t-tests for 2-way ANOVA. Statistical significance is reported at p ≤ 0.05.
RESULTS
General Health of Dams and Reproductive Outcomes
Out of 10 dams bred for each condition, 8/10 control dams and 10/10 CS-exposed dams gave birth. Dam weight gain measured on gestational days (GD) 11, 15, and 19 did not differ significantly between the control and CS exposure group (p = 0.14; Table 1). Although there were fewer pups born to CS-exposed dams compared to control, this did not quite reach statistical significance (p = 0.08; Table 1). This finding was likely influenced by two litters born to CS-exposed dams that consisted of only two pups. To determine whether maternal behavior towards her pups was altered by CS exposure, a pup retrieval task was performed. No significant effect of treatment was found in the latency for the dam to retrieve her pups (Table 1).
Table 1.
Effects of Mainstream Cigarette Smoke Exposure on Gestational Parameters and Maternal Behavior
| Cigarette | ||
|---|---|---|
| Control | Smoke | |
| No. of Litters | 8 | 10 |
| Pups per Litter | 8.25 ± 0.70 | 6.10 ± 0.86 |
| Pup Retrieval (sec) | 31.71 ± 3.29 | 34.40 ± 3.72 |
| Dam weight gain (g) (cumulative GD11-19) | 15.50 ± 0.91 | 14.60 ± 1.40 |
Data represent mean and standard error.
Juvenile Behavior Analysis
Locomotor Activity
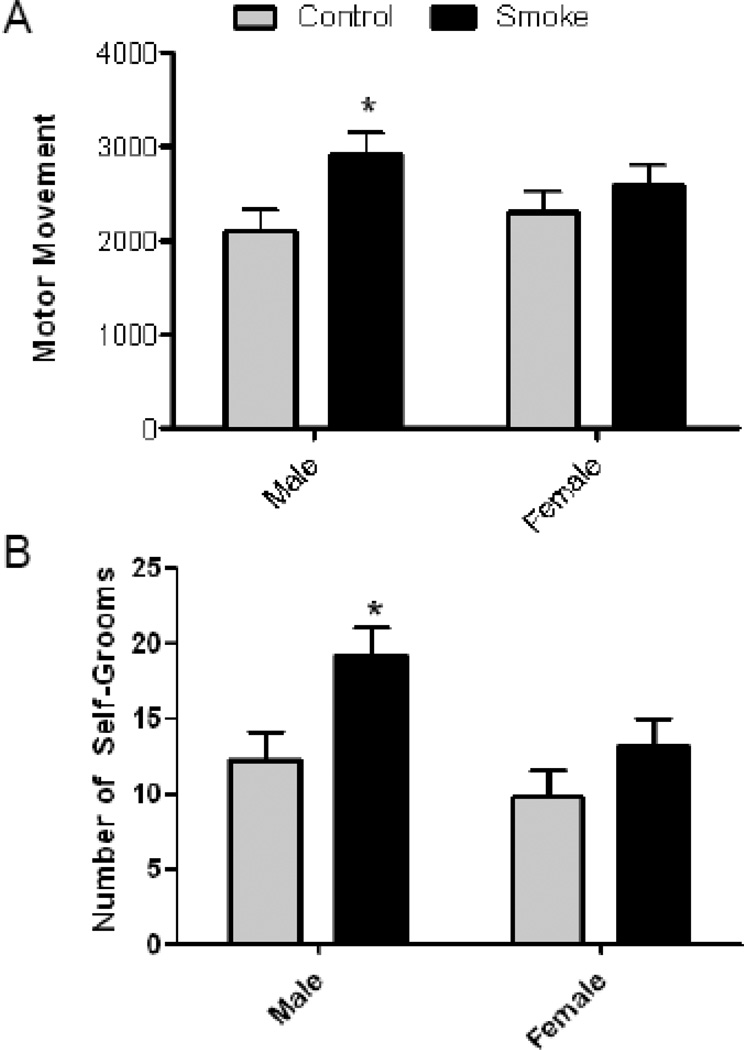
To determine whether the offspring of dams exposed to CS during gestation exhibited altered motor activity levels either in isolation or in a social environment, mice were monitored individually on postnatal day (PND) 30 prior to social testing and during the social test condition. In the individual motor movement condition there was a main effect of treatment, as CS-exposed male offspring exhibited significantly more horizontal motor movements than air treated offspring of both sexes and female cigarette smoke exposed offspring (Fig. 1A) [F(1,20) = 5.939, p = 0.02]. There were no significant differences seen for either sex (2124 for males and 2591 for females) or treatment in the paired (social) condition (data not shown).
Figure 1. Prenatal Cigarette Smoke Exposure Increases Locomotor Activity and Self-Grooms in Juvenile Mice.
Four week old, mice (n=8) were tested in a novel environment for 30 min and the number of photobeam breaks and self-grooms recorded. Male, but not female, offspring exposed in utero to cigarette exhibit (A) increased motor movement and (B) self-grooms. p<0.05 by 2-way ANOVA followed by Boneferroni-corrected t-tests.
Social Interaction and Play
As a further assessment of social behaviors in exposed offspring, treatment matched pairs were observed for social, grooming and play behaviors in an open field environment. There was a significant effect of CS exposure on the number of self-grooms, with the offspring of CS-exposed mice grooming significantly more than air-exposed mice (Fig. 1B) [F (1, 14) = 7.14, p = 0.02]. No significant effects were observed for anogenital sniffs, face sniffs, crawl-under/over behaviors, or allogrooming (data not shown).
Adult Behavior Analysis
Locomotor Activity
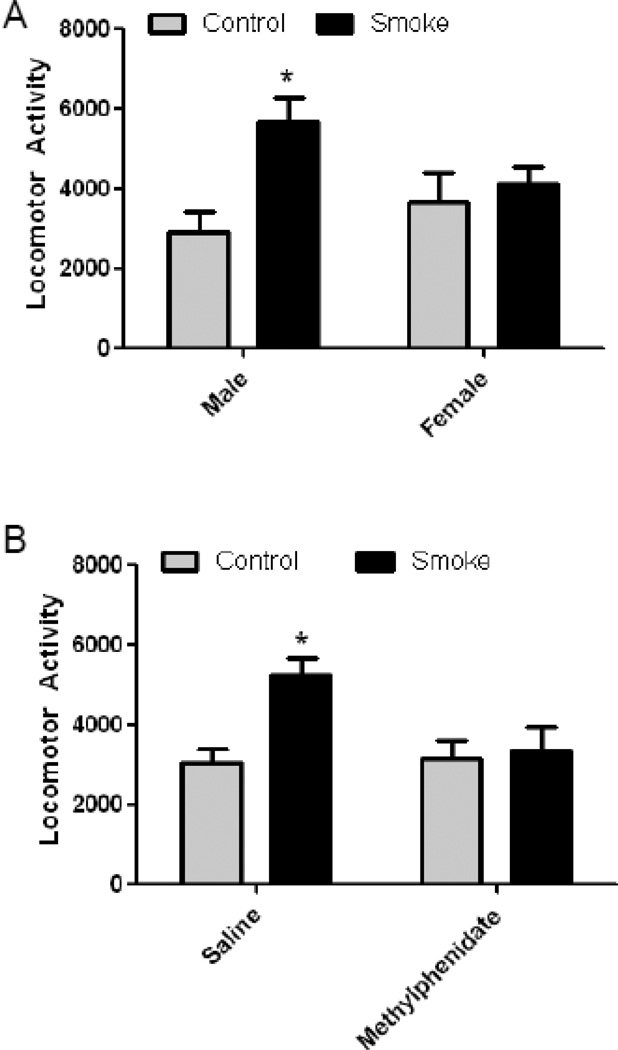
In a second cohort of animals, locomotor activity of 4-month old mice exposed to CS in utero was monitored in sound-attenuating boxes for 60 min and the total distance traveled (cm) determined. Offspring of mice exposed to CS during gestation had significantly increased locomotor activity [F (1, 24) = 7.95, p = 0.001] and there was a significant treatment × sex interaction (p = 0.05). Bonferroni-corrected posthoc tests revealed that only the male offspring of CS-exposed mice exhibited significantly increased locomotor activity and that it was increased by 93% compared to air-exposed males (p < 0.01; Fig. 2A).
Figure 2. Prenatal Cigarette Smoke Exposure Increases Locomotor Activity in Adult Male Mice that is Ameliorated by Methylphenidate.
At 4 months of age, mice in (n=8) locomotor activity (total distance traveled in cm) was measured for 30 min after a 30 min habituation period. (A) Male, but not female, offspring exposed in utero to cigarette smoke (CS) exhibited increased locomotor activity, as determined by total distance traveled. (B) Administration of methylphenidate (1 mg/kg) to the CS-exposed male offspring reduced locomotor activity to control levels. N=8; p<0.05 by 2-way ANOVA followed by Boniferroni-corrected t-tests.
Based on the purported association of prenatal CS and ADHD in humans, we sought to determine whether the increased locomotor activity could be ameliorated by methylphenidate. Mice were first habituated to the open field for 4 consecutive days. There were no differences in rate of habituation between control and CS-exposed mice (data not shown). Following the habituation period, mice were administered saline on day 5 and their locomotor activity monitored. The next day, mice were administered methylphenidate and their locomotor activity monitored. There was a significant effect of CS on locomotor activity [F (1, 24) = 6.18, p = 0.02] and also a significant CS × methylphenidate interaction (p = 0.04). Administration of methylphenidate significantly reduced locomotor activity in CS-exposed offspring back to the levels of air-exposed offspring (Fig. 2B).
Resident Intruder Task for Aggression
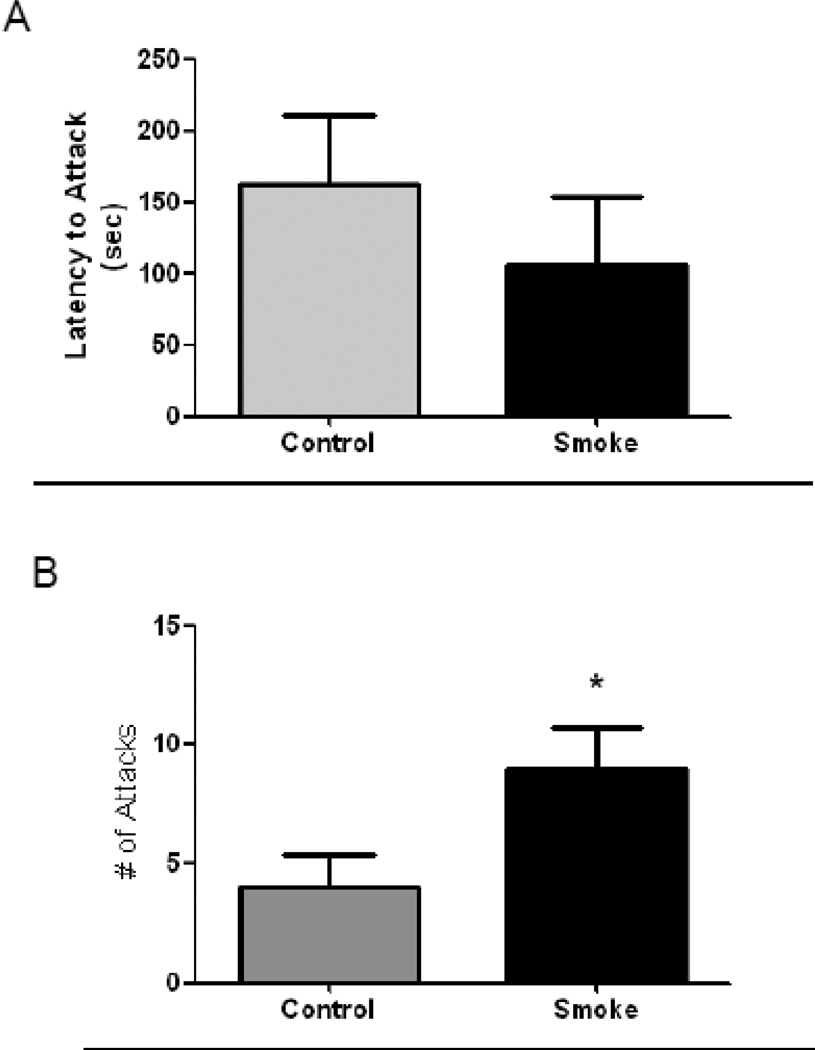
The resident intruder task was used to measure aggression in the male offspring of mice exposed to CS during pregnancy. Although there were clear trends for a decreased latency of CS-exposed resident mice to attack the intruder and an increased total number of attacks, these did not quite reach statistical significance, likely because of a relatively small number of animals performing the task (n = 5). However, there was a significant increase in the total number of attacks by CS-exposed mice compared to control mice (p < 0.05) (Fig. 3A, B).
Figure 3. Prenatal Cigarette Smoke Exposure Increases Aggressive Behavior.
At 4 months of age, aggressive behavior was measured with the resident intruder task. (A) Male offspring exposed in utero to cigarette smoke (CS) exhibited a slight decrease in latency to attack that did not reach statistical significance. (B) CS-exposed male offspring exhibited a significantly higher number of attacks. N=5; p < 0.05 by Students t-test.
Neurochemical Analysis
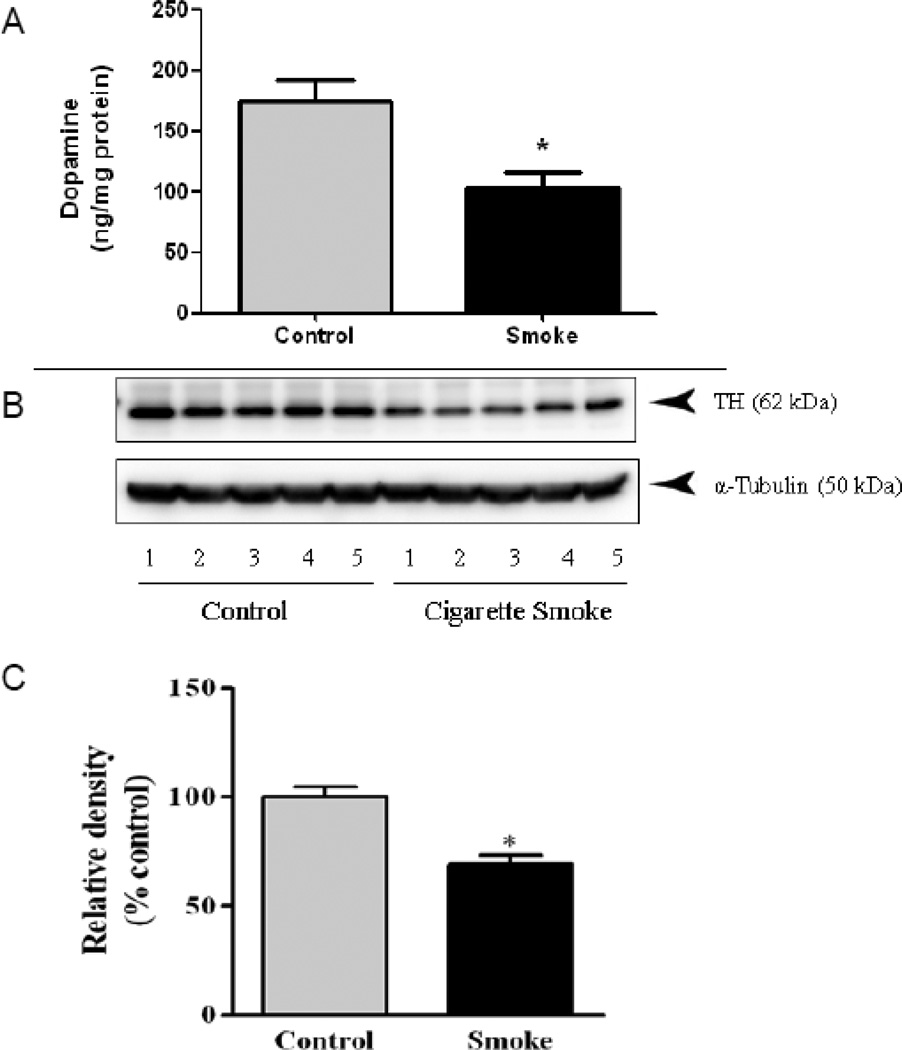
To determine potential mechanisms for the observed behavioral effects of the male offspring of mice exposed to CS during pregnancy, catecholamine levels were measured in the cortex and striatum. CS exposure caused a significant 42% decrease in striatal levels of dopamine [DA; t = 4.96, df = 13, p = 0.0003; Fig. 4A], with no significant changes observed in the levels of the dopamine metabolites, homovanillic acid (HVA) or 3,4-dihydroxyphenylacetic acid (DOPAC; Table 2). Levels of serotonin (5HT) in the striatum were also significantly decreased by 46% [t = 4.21, df = 13, p = 0.001, Table 2]. Similar to that observed with DA, there was no significant effect on the 5HT metabolite 5-hydroxyindoleacetic acid (5HIAA). In the cortex, DA levels were decreased by 34%, although statistical significance was not quite reached (p = 0.051).
Figure 4. Prenatal Cigarette Smoke Exposure Reduces Striatal Dopamine and Tyrosine Hydroxylase Levels.
(A) Striatal dopamine, as measured by HPLC with electrochemical detection, was decreased in 4-month old male offspring exposed in utero to cigarette smoke (CS). (B,C) Western blot analysis of tyrosine hydroxylase (TH) revealed significantly decreased TH protein levels in CS-exposed male offspring. n=5, p < 0.05 Students t-test.
Table 2.
Effects of in Utero Cigarette Smoke Exposure on Catecholamine Levels in Adult Male Mice.
| Cigarette | ||
|---|---|---|
| Control | Smoke | |
| Males | ||
| Striatum | ||
| DA | 174.20 ± 17.96 | 98.02 ± 12.24 |
| HVA | 27.40 ± 2.44 | 28.48 ± 2.66 |
| DOPAC | 13.49 ± 2.15 | 10.81 ± 1.13 |
| 5-HT | 5.30 ± 0.43 | 2.85 ± 0.38* |
| 5-HIAA | 3.50 ± 0.28 | 4.26 ± 0.41 |
| Frontal Cortex | ||
| DA | 5.51 ± 0.53 | 3.63 ± 0.71 |
| DOPAC | 0.38 ± 0.04 | 0.37 ± 0.04 |
| HVA | 1.02 ± 0.18 | 0.93 ± 0.24 |
| 5-HT | 6.69 ± 0.37 | 5.63 ± 0.72 |
| 5-HIAA | 6.00 ± 0.48 | 5.80 ± 0.46 |
| NE | 6.60 ± 0.45 | 6.74 ± 0.54 |
Data represent mean and standard error. N = 5.
To determine whether decreased dopamine levels were accompanied by decreased protein levels of tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis, striatal TH was measured by western immunoblotting. The male offspring of mice exposed to CS during pregnancy resulted in a significant [t = 5.46, df = 4, p = 0.006] 30% decrease in TH protein (Fig. 4B,C). There was no significant change in TH protein levels in the striatum of female mice (Supplemental Fig. 1).
Brain-Derived Neurotrophic Factor (BDNF) Levels
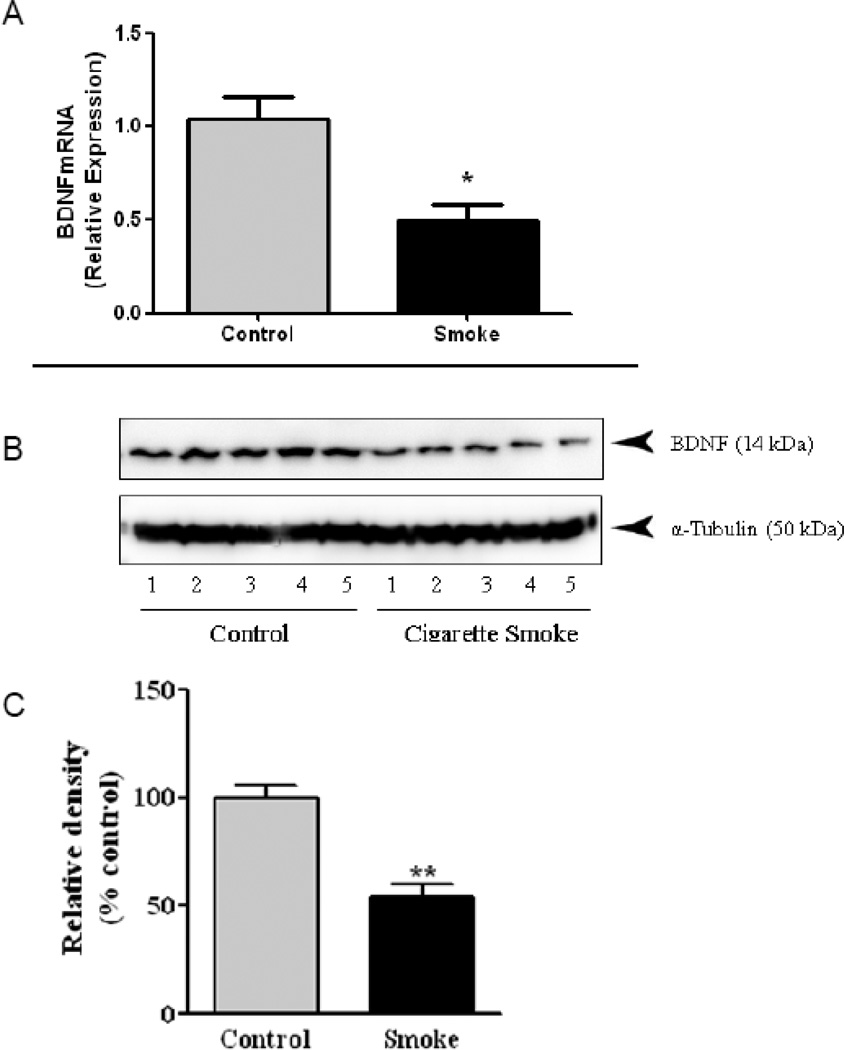
Striatal BDNF mRNA was significantly decreased [t = 3.67, df = 10, p = 0.004] by 50% in the 4-month old male offspring of mice exposed to CS during pregnancy (Fig. 5A), while there was no significant effect in females (Fig. S2). The decreased BDNF mRNA was accompanied by a significant [t = 5.83, df = 4, p = 0.004] 46% decrease in BDNF protein (Fig. 5B,C). In contrast, no significant effect was observed in female offspring exposed to CS (Fig. S2)
Figure 5. Prenatal Cigarette Smoke Exposure Reduces Striatal Brain-Derived Neurotrophic Factor (BDNF) mRNA and Protien.
(A) Striatal BDNFmRNA, as measured by QPCR, was decreased in 4-month old male, offspring exposed in utero to cigarette smoke (CS). (B,C) Western blot analysis of BDNF revealed significantly decreased BDNF protien levels in CS-exposed male offspring. p < 0.05 by Students t-test.
Discussion
Although maternal smoking has been associated with increased risk of behavioral dysfunction, for almost 20 years, recent reports have questioned whether there is a true causal association. This study sought to use a novel animal model of in utero exposure to cigarette smoke (CS), at a concentration reflective of smoking <1 pack of cigarettes/day, to determine whether behavioral alterations were observed similar to that reported in the epidemiological studies. The data reveal that behavioral dysfunction, including hyperactivity and increased aggression, were observed in the offspring of CS-exposed mice and were more apparent in the males, mirroring that observed in epidemiological studies of maternal smoking and offspring behavior. Mechanistically, our data identify alterations in catecholamine levels and neurotrophin signaling that are likely contributors to the behavioral dysfunction observed in mice exposed in utero to CS.
Children of mothers that smoked during pregnancy are reported to have increased risk of a variety of neurobehavioral problems, including hyperactivity, aggression, and externalizing behaviors (Knopik, 2009). Using a mouse model of maternal smoking, we observed that prenatal exposure to CS causes persistent increases in locomotor activity that was observed as early as 4 weeks of age, and persisted through 4-months of age. Prenatal CS exposure also resulted in long-term increases in aggression, as measured in the resident intruder task. These findings are similar to a recent report in humans that examined the long-term effects of prenatal CS exposure that found higher scores on externalizing and aggression scales in 22-year old adults (Cornelius et al., 2012). Other investigators also reported that prenatal CS exposure in humans increased externalizing, internalizing, and ADHD symptoms in adolescence (Indredavik et al., 2007).
Unfortunately, there are few studies in the animal literature that explore prenatal CS exposure and behavior. Two previous studies, one in rats (Gaworski et al., 2004) and one in mice (Amos-Kroohs et al., 2013), exposed rodents to CS throughout gestation and lactation and monitored behavior in the offspring. However, neither of these studies reported increased locomotor activity in the offspring or evaluated aggression. Amos-Kroohs and co-workers (Amos-Kroohs et al., 2013) did observe significant differences in methamphetamine-induced locomotor behavior in the offspring of mice exposed to CS throughout gestation and lactation and reported that these differences were sexually dimorphic in nature, similar to the sex differences observed in our study. The differences between our observation of a basal increase of locomotor activity and the lack of effect in the aforementioned studies may be due, in part, to the fact that CS exposure was extended throughout lactation in these two studies, resulting in direct exposure of the pup to CS smoke components.
Although CS is a complex mixture of > 4,000 chemicals, including polycyclic aromatic hydrocarbons, nitrosamines, metals, and nicotine, all of which may cross the placenta, nicotine is t he most commonly used surrogate for CS exposure (Bruin et al., 2010; Slikker et al., 2005; Slotkin, 2008; Swan and Lessov-Schlaggar, 2007). An early study resported that injection of pregnant rats with nicotine caused increased locomotor activity in PND14 of f spring that was ameliorated by amphetamine (Fung, 1988), but this was not observed in a follow-up study incorporating nicotine infusion (Fung and Lau, 1989). Richardson and Tizabi (Richardson and Tizabi, 1994) reported that maternal infusion of nicotine (6 mg/kg/day) caused an increase in locomotor activity on PND22, and Pauly and co-workers (Pauly et al., 2004) reported that oral nicotine exposure during pregnancy caused sex-specific hyperactivity on PND40 and 60. However, Muneoka and co-workers (Muneoka et al., 1997) found an effect of route of administration (injection vs. infusion), but no effect of prenatal nicotine on locomotor activity. More recently, oral exposure of pregnant rats to nicotine in their drinking water resulted in adolescent hyperactivity in the offspring (Schneider et al., 2012; Zhu et al., 2012). These data suggest that that prenatal nicotine exposure is likely a primary contributor to the hyperactivity observed following prenatal CS exposure.
Prenatal nicotine elicits a variety of effects on developing brain catecholamine systems that appears to be dependent on route of administration (Navarro et al., 1988; Oliff and Gallardo, 1999). Maternal infusion of nicotine in rats decreased striatal dopamine levels in the offspring on PND22 (Richardson and Tizabi, 1994). However, others reported that greater alterations in catecholamine levels and tyrosine hydroxylase activity in the cortex (Navarro et al., 1988). Here, we found the greatest effects on dopamine levels in the striatum, which was accompanied by a decrease in tyrosine hydroxylase protein. Muneoka and co-workers (Muneoka et al., 1997) found that both maternal nicotine infusion and oral exposure altered dopamine and serotonin turnover in the offspring, with no effect on norephinephrine contents, similar to that observed in our study. However, this study did not find changes in striatal dopamine or serotonin, as observed here. Oral exposure of mice to nicotine during pregnancy also produced hyperactivity that was ameliorated by methylphenidate (0.75 mg/kg by oral gavage) (Zhu et al., 2012). However, this study found a significant increase in cortical dopamine levels, with no change in striatal levels. Of interest with our study is that there was no significant effect of CS exposure on the neurotransmitter metabolites, suggesting a potential targeting of biosynthetic enzymes, such as TH. This is supported by our finding of decreased striatal TH protein in the male offspring exposed to prenatal CS. Taken in concert, there appears to be some differences between prenatal nicotine exposure and prenatal CS exposure with regards to catecholamine levels. These differences may be the result of different components in CS or from the different exposure paradigms. Alternatively, these mice were of pure C57 BL/6 background, which may also contribute to the observed differences.
Although the epidemiological literature reported associations between maternal smoking and aggression in children, the animal literature has not explored this relationship in detail. In adult animals, acute nicotine administration was found to decrease aggressive behavior in a variety of paradigms (Driscoll and Baettig, 1981; Silverman, 1971), including the resident intruder task (Johnson et al., 2003), which is supported by a study in humans (Cherek, 1984). The data reported here appear to be the first in the published literature reporting that prenatal exposure of an animal to CS results in increased aggression in the offspring, as evidenced by an increased number of attacks by CS-exposed offspring. This finding may also be related to the alterations in dopamine and serotonin levels observed in these mice, as decreased monoamines, including dopamine and serotonin, are associated with increased aggression (Mosienko et al., 2012; Scholtens et al., 1990; Zagrodzka et al., 1994). Serotonin was also specifically linked to offensive aggression (Vergnes et al., 1986), similar to that observed here.
During the course of our studies, we noted that the behavioral and neurochemical profile of mice prenatally exposed to CS displayed some similarity to that observed in mice deficient in brain-derived neurotrophic factor (BDNF). BDNF plays key roles in neuronal development and synaptic plasticity (Lu and Figurov, 1997), with hyperactivity and increased aggression observed In BDNF heterozygous mice (Lyons et al., 1999; Monteggia et al., 2007). In addition to these roles, BDNF app ears to exert significant effects on the functioning of the dopamine system, as mice with reduced levels of BDNF have deficits in dopamine release (Dluzen et al., 2002). Our data demonstrate that prenatal exposure t o CS causes significant reductions of striatal BDNF at the mRNA and protein level. This is in contrast to data showing increased BDNF levels following gestational exposure to nicotine (Harrod et al., 2011), and in postnatal rats chronically exposed to nicotine during the neonatal period (Son and Winzer-Serhan, 2009). These data suggest that CS exposure and nicotine produce different effects on BDNF.
The decreased BDNF observed in this study have the potential to contribute to behavioral dysfunction observed in the offspring of CS-exposed mice. Alterations of BDNF are considered a potential contributor to a variety of neurodevelopment al disorders based on several lines of evidence. First, elimination of BDNF during development in mice causes alterations of neuronal structure and function that are accompanied by male-specific hyperactivity (Monteggia et al., 2007). Likewise, mice heterozygous for BDNF, forebrain-restricted deletion of BDNF, or conditional BDNF knockouts demonstrate increased aggression. The effects observed here following suggest that alterations in BDNF may play a prominent role in the behavioral effects of prenatal CS exposure and that interventions designed to increase BDNF may ameliorate these behavioral deficits.
Conclusion
Using a novel animal model, we demonstrate for the first time that the offspring of mice exposed to CS during gestation exhibit neurobehavioral deficits including hyperactivity and increased aggression similar to that observed in epidemiological studies of maternal smoking. Importantly, we identified that some of these effects mimic those observed in other studies using prenatal exposure to nicotine. However, there were notable differences, including the effects on aggressive behavior and BDNF levels. These data suggest that additional focus is warranted on other components of tobacco smoke and their interaction with nicotine. Finally, the demonstration that BDNF levels are decreased long after exposure has ceased may provide an avenue for treatment of aberrant behaviors resulting from prenatal exposure to CS.
Supplementary Material
Highlights.
-
➢
Prenatal cigarette exposure (CS) causes hyperactivity and aggressive behavior
-
➢
Behavioral alterations caused by prenatal CS mainly affect the male offspring
-
➢
Prenatal CS exposure reduced striatal and cortical dopamine and serotonin
-
➢
Prenatal CS exposure reduced BDNF mRNA and protein
Acknowledgements
The authors thank Dr. Chen Zhang for excellent technical assistance.
Financial disclosure
This research was supported in part by grants from the National Institutes of Environmental Health Sciences [P30ES000260 (JTZ), P30ES005022 (JRR), T32ES007148 (CY), R01ES015991 (JRR) and a grant from the Institute for Science and Health (JTZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amos-Kroohs RM, Williams MT, Braun AA, Graham DL, Webb CL, Birtles TS, Greene RM, Vorhees CV, Pisano MM. Neurobehavioral phenotype of C57BL/6J mice prenatally and neonatally exposed to cigarette smoke. Neurotoxicology and teratology. 2013;35:34–45. doi: 10.1016/j.ntt.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SW, Gilman SE, Mick E, Fitzmaurice G, Ganz ML, Seidman LJ, Buka SL. Revisiting the association between maternal smoking during pregnancy and ADHD. Journal of psychiatric research. 2010;44(15):1058–1062. doi: 10.1016/j.jpsychires.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96(9):1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Bergman O, Westberg L, Lichtenstein P, Eriksson E, Larsson H. Study on the possible association of brain-derived neurotrophic factor polymorphism with the developmental course of symptoms of attention deficit and hyperactivity. Int J Neuropsychopharmacol. 2011;14(10):1367–1376. doi: 10.1017/S1461145711000502. [DOI] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Wilson WW, Lazo CR, Stout KA, Kim HM, Wang MZ, Walker DI, Pennell KD, Richardson JR, Miller GW, Caudle WM. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: role of dopamine handling in neurotoxicity. Experimental neurology. 2013;241:138–147. doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environmental health perspectives. 2006;114(12):1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicological sciences : an official journal of the Society of Toxicology. 2010;116(2):364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR. Effects of cigarette smoking on human aggressive behavior. Progress in clinical and biological research. 1984;169:333–344. [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, Day NL. Prenatal cigarette smoking: Long-term effects on young adult behavior problems and smoking behavior. Neurotoxicology and teratology. 2012;34(6):554–559. doi: 10.1016/j.ntt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepeaux G, Bouillaud-Kremarik P, Sikhayeva N, Rychen G, Soulimani R, Schroeder H. Late effects of a perinatal exposure to a 16 PAH mixture: Increase of anxiety-related behaviours and decrease of regional brain metabolism in adult male rats. Toxicology letters. 2012;211(2):105–113. doi: 10.1016/j.toxlet.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI, McDermott JL, Kucera J, Walro JM. Striatal dopamine output is compromised within +/− BDNF mice. Synapse. 2002;43(2):112–117. doi: 10.1002/syn.10027. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Baettig K. Selective inhibition by nicotine of shock-induced fighting in the rat. Pharmacology, biochemistry, and behavior. 1981;14(2):175–179. doi: 10.1016/0091-3057(81)90240-9. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. The Psychiatric clinics of North America. 2010;33(1):159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin MC, Aleksunes LM, Richardson JR. Alteration of the expression of pesticide-metabolizing enzymes in pregnant mice: potential role in the increased vulnerability of the developing brain. Drug metabolism and disposition: the biological fate of chemicals. 2013;41(2):326–331. doi: 10.1124/dmd.112.049395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS. Association of tobacco and lead exposures with attentiondeficit/ hyperactivity disorder. Pediatrics. 2009;124(6):e1054–e1063. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of pediatrics & adolescent medicine. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Fung YK. Postnatal behavioural effects of maternal nicotine exposure in rats. The Journal of pharmacy and pharmacology. 1988;40(12):870–872. doi: 10.1111/j.2042-7158.1988.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacology, biochemistry, and behavior. 1989;33(1):1–6. doi: 10.1016/0091-3057(89)90419-x. [DOI] [PubMed] [Google Scholar]
- Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicological sciences : an official journal of the Society of Toxicology. 2004;79(1):157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA pediatrics. 2013;167(3):282–288. doi: 10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Zhu J, Hughes BA, Perna MK, Brown RW. Gestational IV nicotine produces elevated brain-derived neurotrophic factor in the mesocorticolimbic dopamine system of adolescent rat offspring. Synapse. 2011;65(12):1382–1392. doi: 10.1002/syn.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007;96(3):377–382. doi: 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SK, Carlson KM, Lee J, Burr LE, Wagner GC. Effects of nicotine on target biting and resident-intruder attack. Life sciences. 2003;73(3):311–317. doi: 10.1016/s0024-3205(03)00289-3. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Keyes MA, March D, Susser E. Levels of risk: maternal-, middle childhood-, and neighborhood-level predictors of adolescent disinhibitory behaviors from a longitudinal birth cohort in the United States. Mental health and substance use : dual diagnosis. 2011;4(1):22–37. doi: 10.1080/17523281.2011.533445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Developmental neuropsychology. 2009;34(1):1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Heron J, Smith GD, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. American journal of epidemiology. 2012;176(3):261–268. doi: 10.1093/aje/kwr510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva pediatrica. 2005;57(6):359–371. [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicology and teratology. 1999;21(2):109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010;12(4):408–415. doi: 10.1093/ntr/ntq017. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. The American journal of psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen TB. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116(2):462–467. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Reviews in the neurosciences. 1997;8(1):1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(26):15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. Journal of clinical child psychology. 1998;27(3):352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biological psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Moore CA, Baravik J, Meek EC, Richardson JR, Carr RL, Chambers JE. Effects of repeated developmental exposure to chlorpyifos and methyl parathion on choline acetyltransferase and muscarinic receptors in rats. Toxicological Sciences. 2003;72:127-127. [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Translational psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain research. Developmental brain research. 1997;102(1):117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. The Journal of pharmacology and experimental therapeutics. 1988;244(3):940–944. [PubMed] [Google Scholar]
- Ng SP, Steinetz BG, Lasano SG, Zelikoff JT. Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp Biol Med (Maywood) 2006;231(8):1403–1409. doi: 10.1177/153537020623100814. [DOI] [PubMed] [Google Scholar]
- Obel C, Linnet KM, Henriksen TB, Rodriguez A, Jarvelin MR, Kotimaa A, Moilanen I, Ebeling H, Bilenberg N, Taanila A, Ye G, Olsen J. Smoking during pregnancy and hyperactivity-inattention in the offspring--comparing results from three Nordic cohorts. International journal of epidemiology. 2009;38(3):698–705. doi: 10.1093/ije/dym290. [DOI] [PubMed] [Google Scholar]
- Obel C, Olsen J, Henriksen TB, Rodriguez A, Jarvelin MR, Moilanen I, Parner E, Linnet KM, Taanila A, Ebeling H, Heiervang E, Gissler M. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?-- Findings from a sibling design. International journal of epidemiology. 2011;40(2):338–345. doi: 10.1093/ije/dyq185. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Gallardo KA. The effect of nicotine on developing brain catecholamine systems. Frontiers in bioscience : a journal and virtual library. 1999;4:D883–D897. doi: 10.2741/oliff. [DOI] [PubMed] [Google Scholar]
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital and health statistics. Series 10, Data from the National Health Survey. 2008;(237):1–14. [PubMed] [Google Scholar]
- Pauly JR, Sparks JA, Hauser KF, Pauly TH. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2004;22(5–6):329–337. doi: 10.1016/j.ijdevneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Peacock JL, Cook DG, Carey IM, Jarvis MJ, Bryant AE, Anderson HR, Bland JM. Maternal cotinine level during pregnancy and birthweight for gestational age. International journal of epidemiology. 1998;27(4):647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology. 2008;29(5):855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacology, biochemistry, and behavior. 1994;47(2):331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology. 2012;223(4):401–415. doi: 10.1007/s00213-012-2728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens J, Roozen M, Mirmiran M, van de Poll NE. Role of noradrenaline in behavioral changes after defeat in male and female rats. Behavioural brain research. 1990;36(3):199–202. doi: 10.1016/0166-4328(90)90057-l. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Richardson JR, Gupta RK, Flaws JA, Fiskum G. Effects of the organochlorine pesticide methoxychlor on dopamine metabolites and transporters in the mouse brain. Neurotoxicology. 2009;30(2):274–280. doi: 10.1016/j.neuro.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheleg M, Yochum CL, Wagner GC, Zhou R, Richardson JR. Ephrin-A5 deficiency alters sensorimotor and monoaminergic development. Behavioural brain research. 2013;236(1):139–147. doi: 10.1016/j.bbr.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AP. Behaviour of rats given a "smoking dose" of nicotine. Animal behaviour. 1971;19(1):67–74. doi: 10.1016/s0003-3472(71)80136-7. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Critical reviews in toxicology. 2005;35(8–9):703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicology and teratology. 2008;30(1):1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Soane L, Siegel ZT, Schuh RA, Fiskum G. Postnatal developmental regulation of Bcl-2 family proteins in brain mitochondria. Journal of neuroscience research. 2008;86(6):1267–1276. doi: 10.1002/jnr.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases mRNA expression of neurotrophic factors in the postnatal rat hippocampus. Brain research. 2009;1278:1–14. doi: 10.1016/j.brainres.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology review. 2007;17(3):259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. The American journal of psychiatry. 2003;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biological psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Depaulis A, Boehrer A. Parachlorophenylalanine-induced serotonin depletion increases offensive but not defensive aggression in male rats. Physiology & behavior. 1986;36(4):653–658. doi: 10.1016/0031-9384(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. Journal of toxicology and environmental health. Part B, Critical reviews. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DS, Sherer TB, Richardson JR, Seo B, Miller GW, Matsuno-Yagi A, Yagi T, Greenamyre JT. Pesticides that inhibit complex I kill cells through an oxidative mechanism. Society for Neuroscience Abstract Viewer and Itinerary Planner 2003, Abstract No. 95.17-Abstract No. 95.17. 2003 [Google Scholar]
- Zagrodzka J, Wieczorek M, Romaniuk A. Social interactions in rats: behavioral and neurochemical alterations in DSP-4-treated rats. Pharmacology, biochemistry, and behavior. 1994;49(3):541–548. doi: 10.1016/0091-3057(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(27):9410–9418. doi: 10.1523/JNEUROSCI.1041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li G, Dong Y, Zhou HH, Kong B, Aleksunes LM, Richardson JR, Li F, Guo GL. Modulation of farnesoid X receptor results in posttranslational modification of poly (ADP-ribose) polymerase 1 in the liver. Toxicology and Applied Pharmacology. 2013;266(2):260–266. doi: 10.1016/j.taap.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.