Abstract
Surface enhanced Raman spectroscopy (SERS) has been established as a powerful tool to detect very low-concentration bio-molecules. One of the challenging problems is to have reliable and robust SERS substrate. Here, we report on a simple method to grow coherently embedded (endotaxial) silver nanostructures in silicon substrates, analyze their three-dimensional shape by scanning transmission electron microscopy tomography and demonstrate their use as a highly reproducible and stable substrate for SERS measurements. Bi-layers consisting of Ag and GeOx thin films were grown on native oxide covered silicon substrate using a physical vapor deposition method. Followed by annealing at 800°C under ambient conditions, this resulted in the formation of endotaxial Ag nanostructures of specific shape depending upon the substrate orientation. These structures are utilized for detection of Crystal Violet molecules of 5 × 10−10 M concentrations. These are expected to be one of the highly robust, reusable and novel substrates for single molecule detection.
The discovery of Surface Enhanced Raman Scattering (SERS) lead to the solutions for many challenges due to its power as an analytical tool for the sensitive and selective detection of molecules adsorbed on noble metal nanostructures1,2,3,4,5. In SERS, enormous field enhancement occurs at the noble metal junctions due to electromagnetic field localization coupling resonantly with the surface plasmon6,7,8. One of the major challenges in the field of SERS is to have an appropriate and effective substrate to take care of low signal enhancement, poor selectivity, unstable and irreproducible signals5. Highly reproducible and stable substrates can efficiently be used as SERS based sensors for label free immunoassays9, biosensing10 and other applications5.
The success and the usefulness of the SERS method depends on the optimization of the interaction between adsorbed molecules and the surface plasmonic structures5. To maximize the enhancement factors for the SERS signal, various shapes and combinations of gold and silver nanostructures, such as SiO2-encapsulated gold particles11, nanorods of Ag deposited using oblique angle vapor deposition12, 2D Au nano-mushroom arrays13, polyhedral Ag mesocages14, and film over nanospheres (FON's)15 have been tried out and obtained better stability and reproducibility in some cases. Besides plasmonic applications, silver nanostructures can also be used as antennas to convert light into localized electric fields or as wave guides to route light to specified locations with a precision of few nanometers16, photonic crystals and in infrared polarizers17,18. Embedded Ag nanoparticles have been found to enhance the light absorption in semiconductors, due to their strong plasmonic near-field coupling5. Owing to the large contingent of applications of Ag nanostructures, it is a challenge to control the shape, size, composition and placement/position of Ag nanostructures. Wiley et al., reported a solution-phase polyol synthesis for controlled shapes of Ag nanostructures, such as, pentagonal nanowires, cuboctahedra, nanocubes, nanobars etc.19. Recently we have reported the possibility of growth of endotaxial Ag nanostructures by chemical vapor deposition (CVD) method20.
In the present work, we report on the growth of various shapes of coherently embedded or/and endotaxial Ag nanostructures on silicon substrates using a physical vapor deposition method (PVD). It is important to know that both the CVD and PVD are two different methods but the yielding endotaxial structures in the both processes indicate an interesting phenomenon of growth. The present paper also presents control over the position and shape of Ag structures by introducing the Ag thin film sandwiching between the GeOx and SiOx. Such control is not possible in CVD method. We present a simple process to grow substrate symmetry-driven silver nanostructures on silicon substrate by annealing the samples at ≈800°C in air. The very nature of Ag nanostructures (i.e. embedding coherently in substrate) would provide a stable substrate for SERS applications. Our results reveal an interesting process involving a low-temperature etching of native oxide of the silicon substrate using GeOx as an intermediate layer to help the growth of the endotaxial Ag nanostructures. The term “endotaxy” here refers to the growth of precipitate phases in a bulk matrix, with coherent interfaces surrounding the precipitate21. We present 3D imaging of these embedded structures using scanning transmission electron microscopy (STEM) based tomography in addition to the use of different procedure of the growth (i.e., PVD). Using traditional gas phase or solution phase methods, formation of various shapes and sizes of Ag nanostructures has been reported, but they are not endotaxial in nature. It is also be noted that, traditionally, endotaxial structures were prepared with molecular beam epitaxy (MBE) in ultra high vacuum conditions (UHV)22. Earlier reports indicated that endotaxial structures have potential applications in thermoelectric, magnetic systems, spin polarized contacts, opto-electronic components and nanoelectronics22.
Results
Growth of Endotaxial Ag nanostructures: a simple PVD method
Using physical vapor deposition and annealing in ambient conditions, we have succeeded in growing the coherently embedded nanostructures of Ag in Si. The results presented in figure 1 depict the shape variation of Ag nanostructures depending on the substrate orientation. For (100), (110) and (111), the surface unit cell has 4 – fold, 2 – fold and 3 – fold symmetry, respectively. The shape of Ag nanostructures is commensurate with the substrate surface symmetry (4, or 2 and 3 fold symmetry for (100), (110) and (111) orientations, respectively) as shown in figure 1. Figure 1 (a) depicts a bright field (BF) planar TEM micrograph for 2 nm Ag/17 nm GeOx/SiOx/Si (100) annealed at ≈800°C in air for 30 minutes. This shows formation of square/rectangular shaped silver nano-structures on Si(100) substrate following the four-fold symmetry of the substrate. By considering about 220 particles from many TEM micrographs the average length of the square/rectangle shape Ag nanostructures is found to be 110 nm ± 50 nm and average breadth is 100 nm ± 40 nm with an aspect ratio of 1.1. Also, figure 1 (b) reveals the formation of silver nanorod structures for the 2 nm Ag/17 nm GeOx/SiOx/Si (110) annealed at ≈800°C in air for 30 minutes, following the substrate's two-fold symmetry. The average length and width of the rod shaped Ag nanostructures is found to be 150 nm ± 80 nm, 30 nm ± 10 nm with an aspect ratio of ≈ 5.0. From the associated error it is clear that a wide distribution of sizes is observed. Figure 1 (c) is for the 2 nm Ag/17 nm GeOx/SiOx/Si (111) annealed at ≈800°C in air for 30 minutes and this reveals the triangular nanostructures formed by following a three-fold symmetry of the Si(111) substrate. The process of Ag diffusion from thin films through the reactive interfaces of GeOx and SiOx which resulted in a clean Ag - Si interface is an interesting result from the present measurements.
Figure 1. Planar TEM Micrographs of 2 nm Ag/17 nm GeOx/SiOx on (a) Si(100), (b) Si(110) and (c) Si(111) annealed at 800°C in air.
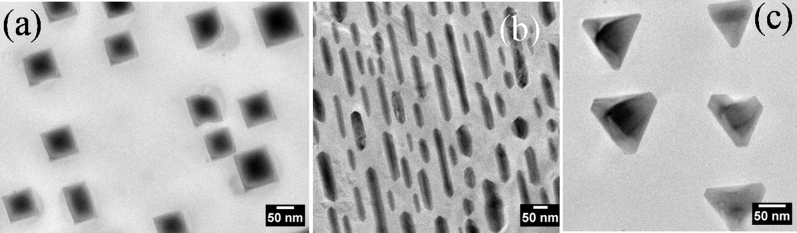
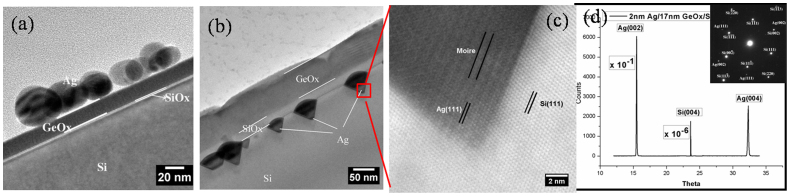
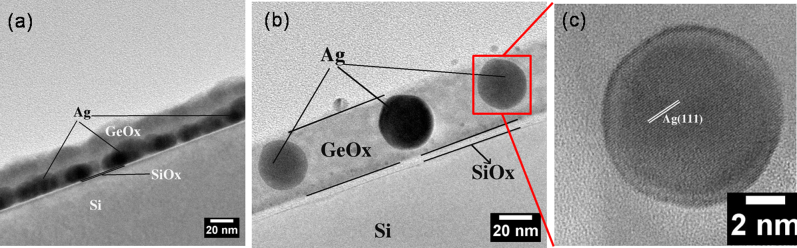
Figure 2 (a) shows a low magnification cross-sectional TEM (X-TEM) bright field (BF) image of as-deposited 2 nm Ag/17 nm GeOx/SiOx/Si(100). This confirms the presence of spherical silver nanoparticles on top of an amorphous GeOx layer. It should be noted that a physical vapor deposition of Ge in high vacuum condition yielded GeOx layer. The as-deposited Ag thin film showed isolated irregular nanostructures. From figure 2 (a), the thicknesses of the GeOx and the native oxide (SiOx) layers were found to be ≈17 nm and ≈2 nm, respectively. Figure 2 (b) depicts a low magnification BF X-TEM micrograph of 2 nm Ag/17 nm GeOx/SiOx/Si (100) at ≈800°C (annealing done in air for 30 minutes). From this micrograph, the thickness of GeOx is found to be ≈75 nm where SiOx is ≈20 nm thick. Following the annealing in air, the GeOx and SiOx layer thicknesses have been increased by a factor of ≈3.4 and ≈9, respectively. The cross-sectional image taken (for 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800°C), shown in figure 2 (b), confirms the intrusion of silver into the silicon substrate. Figure 2 (c) represents a high resolution lattice image of a small area shown in Figure 2 (b). This lattice image confirms the presence of Ag (111) (0.238 ± 0.005 nm) and Si (111) (0.315 ± 0.005 nm) lattice planes as an endotaxial structure. The lattice images depict the presence of Moiré fringes in the structures, which confirms the presence of the coherent embedment nature of silver nanostructures in silicon. The Moiré fringe spacing can be determined by
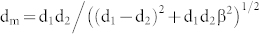 |
where d1,d2 are lattice spacings of the planes which are constituents of Moiré fringes and β is the angle between the planes23. The Moiré fringe spacing of 0.96 nm was calculated between Ag(111) and Si(111) planes with β = 0. This matches well with the measured fringe spacing from our measurements (Figure 2 (c)) is 0.95 ± 0.01 nm. Figure 2 (d) is the synchrotron XRD spectrum of the 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800°C in air for 30 minutes, which depicts the single crystalline nature of the silver nanostructures on silicon (100) substrate. In the XRD spectrum, only Ag(002) and Ag(004) peaks were observed along with the Si(004) peak that belongs to the substrate. Due to the larger x-ray beam size (250 μm × 250 μm), the XRD show the macroscopic ordering. Hence, the XRD data confirms the presence of crystalline Ag structures over a larger scale compared to TEM. The inset of figure 2(d) shows the selected area electron diffraction (SAED) pattern taken on a single endotaxial nanostructure, confirming that the silver nanostructures are single crystalline in nature. XRD and SAED confirm the macroscopic and microscopic coherent nature of the Ag nanostructures, respectively.
Figure 2. X-TEM Micrographs of 2 nm Ag/17 nm GeOx/SiOx/Si (100) (a) Low Mag of as-deposited (b) Low Mag of 800 °C annealed in air (c) HR-XTEM depicts endotaxial structures (with Moire fringes) and (d) X-Ray Diffraction Pattern showing the single crystalline nature of the Ag nano structures which also complimented by a Selected Area Electron Diffraction (SAD) pattern taken on a single structure.
Plausible mechanism
We propose a plausible mechanism involved in the enhanced desorption of SiOx and GeOx at initial stages20. During annealing of the sample, GeOx desorbs into volatile GeO from the metal edges by forming intermediate solid GeO(s)24. It has been reported that GeO desorbs from a solid GeO layer at >300°C25,26. In our case, the metal could be a catalyst for the formation of GeO(s) which then desorbs as GeO gas24.
 |
Wang et al. reported the disproportionation of GeO into Ge and GeO227, and they have also shown the formation of crystalline germanium above 600°C annealing.
 |
Ge formed in the disproportionation of GeO thus has a possibility to react with native SiOx to form volatile SiO around 750°C. Furthermore, Yun et al., reported the desorption of SiO from SiO2 in the vicinity of Ge28:
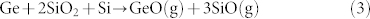 |
In a proposed cyclic process the GeO formed in this reaction (3) also disproportionate into GeO2 and Ge and the Ge will help desorption of SiO2 into volatile SiO resulting a competition between GeO disproportionation, SiO desorption and SiO2 formation.
It is well known from literature that silver diffuses into silicon through an interstitial-substitution mechanism29,30. Ag diffusion to reach the silicon surface has been enhanced due to desorption of SiOx and GeOx. This would presumably happen during annealing. During cooling, initially, oxidation of the silicon substrate occurs which might be a reason for increased thickness of the SiOx layer, the condensation of GeOx might take place at a lower temperature compared to SiOx. The increase of the GeOx layer is also observed from the cross-sectional TEM micrograph (figure 2(b)) which might be explained through the stochiometric oxide formation.
Chemical analysis and 3D imaging
In order to understand the chemical nature of the system, two-dimensional energy-dispersive x-ray (EDX) analysis of a typical endotaxial structure has been performed in STEM mode as shown in Figure 3. The high-angle annular dark field (HAADF) Z-contrast STEM micrograph in figure 3 (a) depicts the investigated region containing a 50 nm wide pyramidal structure. As shown by the Oxygen map in figure 3(b), the endotaxial structure itself is free of oxides while the presence of oxygen is clearly confirmed in the top two oxide layers. Together with the silicon map in figure 3(c) and the germanium distributions in figures 3(d,e), the first oxide layer from the substrate side is identified as SiO. From the line-profile analysis the EDX signal, the Si signal decreases with increasing silver signal, and that SiOx is formed at the surface where no Ge is present and is followed by a mixed layer with SiOx and GeOx. Moreover, figure 3(c) exhibits a significant silicon deficiency in the endotaxial formation, suggesting the substitution of silicon here. In fact, the silver map in figure 3(f) reveals that the investigated structure consists of silver which is also in agreement with the high Z-contrast observed in the HAADF image (as in figures 3 (a), 4(a)). Together with the SAED pattern shown in figure 2(d), we can preclude the presence of silver silicide because silver and silicon lattice sapcings constitute a ratio of ¾ which matches with the expectation for the pure materials exactly.
Figure 3. STEM- EDX Elemental of mapping 2 nm Ag/17 nm GeOx/SiOx/Si (100) @800°C (a) STEM Micrograph, (b) Oxygen mapping, (c) Silicon mapping, (d),(e) Germanium K,L mapping and (e) Silver mapping.
Figure 4.
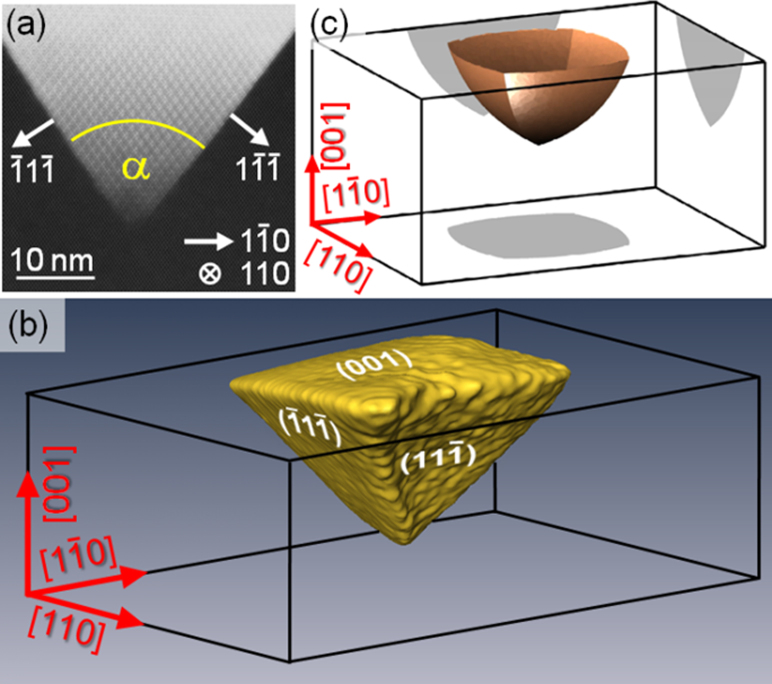
(a) STEM – HAADF micrograph of 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800 C annealed in air, with selected directions indexed from this 2D projection. (b) Reconstructed 3D tomography view of one of the endotaxial islands, showing that the silver embedment is terminated by {111} facets and an (001) base. (c) Result of the Wulff construction, explaining this pyramidal shape and its orientation theoretically in terms of interface energy.
The plane- and cross-sectional TEM studies in figures 1(a) and 2(b,c) already imply that the silver nanostructures obey a pyramidal shape with a quadratic base which has a [100] surface normal. Moreover, figure 4(b) exhibits an angle of α = 70.0° between the projected Ag/Si interface which agrees well with the theoretical angle of 70.5° for {111} facets. However, to prove that the silver nanostructures are indeed terminated by four microscopically flat {111} facets in addition to the quadratic base, STEM tomography has been performed to allow for the 3D reconstruction shown in figure 4(b). This demonstrates the termination by (-11-1) and (11-1) facets in this particular perspective. Finally, we calculated the equilibrium shape of silver embedments in silicon theoretically by considering energies for different crystallographic orientations of the Ag/Si interface. We took the energies from Xin et al.31, who computed energies for Ag(001)/Si(111), Ag(011)/Si(111) and Ag(111))/Si(111) twisted interfaces. By Wulff's construction32,33, we then found the terminating facets of the Ag embedments in figure 4(c) for the case that the Ag facets are lying on Si (111) facets. As the shape agrees well with the reconstruction in figure 4(b), we conclude that the observed pyramid formation is driven by Ag/Si interface energy minimization and takes the shape close to thermodynamic equilibrium.
SERS applications
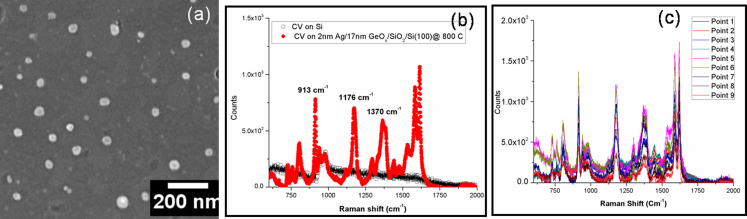
Silver nano square/rectangles formed in the 2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800°C substrate were used to detect the Crystal Violet organic dye molecules. CV is a commonly used molecule for SERS study because of its high Raman scattering cross-section and it chemisorbs on the silver surfaces due to the amino group3,34,35. The SERS spectra reveal the characteristic peaks of CV at 1172, 1371, and 1619 cm−1 36. Figure 5 (a) depicts the SEM micrograph of 2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800°C annealed in air, which was treated with HF for 90 minutes to remove the top oxide layers and expose the Ag nanoparticles in order to use it for SERS. Figure 5 (b) is the corresponding SERS spectra of CV with 5 × 10−10 molar (0.5 nM) concentration drop casted on this HF treated 2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800°C, the same concentration of CV was drop casted on plain silicon in order to compare the enhancement due to the endotaxial square nanostructures. Analytical enhancement factors (AEF) were calculated for the characteristic peaks of crystal violet using the following equation
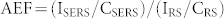 |
Figure 5. (a) SEM Micrographs of 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800 °C in air etched with hydro fluoric acid for 90 minutes, (b) SERS spectrum of Crystal Violet with a concentration of 5 × 10−10 Molar drop casted on 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800 °C in air etched with HF for 90 min, and (c) SERS spectrum of Crystal Violet of above sample at different points showing the consistency of the substrate with uniform enhancement.
The analytical enhancement factors (AEF) for the CV for peak positions of 913, 1172 and 1371 are 1.7 × 107, 1.8 × 107 and 1.8 × 107 respectively, for nanosquare/rectangular structured sample i.e. for 2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800 C annealed in air and HF-treated, where as for as deposited case the AEF's are as follows: 2.9 × 104, 3.0 × 104 and 2.9 × 104, respectively. This enhancement of three (3) orders of magnitude compared to the as-deposited is attributed to the shape and placement of Ag nanostructures. Figure 5(c) SERS spectrum of Crystal Violet on HF treated 2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800°C at different points showing the consistency of the substrate with uniform enhancement. A SERS substrate can be readily obtained as and when required by etching out the oxide layer with HF and use it for SERS recording. In their detailed paper, Le Ru et al., reported average enhancement factor of ≈105 for 5 nM CV (page no 13798, Table 2 in ref. 3) for an analyte in a partially aggregated Ag colloid solution. Our average enhancement factor are two orders of magnitude higher than the ones reported for similar concentration CV in reference 3. It should be noted that we have used a concentration of ≈ 0.5 nM CV while the measurements in ref. 3 are for 5.0 nM. It is expected to have variation in AEF depending on the concentration3. It is worth noting that if one could position all the molecules at these hot-spots, then much larger average enhancements could be obtained. Hence, we need to note that the values mentioned here are the average enhancement factors.
In order to look at its reusability, we recorded the SERS spectra with fairly reproducible signals after slightly etching the substrate with HF in the case of CV molecule, as these molecules strongly adhere to the Ag nanoparticles on the substrate through a covalent band. However, if the molecule of interest can easily be removed with a proper solvent, then these substrates can be used several times. The most important thing of robustness is that one does not have to bother about the oxidation of the substrate as Ag particles are covered by SiOx, GeOx layers. The second major advantage is that this technique provides the nanostructures that have sharp ordered edges that can be obtained for high field enhancements. The third advantage is that it is highly cost effective.
Role of GeOx layer
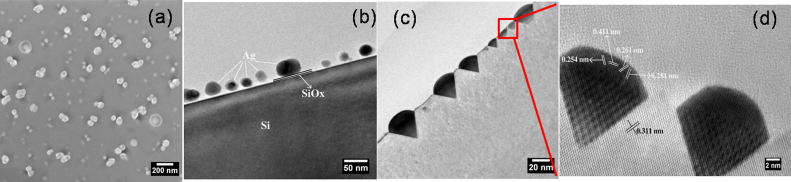
In order to understand the role of GeOx to form substrate symmetric endotaxial silver nano structures, samples of silver on native oxide silicon substrate are also prepared and annealed at ≈800°C under the same conditions, like 2 nm Ag/17 nm GeOx/SiOx/Si(100). Figure 6 (a) is the SEM micrographs of 2 nm Ag/2 nm SiOx/Si(100) annealed at ≈800°C. Interestingly, no ordered structures have been observed. Figure 6(b) is the low magnification XTEM (cross-sectional TEM) micrograph of as deposited 2 nm Ag/SiOx/Si(100) revealing the silver nanoparticles on silicon substrate separated by native oxide layer. Figure 6 (c), (d) are the XTEM images of low magnification and high resolution of 2 nm Ag/2 nm SiOx/Si(100) annealed at ≈800°C in air revealing the intrusion of silver into silicon substrate like 2 nm Ag/17 nm GeOx/SiOx/Si(100) @800°C. From the HR-XTEM image, inter planar spacings of 0.254 nm, 0.261 nm, 0.281 nm and 0.411 nm on the structures and 0.311 nm on the substrate have been measured. From these values, 0.254 nm, 0.261 nm are matching with the (131) planes of Ag2Si, 0.281 nm is matches with the (200) planes of Ag2Si phase. HR-XTEM images depicts the presence of Moire fringes in the structures, the Moire fringe spacings using Ag2Si (131) and Si(111) planes with zero angle yielding a value of 1.47 nm, which matches with the measured value 1.46 nm ± 0.02 nm. In this case, formation of Ag2Si was found within the structures and these structures are also endotaxial in nature (figure 6(d)). This can be explained through the consumption of thermal energy for alloy formation. Mc Brayer et al., reported that silver inter diffuses through SiO2 layer in the form of Ag+ ion37, which will react with the silicon atoms to form alloy at lower temperatures compared to silver atoms. From the observations of 2 nm Ag/2 nm SiOx/Si(100)@800°C, presence of GeOx plays a key role in the formation of substrate symmetric single crystalline silver endotaxial nanostructures. We now address the position of GeOx layer in the formation of these nanostructures.
Figure 6. (a) SEM Micrographs of 2 nm Ag/SiOx/Si (100)@ 800°C in air, X-TEM Micrographs of 2 nm Ag/SiOx/Si (100)(b) as deposited and annealed at @ 800 °C in air (c) Low Magnification (d) HRTEM of selected area depicts endotaxial structures.
In order to understand the role of GeOx position at Ag and Si interface, samples of 17 nm GeOx/2 nm Ag/2 nm SiOx/Si were prepared (the Ag layer is sandwiched between GeOx and SiOx) and annealed under the same condition as above. Figure 7 (a) is the low magnification X-TEM micrograph of as deposited 17 nm GeOx/2 nm Ag/2 nm SiOx/Si(100) shows the irregular Ag structures on top of native oxide are covered along with the inter particle space is filled by GeOx layer. Figure 7 (b) and (c) are the XTEM micrographs of low magnification and HR-XTEM of 17 nm GeOx/2 nm Ag/2 nm SiOx/Si(100) annealed at ≈800°C. XTEM micrograph reveal the formation of embedded Ag nanostructures in the GeOx layer and the presence of oxide layer in between the silicon substrate and nanostructures. From HR-XTEM image, inter planar distance of 0.239 nm on spherical nano particles which corresponds to Ag (111) plane was measured. Oniki et. al., reported that desorption of GeO2 into volatile GeO occurs at lower temperatures in presence of metals compared to the absence of metal at the SiO2/Si interface. This could be one of the possible reasons for the embedded Ag nano structure formation because of the incomplete desorption of GeOx within the time and temperature available for the system to react with SiOx. From above observations it is evident that the position and presence of GeOx layer plays a key role in the formation of substrate symmetric single crystalline silver endotaxial nanostructures.
Figure 7. X-TEM Micrographs of (a) low mag of as deposited 17 nm GeOx/2 nm Ag/SiOx/Si (100) (b) Low Mag (c) HR-X-TEM of 17 nm GeOx/2 nm Ag/SiOx/Si (100) @ 800°C in air respectively.
Conclusion
Substrate symmetry driven silver nano structures are formed on silicon surface by annealing the 2 nm Ag/17 nm GeOx/SiOx/Si samples in ambient conditions at high temperatures. Endotaxial nano squares/rectangles are formed in the case of Si(100), endotaxial nanorods are formed on Si(110) and endotaxial nano triangles are formed for Si(111) substrate according to their 4-fold, 2-fold and 3-fold symmetries respectively. These results show an elegant procedure to fabricate controlled shapes of Ag or AgxSiy endotaxial nanostructures. 3-D structural analysis was carried out using STEM-tomography. These substrates are reusable if the molecules of interest can be removed by a proper solvent without affecting the Ag/Si layer. We believe these endotaxial Ag structures have potential to be robust SERS substrates.
Methods
Silicon wafers of various orientations i.e., (100), (110) and (111) were cleaned by standard procedure of rinsing and sonication with alcohol and de-ionized water. A ≈ 2 nm thick SiOx (native oxide) layer is also present on all the silicon substrates. Deposition of GeOx and silver was done by physical vapor deposition (PVD) system. We have used three configurations: (a) a ≈ 17 nm thick GeOx was deposited on Si(100), Si(110) and Si(111), then the deposition of 2 nm silver was done on 17 nm GeOx/SiOx/Si. (b) a ≈ 2 nm thick Ag was deposited on Si followed by GeOx deposition of about 17 nm thick and (c) a 2 nm thick Ag was deposited on Si. All these three types of samples are annealed in a horizontal tube furnace up to 800°C in atmosphere with a ramp rate of 7°C/minute and hold time is 30 minutes at 800°C. A Carl Zeiss Neon 40 Field Emission Gun Scanning Electron Microscope (FEGSEM) and JEOL JEM-2010 high resolution transmission electron microscope (HRTEM) were used to study the morphology and structure. An FEI Titan 80/300 TEM/STEM microscope operated at 300 keV was used for all STEM measurements. The HAADF detector covered an angular range of 33–200 mrad. For tomography, a single-tilt Fischione tomography sample holder was used and images have been acquired between −66° and +66° of tilt around the substrate surface normal in steps of 2°. The simultaneous iterative reconstruction technique (SIRT) was used for reconstruction with 10 iterations.
Two types of samples (Planar and Cross-sectional) were prepared for TEM observations. For planar samples ≈3 mm disc was cut from the desired sample using ultrasonic disc cutter followed by lapping from the back side to a thickness of ≈100 μm. The disc was dimpled using dimple grinder to a thickness of ≈30 μm at the dimple centre. Electron transparency was achieved using precise ion polishing system (PIPS) with low energy Ar+ ions. For cross-sectional samples ≈2.5 mm rectangular slabs were cut from the sample and bonded face to face (in order to protect the desired area) using epoxy glue and inserted in a stainless steel (SS) tube of inner diameter of ≈2.5 mm and outer diameter of ≈3 mm using epoxy glue. A ≈500 μm thick disc is cut from the above packed SS tube using diamond wheel saw. This disk is lapped from both the sides to a thickness of ≈100 μm, which is polished from both sides and dimpled from one side to a thickness of ≈30 μm at dimple centre. Electron transparency was achieved using 6.0 keV energy Ar+ ions.
Samples (2 nm Ag/17 nm GeOx/SiOx/Si(100) @ 800°C) were cleaned using Piranha solution for 30 minutes and dipped in 5% Hydroflouric acid for 90 minutes to remove the top oxide layers. After removing from the HF solution samples were allowed to dry in air for 30 minutes and then C V solution with various concentrations was drop casted on top of the substrate. SERS spectra were recorded using micro-Raman spectrometer (LABRAM-HR) using laser excitation lines of 514.5 nm at room temperature. The reason for recording SERS spectra with this excitation wave length was to study resonance of adsorbate on Ag nanostructures. All the measurements were made in a backscattering geometry, using a 50× microscope objective lens with a numerical aperture of 0.7. Typical laser power at the sample surface was ≈1.0 mW with a spot size of ≈2 μm. A 20 μl of the CV solution (5 × 10−10 M) was dropped onto the SERS substrate and allowed it to dry naturally. A fixed volume micro-pipette with disposable tips was used to prevent contamination. In our tests the dropped solution soon spread over the whole SERS substrate but remained confined to the substrate. Synchrotron based x-ray diffraction measurements have been carried out at BL-18B, Photon Factory (PF), KEK using 11.38 keV x-rays and with a beam size of 250 μm × 250 μm.
Author Contributions
R.R.J., A.Rath and P.V.S. took part in conceiving the idea, performed experiments, and R.R.J. and P.V.S. wrote the main manuscript; A.G. and A.B. took part in the growth of the samples and preparation of the specimen; R.S. and D.N.R. took performed the Raman measurements and wrote part of the manuscript; K.M., M.S., K.F., T.G., F.K. and A.R., performed STEM measurements, STEM-Tomography measurements and wrote part of the manuscript. All authors reviewed the manuscript.
Acknowledgments
Prof PVS's work has been funded by the Department of Atomic Energy, Government of India for project No. 12-R&D-IOP-5.09-0100 for whole project. The authors acknowledge Department of Science and Technology, India for the financial support and Saha Institute of Nuclear Physics, India for facilitating the experiments at the Indian Beamline, Photon Factory, KEK, Japan. DNR's work has been funded by Department of Science and Technology – ITPAR – Phase – 3 project and RS's work has been funded by Department of Science and Technology PURSE Fellowship.
References
- Kneipp J., Kneipp H., Wittig B. & Kneipp K. Novel optical nanosensors for probing and imaging live cells. Nanomed.: Nanotech. Bio. Med. 6, 214–226 (2010). [DOI] [PubMed] [Google Scholar]
- Stiles P. L., Dieringer J. A., Shah N. C. & Van Duyne R. P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008). [DOI] [PubMed] [Google Scholar]
- Le Ru E. C., Blackie E., Meyer M. & Etchegoin P. G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 111, 13794–13803 (2007). [Google Scholar]
- Qian X. M. & Nie S. M. Single-Molecule and Single-Nanoparticle SERS: from Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 37, 912–920 (2008). [DOI] [PubMed] [Google Scholar]
- Sharma B., Frontiera R. R., Henry A. I., Ringe E. & Van Duyne R. P. SERS: Materials, Applications and the Future. Materials Today 15, 16–25 (2012). [Google Scholar]
- Otto A. What is observed in single molecule SERS, and why? J. Raman Spectroscopy 33, 593–598 (2002). [Google Scholar]
- Camaden J. P. et al. Probing the Structure of Single-Molecule Surface-Enhanced Raman Scattering Hot Spots. J. Am. Chem. Soc. 130, 12616–12617 (2008). [DOI] [PubMed] [Google Scholar]
- Stender A. S. et al. Single Cell Optical Imaging and Spectroscopy. Chem. Rev. 113, 2469–2527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S., Smith D., Mock J. J. & Schultz D. A. Single Target Molecule Detection with Nonbleaching Multicolor Optical Immunolabels, Proc. Natl. Acad. Sci., USA 97, 996–1001 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J. N. et al. Biosensing with Plasmoic Nanoosensors. Nat. Mater. 7, 442–453 (2008). [DOI] [PubMed] [Google Scholar]
- Wustho lz K. L. et al. Structure−Activity Relationships in Gold Nanoparticle Dimers and Trimers for Surface-Enhanced Raman Spectroscopy. J Am Chem. Soc. 132, 10903–10910 (2010). [DOI] [PubMed] [Google Scholar]
- Negri P. & Dluhy R. A. Ag nanorod based surface-enhanced Raman spectroscopy applied to bioanalytical sensing. J. Biophotonics 6, 20–35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya M., Tani T., Tomaru Y., Li J. & Murakami N. Nanophotonics bio-sensor using gold nanostructure. Proc SPIE 7032, 70321Q/1 (2008). [Google Scholar]
- Fang J., Liu S. & Li Z. Polyhedral silver mesocages for single particle surface-enhanced Raman scattering-based biosensor. Biomaterials 32, 4877–4884 (2011). [DOI] [PubMed] [Google Scholar]
- Biggs K. B., Camden J. P., Anker J. F. & Van Duyne R. P. Surface-Enhanced Raman Spectroscopy of Benzenethiol Adsorbed from the Gas Phase onto Silver Film over Nanosphere Surfaces: Determination of the Sticking Probability and Detection Limit Time. J. Phys. Chem. A 113, 4581–4586 (2009). [DOI] [PubMed] [Google Scholar]
- Barnes W. L., Dereux A. & Ebbesen T. W. Surface plasmon subwavelength optics. Nature 424, 824–830 (2003). [DOI] [PubMed] [Google Scholar]
- Hu X. & Chan C. T. Photonic Crystals with Silver Wires as a Near-Infrared Superlens. Appl. phys. lett. 85, 1520–1522 (2004). [Google Scholar]
- Pang Y. T., Meng G. W., Fang Q. & Zhang L. D. Silver Nanowire Array Infrared Polarizers. Nantechnology. 14, 20–22 (2003). [Google Scholar]
- Wiley B., Sun Y. & Xia Y. Synthesis of Silver Nanostructures with Controlled Shapes and Properties. Acc. Chem. Res. 40, 1067–1076 (2007). [DOI] [PubMed] [Google Scholar]
- Juluri R. R., Rath A., Ghosh A. & Satyam P. V. Substrate Symmetry Driven Endotaxial Silver Nanostructures by Chemical Vapor Deposition. J. Phys. Chem. C. 117, 13247–13251 (2013). [Google Scholar]
- Bonev I. On the Terminology of the Phenomena of Mutual Crystal Orientation. Acta. Cryst. A 28, 508–512 (1972). [Google Scholar]
- Bennet P. A., He Z., Smith D. J. & Ross F. M. Endotaxial Silicide Nanowires: A Review. Thin Solid films 519, 8434–8440 (2011). [Google Scholar]
- Williams D. B. & Carter C. B. Transmission Electron Microscopy: A Textbook for Materials Science. (Springer, 1996).
- Oniki Y., Koumo H., Iwazaki Y. & Ueno T. Evaluation of GeO Desorption Behavior in the Metal/GeO2/Ge Structure and Its Improvement of the Electrical Characteristics. J. Appl. Phys. 107, 124113-1–124113-5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata Y., Takashima A. & Tsutomu T. Material Properties, Thermal Stabilities and Electrical Characteristics of Ge MOS Devices, Depending on Oxidation States of Ge Oxide: Monoxide [GeO(II)] and Dioxide [GeO2(IV)]. Mat. Res. Soc. Symp. Proc. 1155, C02–04 (2009). [Google Scholar]
- Bernstein R. B. & Cubicciotti D. The Kinetics of the Reaction of Germanium and Oxygen. J. Am. Chem. Soc. 73, 4112–4114 (1951). [Google Scholar]
- Wang S. K., Liu H. G. & Toriumi A. Kinetic study of GeO Disproportiantion into GeO2/Ge System using X-ray Photoelectron Spectroscopy. Appl. Phys. Lett. 101, 061907-1–061907-4 (2012). [Google Scholar]
- Yun S. J., Lee S. C., Kim B. & Kang S. W. Study of Interaction between Incident Silicon and Germanium Fluxes and SiO2. J. Vac. Sci. Technol., B 12, 1167–1169 (1994). [Google Scholar]
- Nason T. C., Yang G. R., Park K. H. & Lu T. M. Study of Silver DIffusion into Si(111) and SiO2 at Moderate Temperature. J. Appl. Phys. 70, 1392–1396 (1991). [Google Scholar]
- Rollert F., Stolwijk N. A. & Mehrer H. Solubility, Diffusion and Thermodynamic Properties of Silver in Silicon. J. phys. D: Appl. Phys. 20, 1148–1155 (1987). [Google Scholar]
- Xin H., Zhang J.-M., Wei X.-M. & Xu K. W. Anisotropy Analysis of Energy in Ag/Si Twist Interface. Surf. and Inter. Anal. 37, 608 (2005). [Google Scholar]
- Wulff G. Zur Frage der Geschwindigkeit des Wachsthums und der Auflosung der Krystallflachen. Zeitschrift für Krystallographie und Mineralogie 34, 449 (1901). [Google Scholar]
- Laue M. v. Der Wulffsche Satz fur die Gleidigewichtsform von Kristallen. Zeitschrift für Kristallographie 105, 124 (1943). [Google Scholar]
- Leopold N. & Lendl B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 107, 5723–5727 (2003). [Google Scholar]
- Kneipp K. et al. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 78, 1667–1670 (1997). [Google Scholar]
- Cho W. J., Kim Y. & Kim J. K. Ultrahigh-Density Array of Silver Nanoclusters for SERS Substrate with High Sensitivity and Excellent Reproducibility. ACS Nano 6, 249–255 (2012). [DOI] [PubMed] [Google Scholar]
- Mc Brayer J. D., Swanson R. M., Sigmon T. W. & Bravman J. Observation of Rapid Field Aided Diffusion of Silver in Metal Oxide Semiconductor Structures. Appl. Phys. Lett., 43, 653–654 (1983). [Google Scholar]