Abstract
This study examined the association of menstrual cycle phase with stress reactivity as well as the hormonal and neuroendocrine mechanisms contributing to cycle effects. Fifty-seven women underwent a modified Trier Social Stress Test (TSST) during the early follicular, late follicular and luteal phases of the menstrual cycle. Greater increases in cardiac index (CI) and greater decreases in vascular resistance index (VRI) during speech were observed in the luteal phase relative to other phases, while greater increases in epinephrine (EPI) was observed during the late follicular and luteal phases compared to the early follicular phase. Luteal phase estradiol predicted luteal EPI reactivity but not CI or VRI reactivity while luteal phase EPI reactivity predicted luteal phase CI and VRI reactivity. Thus, cycle-related changes in EPI reactivity may be a stronger determinant of cycle effects on hemodynamic reactivity than sex hormones per se.
Keywords: menstrual cycle, estradiol, progesterone, stress reactivity, catecholamines, hemodynamics, cortisol, blood pressure, heart rate
Cardiovascular disease (CVD) is the leading cause of mortality for women in the United States (Center for Disease Control, 2009). However, women are relatively protected from CVD until they reach the menopause transition, after which they experience a doubling of their CVD risk (Gordon, Kannel, Hjortland, & McNamara, 1978). That young women experiencing premature ovarian failure also exhibit an increased risk for CVD (Hu et al., 1999; Jacobsen, Knutsen, & Fraser, 1999) strongly suggests that sex steroid hormone withdrawal, independent of advancing age, is responsible for this observation.
Female sex hormones may act to confer CVD protection in part via beneficially modifying physiologic reactivity to stress. Exaggerated stress reactivity has been shown to predict the development of CVD risk factors, including hypertension, increased left ventricular mass, carotid atherosclerosis and coronary calcification (Gianaros et al., 2005; Matthews et al., 2004; Treiber et al., 2000) as well as future CVD risk itself (Chida & Steptoe, 2010). Among postmenopausal women, combined estrogen and progesterone therapy lowers blood pressure (BP) reactivity to stress and beneficially modifies the hemodynamic and neuroendocrine determinants of BP reactivity, namely vascular resistance stress reactivity, endothelial function, and norepinephrine stress reactivity (Del Rio et al., 1998; Girdler et al., 2004; Light et al., 2001). While these studies suggest that estrogen and progesterone may serve to protect premenopausal women from CVD via their positive effect on cardiovascular and neuroendocrine stress reactivity, with few exceptions, the majority of these studies have examined combined (estrogen plus progesterone) therapy and therefore cannot differentiate the cardioprotective effects of estrogen from progesterone.
Studies in premenopausal women that have attempted to do so by examining stress reactivity at different phases of the menstrual cycle have yielded inconsistent findings (Childs, Dlugos, & DeWit, 2010; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999; Lustyk, Douglas, Shilling, & Woods, 2012; Lustyk, Olson, Gerrish, Holder, & Widman, 2010; Manhem, Jern, Pilhall, Shanks, & Jern, 1991; Polefrone & Manuck, 1988; Pollard et al., 2007), that may be attributable to at least two methodological limitations in the existing research. First, these studies have compared stress reactivity in the luteal phase, characterized by elevated estradiol and progesterone, with reactivity in the follicular phase, assumed to reflect a lower hormone state. However, only the early follicular phase is characterized by both low estradiol and progesterone while the mid to late follicular phase is associated with markedly elevated estradiol concentrations. Thus, the existing menstrual cycle literature, with one exception (Pollard et al., 2007), is unable to differentiate the influence of estradiol alone from estradiol plus progesterone on stress reactivity. Pollard et al., (2007) showed that HR reactivity to perceived work stress is greater in the late follicular phase compared to the early follicular phase, underscoring the importance of refining the follicular phase window of testing.
The second major limitation of many of the referred menstrual cycle studies is the reliance on a between-subjects design. The substantial inter-individual differences in absolute sex steroid concentrations across the menstrual cycle obscures the ability to examine menstrual cycle phase effects on stress reactivity with a between-subjects design (Gravetter & Forzano, 2011).
These limitations notwithstanding, the majority of studies in this field find an absence of menstrual cycle effects on BP reactivity to stress (Collins, Eneroth, & Landgren, 1985; Girdler & Light, 1994; Girdler, Pedersen, Stern, & Light, 1993; Kajantie & Phillips, 2006; Stoney, Langer, & Gelling, 1986; Stoney, Owens, Matthews, Davis, & Caggiula, 1990). That is not to say, however, that the menstrual cycle does not influence the underlying hemodynamic determinants of BP reactivity, namely stroke volume (SV), cardiac output (CO) and vascular resistance (VR). The relative contribution that the myocardium versus the vasculature makes to BP responses, which is relatively stable within an individual over time (Kasprowicz, Manuck, Malkoff, & Krantz, 1990; Sherwood, Turner, Light, & Blumenthal, 1990), may be an important pathogenic influence in CVD development (Manuck, 1990). Estradiol has been shown to exert inotropic (contractile) actions on the myocardium, thereby increasing SV (Girdler et al., 2004; Pines et al., 1991; Ren et al., 2003), and to promote greater vasodilation to stress, thereby decreasing VR (Girdler, et al., 2004; Pines, et al., 1991). This combination of enhanced myocardial reactivity and greater decreases in VR is a hemodynamic stress response profile seen in groups at lower risk for CVD relative to their higher risk counterparts (Allen, Stoney, Owens, & Matthews; Girdler, Turner, Sherwood, & Light, 1990; Light, Turner, Hinderliter, & Sherwood, 1993; Sherwood, & Light, 1990; Treiber et al., 2000). Moreover, greater VR contributes to left ventricular hypertrophy (D. Levy et al., 1988), an independent predictor of CVD morbidity/mortality (Ghali et al., 1992; Koren, Devereux, Casale, Savage, & Laragh, 1991).
To date, there have been only four studies examining menstrual cycle influences on hemodynamic stress reactivity. In two prior within-subjects studies from our group, the luteal phase was associated with greater increases in SV coupled with greater decreases in VR to mental stress relative to the early follicular phase (Girdler & Light, 1994; Girdler, et al., 1993). The third study (McFetridge & Sherwood, 2000), using a between-subjects design, similarly found greater decreases in VR to mental stress during the luteal compared to the early follicular phase, though SV did not differ. Finally, in the fourth study (Sita & Miller, 1996), a within-subjects design, there were no differences in SV, CO or VR between the luteal phase and the late follicular phase. However, the relative equivalence of estradiol concentrations in luteal and late follicular phases may have accounted for the absence of a phase effect in that study.
The influence of the endogenous steroid hormones on hypothalamic-pituitary-adrenal (HPA) axis and sympatho-adrenal axis reactivity is also relevant to CVD risk (Christensen & Schultz-Larsen, 1994; Whitworth, Williamson, Mangos, & Kelly, 2005; Zoccali et al., 2002). Where phase effects on HPA axis reactivity have been observed, cortisol reactivity, but not ACTH reactivity, is greater in the luteal than the follicular phase (Childs, et al., 2010; Kirschbaum, et al., 1999). While fewer studies on catecholamine stress reactivity across the menstrual cycle exist, the data suggest greater catecholamine resting levels (Girdler et al., 2003) and reactivity in the luteal versus the early to mid-follicular phase (Childs, et al., 2010; Collins, et al., 1985). Exceptions to this general finding do exist, however: for example, one study by McFetridge & Sherwood (2000) found that women in the luteal phase exhibited lower catecholamine responses to laboratory stressors compared to women in the follicular phase.
Finally, no studies of which we are aware have examined the hormonal and neuroendocrine predictors of hemodynamic stress reactivity across the menstrual cycle. Thus, it is unknown whether estradiol and progesterone influence hemodynamic reactivity directly or indirectly (e.g., through alterations in neuroendocrine reactivity). The aims of the present study were therefore: 1) to examine menstrual cycle effects on hemodynamic and neuroendocrine stress reactivity in the early follicular (low hormones), late follicular (high estradiol, low progesterone) and luteal (high estradiol and high progesterone) phases of the menstrual cycle; and 2) to explore the hormonal and neuroendocrine predictors of these effects.
Method
Participants
Participants included 57 women who were medically healthy non-smokers, aged 18–40, recruited through newspaper advertisements. Exclusion criteria included: blood pressure >160/90 mmHg, prescription medication use (including oral contraceptives), regular over-the-counter medication use (e.g., non-steroidal anti-inflammatory agents), a chronic pain condition (e.g. temporomandibular joint disorder, fibromyalgia, arthritis) or other medical illness (e.g. cardiovascular, respiratory, neuroendocrine and gastrointestinal disorders), irregular menstrual cycles (<24 days or >32), self-reported alcoholism or drug abuse, a history of more than one depressive episode, the presence of a depressive episode in the last year, or self-reported moderate to severe emotional premenstrual symptoms. Due to the association of both depression (Gordon et al., 2008) and anxiety (Watkins, Blumenthal, & Carney, 2002) with cardiac-autonomic dysfunction, participants with Hamilton Depression Scale scores > 7 or Hamilton Anxiety scores > 9 (administered by a trained interviewer) were excluded., The study protocol was approved by the institution’s Institutional Review Board. All participants provided informed, written consent prior to participating and received $150 in compensation.
Procedure
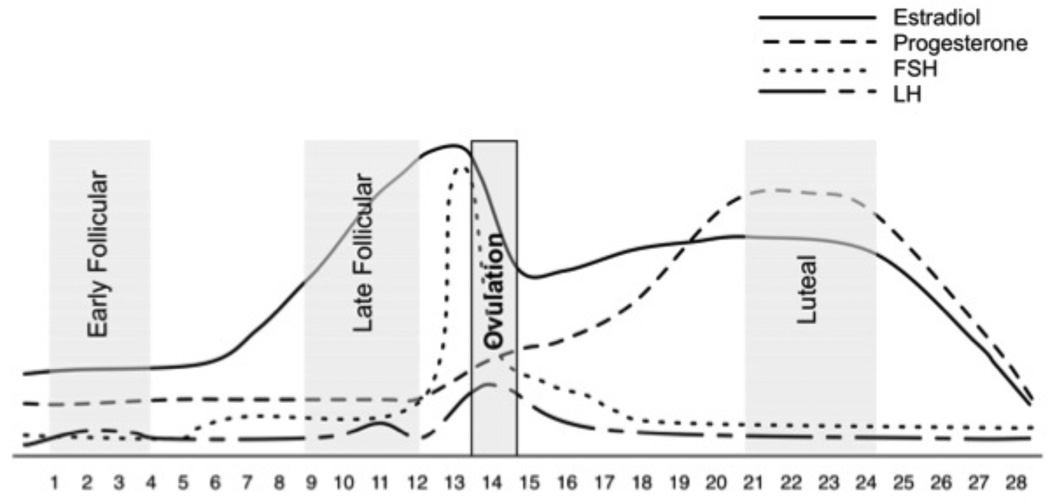
All laboratory sessions began at 8:00 a.m. Participants were asked to refrain from all over-the-counter medications 24 hours before testing as well as refrain from caffeine the day of testing. Participants were tested at three time points throughout their menstrual cycle (Figure 1): the early follicular phase (days 1–4, when both estradiol and progesterone are low), the late follicular phase (days 9–12, when estradiol is elevated and progesterone is low) and the luteal phase (5–10 days after ovulation, when both estradiol and progesterone are elevated). The timing of the luteal phase was based upon the detection of the luteinizing hormone (LH) surge that occurs 24–36 hours prior to ovulation using a home urine ovulation predictor test. All cycles were later confirmed to be ovulatory based on serum progesterone and estradiol levels, taken during the baseline period of each testing session (Table 1). The order of menstrual cycle phase was randomized for each woman.
Figure 1.
Table 1.
Demographic and baseline characteristics.
| Mean (SD) or % | |
|---|---|
| Age (yrs) | 27.5 (0.8) |
| BMI (kgs/m2) | 26.9 (0.8) |
| Race | |
| % Caucasian | 60 |
| % African American | 31 |
| % Other | 9 |
| BDI Score | 3.0 (0.4) |
| Spielberger Trait Anxiety | 31.7 (6.5) |
| Spielberger State Anxiety | 27.8 (5.9) |
| Estradiol (ng/ml) | |
| Early follicular | 23.3 (6.4) |
| Late follicular | 44.6 (22.6) |
| Luteal | 54.5 (22.4) |
| Progesterone (ng/ml) | |
| Early follicular | 0.2 (0.1) |
| Late follicular | 0.2 (0.2) |
| Luteal | 13.7 (7.8) |
BMI, body mass index; BDI, Beck Depression Inventory
Upon arrival, participants were instrumented with a BP cuff and impedance band electrodes for later monitoring of cardiovascular stress reactivity. A catheter was then placed into a forearm vein, to be used for blood draws using a non-heparinized, multi-stop-cock system allowing for blood samples to be taken at precise time intervals throughout testing. A curtain was drawn to prevent the subject from viewing the catheter and the blood draws.
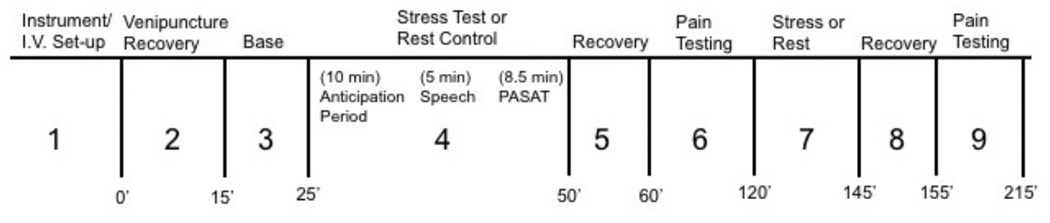
A secondary goal of the current study was to examine the effect of the menstrual cycle on pain sensitivity to a short battery of pain tests. These results are reported elsewhere (Klatzkin, Mechlin, & Girdler, 2010). Thus, subjects were exposed to both a mental stressor battery and a pain testing battery. The order of stress and pain testing was counterbalanced across subjects but constant within subject (see Figure 2).
Figure 2.
Figure 2 depicts the sequence of laboratory events: 1) Instrumentation and intravenous set-up; 2) recovery from venipuncture (15 min); 3) baseline (10 min quiet rest); 4) Trier social stress test (TSST) (23.5 min); 5) recovery (10 min); 6) pain testing; 7) rest control (or TSST if event #4 was rest control) (23.5 min); 8) recovery (10 min); and 9) pain testing. The current study’s findings are based on events #3, #4 and #5 for those who had the TSST first, or events #3, #7 and #8 for those who had pain testing first. These events are described below.
Baseline
Twenty-five minutes of quiet rest followed the i.v. setup, the first 15 min being recovery from venipuncture and the final 10 min constituting baseline. Blood pressure and impedance-derived measures were taken at minutes 1, 3, 6, 8 and 10 and averaged to yield a mean baseline value. Blood was sampled at minute 10 for baseline ACTH, cortisol, epinephrine (EPI), norepinephrine (NE), estradiol and progesterone concentrations.
The Trier Social Stress Test (TSST)
The TSST used was modified to account for the fact that participants were not ambulatory and to include a serial addition rather than a serial subtraction task. The TSST is a stress test that has been shown to induce reliably large and consistent activation of the SNS and HPA axes (Kirschbaum, Pirke, & Hellhammer, 1993). The TSST involves four components: 1) Pre-task instructions (5 minutes) during which participants are introduced to the committee who will later listen to their job talk, and are given instructions for the mental arithmetic task; 2) Speech preparation period (5 minutes) during which time participants were left alone to prepare their job talk; 3) Job speech (5 minutes) immediately following the preparation period, the selection committee returned to the testing room and asked the participant to deliver his/her job talk on why they would be the perfect candidate for the job. If the participant ended their talk before 5 minutes, the selection committee questioned the participant in a systematic fashion to ensure the participant would speak the entire 5 minutes. Participants were tape-recorded throughout their performance. 4) Paced auditory serial addition test (PASAT) (8.5 minutes) involves a tape-recorded presentation of numbers from 1 to 9 (for greater detail, see Tombaugh, 2006). Participants added each number presented on the tape to the immediately preceding number and stated the answer aloud. There are four series of numbers, with progressively shorter inter-digit intervals. The committee remained in the room to monitor performance.
Cardiovascular measures were obtained at minutes 1, 3 and 5 of both the speech preparation period and the job speech and once during each of the four series of the PASAT. These measures were then averaged to obtain a mean preparation, speech and math task value for each cardiovascular measure. Plasma EPI and NE were sampled at the end of minute 2 of both the job speech and PASAT since catecholamines peak within the first two minutes of mental stress (Dimsdale & Ziegler, 1991).
Post-Stress Cortisol Sampling
Participants rested quietly alone. Plasma cortisol was measured at the end of the recovery period to capture the delayed plasma cortisol response to the TSST (Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Kirschbaum et al., 1995).
Physiological Recording Procedures
The SunTech Exercise BP monitor, Model 4240 (SunTech Medical Instruments, Inc., Raleigh, NC) provided BP measurements during baseline and mental stress testing. Five standard stethoscopic and automated BP measurements were taken simultaneously to ensure correct microphone placement and cuff position.
Impedance cardiography was used to noninvasively monitor cardiovascular activity (Sherwood et al., 1990), including CO, SV, total vascular resistance (VR) and HR. A custom-designed impedance cardiograph (HIC-100 Bioimpedance Technology, Inc., Model 100, Chapel Hill, NC, USA) was used in conjunction with a tetrapolar band electrode configuration to record impedance dZ/dt and Zo signals. Impedance and electrocardiogram signals were processed online by specialized computer software (BIT, Chapel Hill, NC) with subsequent manual editing to improve accuracy. For each minute of interest, a 30 second continuous sample of waveforms (obtained concurrently with BP) was processed to generate an ensemble-averaged cardiac cycle, from which SV was determined using the Kubicek et al. (1966) equation and HR was determined using the mean interbeat interval. CO and VR for these same minutes were then calculated using standard formulae (Sherwood et al., 1990). CO, SV and VR were adjusted for individual variations in body size by using body surface areas to derive cardiac index (CI), stroke volume index (SVI) and vascular resistance index (VRI).
Hormone and Neuroendocrine Assays
Plasma cortisol, serum estradiol and progesterone were determined using radioimmunoassay (RIA) techniques (ICN Biomedical Inc.). The specificity of the antiserum for progesterone is very high, showing only 0.01–2.5% cross-reactivity with other steroid compounds. The specificity of the antiserum for estradiol is also high, showing only 0.01–1.45% cross-reactivity with steroid hormones with the exception of estrone, for which there is up to 6% cross-reactivity. For cortisol, the sensitivity of the assay is excellent at 0.07 µg/dl and the specificity high, showing 0.05–2.2% cross-reactivity with similar compounds, except prednisolone, where 94% cross-reactivity is obtained.
Plasma ACTH was determined using RIA techniques from commercially available kits (Nichols Institute Diagnostics). The sensitivity of this assay is high at 1 pg/ml and the selectivity is excellent showing only 0–0.02% cross-reactivity with steroid compounds.
Plasma EPI and NE concentrations were determined using the high performance liquid chromatography (HPLC) technique. All HPLC procedures were conducted in the Core Laboratory of the institution’s General Clinical Research Center (GCRC). The lower limit of quantification is 25 pg/ml and the intra- and inter- day coefficients are <10%.
Statistical Analyses
As per Warner (2012), sensitivity analyses were used to identify extreme outliers prior to data analysis, defined as values 3 or more interquartile ranges below the first quartile or above the third quartile (SAS Institute Inc., 2011). Although the analyses presented are those with the outliers removed, analyses with the outliers retained yielded an identical pattern of results.
For each cardiovascular or neuroendocrine measure, reactivity was defined as the difference between mean stress level and mean baseline level. T-tests were used to confirm a significant effect of the TSST on hemodynamic and neuroendocrine measures.
Apart from cortisol and ACTH, which were measured only in response to the TSST, reactivity of all other measures was calculated separately in response to the job speech and the PASAT in consideration of the literature suggesting that women are particularly responsive to social rejection over performance-type tasks (Darnall & Suarez, 2009).
For each physiologic dependent measure, a repeated measures ANOVA, with cycle phase as the repeated measure was then used to examine phase effects on baseline and reactivity values in the early follicular, late follicular and luteal phases. Within-subjects paired t-tests were then used to explore significant phase effects. The Huynh-Feldt correction was used whenever appropriate to correct for sphericity.
Where significant phase effects on stress reactivity were observed, stepwise regression analyses were conducted in order to examine predictors of the phase effects. In stepwise regression each independent variable specified is entered into the regression one at a time until all variables have been added with the provision that each meets the specified criterion. For this study, the criterion used was one of significance level p<.100, a more conservative significance level than the p-value of .150 that is conventional for stepwise regression (SAS Institute, 2011). Furthermore, the stepwise approach involves an additional procedure in which all variables are reexamined after the addition of other variables to verify that each remains a significant and independent predictor. Thus, this approach helps to circumvent the problem of multicolinearity of independent variables.
All analyses were conducted using SAS v. 9.3.
Results
Participant Characteristics
As reflected in Table 1, the participants were relatively young, had low depression and anxiety scores and exhibited estradiol and progesterone concentrations in each menstrual cycle phase that were consistent with expected values.
Menstrual Cycle Effects on Baseline Measures
A significant effect of phase was found for baseline HR (F(2, 98) = 3.91, p = .024) such that HR during the luteal phase was significantly higher than the early (t(49) = 2.27, p = .027) and late follicular phase (t(51) = 2.56, p = .014). Second, a significant effect of menstrual cycle phase was seen for baseline VRI (F(2, 94) = 3.06, p = .052) such that VRI was significantly lower during the luteal phase compared to the early follicular phase (t(47) = −2.14, p = .038). No other baseline cardiovascular or neuroendocrine measures differed according to menstrual cycle phase.
Overall Efficacy of the Stress Protocol
Systolic BP, Diastolic BP and HR all significantly increased in response to the mental stressors (t(57) = 21.1, 24.6 and 19.2, p<.001, respectively) as did plasma EPI, NE (t(55) = 6.8 and 6.7, p<.001, respectively), ACTH (t(55) = 2.0, p = .047) and CI (t(55) = 10.6, p<.001). SVI tended to decrease (t(55) = −1.9, p=.070) while no effect of the mental stress was seen for VRI or plasma cortisol.
Menstrual Cycle Effects on Stress Reactivity
Blood pressure (BP) and heart rate (HR)
No phase effects on BP or HR reactivity were observed in response to either mental stressor.
Hemodynamic measures
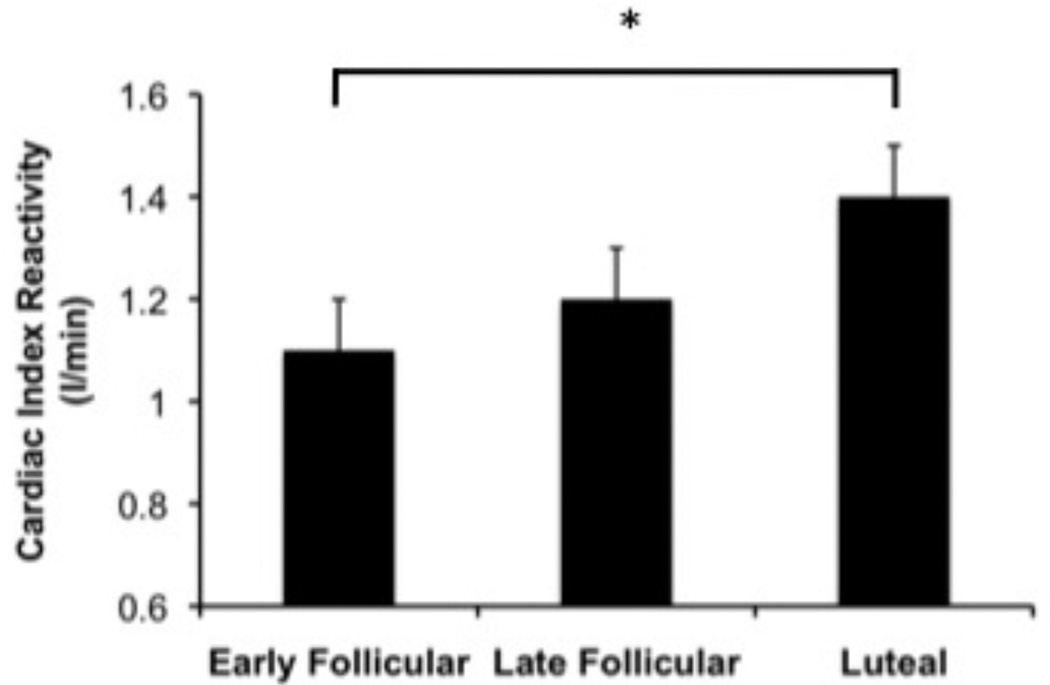
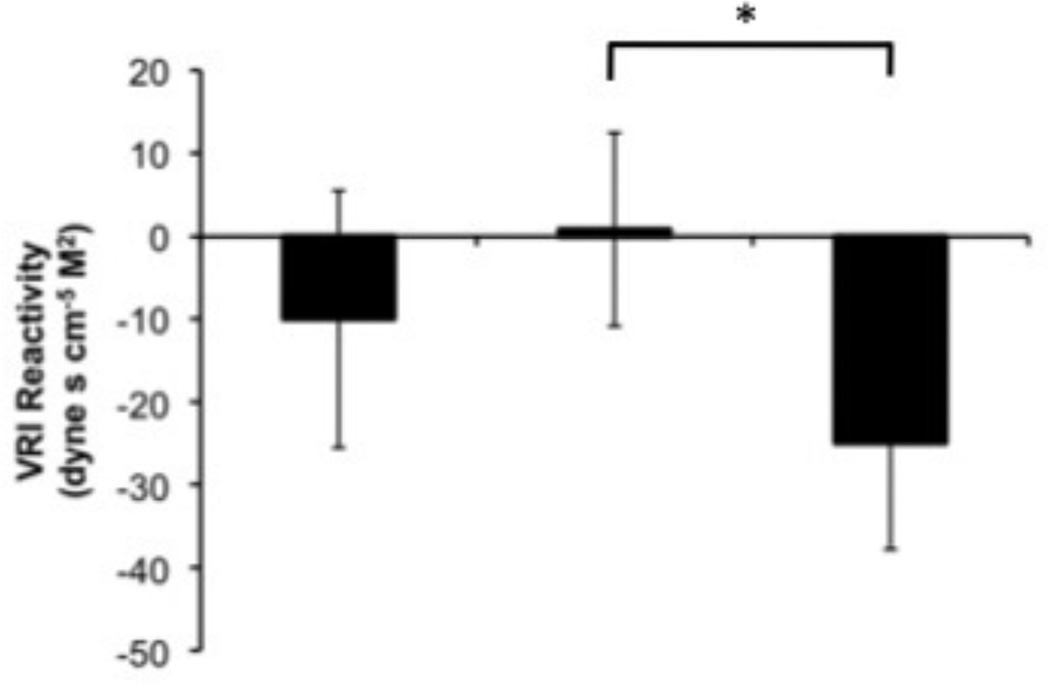
Menstrual cycle phase effects were seen for CI reactivity (F(2, 94) = 3.6, p = .033) such that during the speech, reactivity was greater during the luteal phase than the early follicular phase (t(47) = −2.6, p = .014) (Figure 3). Although the omnibus test for a cycle effect on VRI reactivity to speech did not reach statistical significance (F(2, 94) = 2.2, p = .123), because of the prior literature indicating that such an effect is consistently observed, we compared the individual phases in response to the speech. Consistent with prior research, VRI decreases in the luteal phase was significantly different from essentially no change in VRI in the late follicular phase (t(49) = 2.4, p = .019) (Figure 4). No cycle effects were observed for SVI reactivity.
Figure 3.
Figure 4.
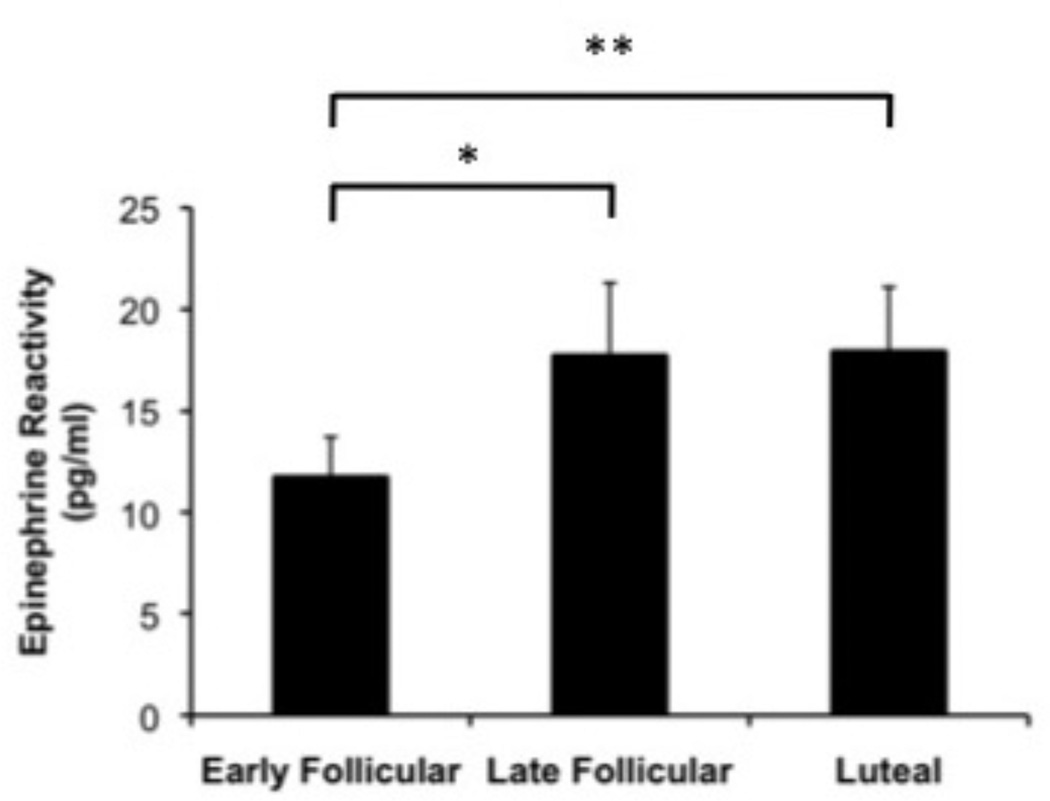
Epinephrine (EPI)
Consistent with the pattern seen for CI reactivity, a significant effect of menstrual cycle phase was seen for EPI reactivity (F(2, 86) = 3.3, p = .048) such that reactivity in response to the speech was greater in the late follicular (t(46) = −2.2, p = .031) and luteal phases (t(45) = −2.7, p =.009) compared to the early follicular phase (Figure 5).
Figure 5.
Norepinephrine (NE), ACTH and Cortisol
There were no significant effects of cycle phase on stress reactivity of these neuroendocrine measures.
Hormonal and Neuroendocrine Predictors of Menstrual Cycle Phase Effects
In order to examine predictors of speech stress reactivity measures that were influenced by the menstrual cycle (EPI, CI and VRI reactivity), stepwise multiple regression analyses were conducted. Estradiol, progesterone, baseline and reactivity values for cortisol, EPI and NE were entered as possible predictors into two separate models, the first predicting CI reactivity to the speech stress and the second predicting VRI reactivity to the speech stress. These analyses were conducted separately for each menstrual cycle phase. A third model, including the same predictors as above, with the exception of baseline and reactivity EPI, was similarly examined as potential predictors of EPI reactivity for each menstrual cycle phase (see Table 4).
Table 4.
Predictors of CI, VRI and EPI reactivity to speech stress on the basis of multiple regression analyses.
| Early Follicular Phase Reactivity | Late Follicular Phase Reactivity | Luteal Phase Reactivity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor Variables | CI | VRI | EPI | CI | VRI | EPI | CI | VRI | EPI |
| Estradiol (ng/ml) | - | - | - | - | - | - | - |
R2 = .08 β = .32 |
|
| Progesterone (ng/ml) | - | - | - | - | - | - | - | - | - |
| Baseline EPI (pg/ml) |
R2 = .31 β = .02 |
- | NA | - | - | NA | - | - | NA |
| EPI Reactivity (pg/ml) |
R2 = .05 β = .03 |
- | NA |
R2 = .05 β = .01 |
- | NA |
R2 = .43 β = .03 |
R2 = .10 β = −1.24 |
NA |
| Baseline NE (pg/ml) | - | - |
R2 = .06 β = .09 |
- | - | - | - |
R2 = .07 β = -.34 |
- |
| NE Reactivity (pg/ml) | - | - |
R2 = .12 β = .08 |
R2 = .21 β = .01 |
- |
R2 = .34 β = .33 |
- | - |
R2 = .39 β = .29 |
| Baseline Cortisol (pg/ml) | - | - | - | - | - | - | - | - | - |
| Cortisol Reactivity (pg/ml) | - | - | - | - | - | - | - | - |
R2 = .04 β = 2.19 |
| Total model R2 |
R2 = .36 p = <.001 |
- |
R2 = .18 p = .015 |
R2 = .26 p <.001 |
- |
R2 = .34 p < .001 |
R2 = .43 p <.001 |
R2 = .17 p = .019 |
R2 = .51 p < .001 |
CI
In the early follicular phase, greater baseline EPI and greater EPI reactivity predicted greater CI reactivity, accounting for 36% of its variance (F(2, 45) = 11.9, p<.001). In the late follicular phase, greater EPI reactivity and NE reactivity predicted greater CI reactivity, accounting for 26% of its variance (F(2, 51) = 8.9, p<.001). Finally, in the luteal phase, greater EPI reactivity predicted greater CI reactivity, accounting for 43% of its variance (F(1, 46) = 34.4, p<.001).
VRI
A significant predictive model was found for VRI reactivity during the luteal, but not the early or late follicular phases. In this model, both greater luteal EPI reactivity and baseline NE predicted greater decreases in VRI. Together, they accounted for 17% of the variance in VRI reactivity (F(2, 46) = 4.4, p = .019).
EPI
In the early follicular phase, greater baseline NE and greater NE reactivity predicted greater EPI reactivity, accounting for 18% of its variance (F(2, 46) = 4.6, p = .015). In the late follicular phase, greater NE reactivity predicted greater EPI reactivity and accounted for 34% of its variance (F(1, 52) = 25.9, p<.001). Finally, in the luteal phase, greater concentrations in luteal phase estradiol and greater NE reactivity and cortisol reactivity predicted greater EPI reactivity, accounting for 51% of its variance (F(3, 47) = 15.4, p<.001).
Discussion
The current study aimed to clarify the effects of estradiol versus progesterone in various phases of the menstrual cycle on stress reactivity as well as to identify the hormonal and neuroendocrine mechanisms predicting these effects. Results indicated that EPI, CI and VRI reactivity to stress differ by menstrual phase, with the greatest increases in EPI and CI, and the greatest decreases in VRI (vasodilatory responses) occurring during the luteal phase, when both estradiol and progesterone are elevated. While it must be acknowledged that the overall effect of cycle phase on VRI did not reach conventional levels of statistical significance, this likely resulted from a Type II error as suggested by our posthoc tests comparing the luteal and late follicular phases, where differences in VRI were substantial and statistically significant.
It was found that EPI reactivity increased in the late follicular phase and remained elevated during the luteal phase, mirroring menstrual increases in estradiol rather than progesterone. Furthermore, stepwise regression analyses demonstrated that estradiol, but not progesterone, was an independent predictor of EPI reactivity during the luteal phase, as was cortisol reactivity, the latter potentially reflecting the positive feedback effects involving the HPA and SNS axes (Calogero, Gallucci, Chrousos, & Gold, 1988; Valentino, Foote, & Aston-Jones, 1983). That EPI but not NE reactivity was associated with menstrual cycle phase may be related to the fact that estradiol and plasma EPI share the adrenal gland as a common origin whereas NE’s release is more dispersed throughout the body. However, this explanation is inconsistent with the only other study measuring catecholamine reactivity to the TSST across the menstrual cycle (Childs et al., 2010), which found NE reactivity to be greatest in the luteal relative to the early follicular phase. While both the current study and the study by Childs et al. find greater catecholamine reactivity in the luteal phase, these cycle effects run counter to the literature examining the effect of exogenous estradiol administration in perimenopausal (Komesaroff, Esler, & Sudhir, 1999) and postmenopausal women (Girdler, et al., 2004; Light et al., 2001; Lindheim et al., 1992) and in men (Del Rio et al., 1994), which find a catecholamine reactivity blunting effect of estradiol.
The human and animal literature suggests that estradiol attenuates reactivity of the sympatho-adrenal nervous system through a variety of mechanisms (Hinojosa-Laborde, Chapa, Lange, & Haywood, 1999), including reduced sensitivity of α2 adrenergic receptors to inhibitory signals (Del Rio, Verlardo, Zizzo, Marrama, & Della Casa, 1993) and decreased release and increased degradation of catecholamines at the adrenal medulla (Fernandez-Ruiz, Bukhari, Martinez-Arrieta, Tresguerres, & Ramos, 1988). However, the estradiol-mediated effects on sympathetic mechanisms that are observed when exogenous estradiol is delivered in isolation may be less evident in the presence of other hormones or processes occurring in the late follicular or luteal phases of the menstrual cycle. For example, relative to control and estradiol treatment, combined estradiol and progesterone treatment in postmenopausal women has been associated with greater catecholamine reactivity to mental stress (Burleson et al., 1998). However, our study found increased EPI reactivity in the late follicular phase relative to the early follicular phase, suggesting that the association between elevated estradiol concentrations and increased catecholamine reactivity is not solely explained by concurrently elevated progesterone concentrations. Clearly, the context in which reproductive steroid hormones act on the sympathetic nervous system, including the age, sex, and menopausal status of the individual, is important. Furthermore, the acute actions of an exogenously-administered hormone may differ from the effects of long-term endogenous hormone exposure.
In the case of CI, although reactivity was greater during the luteal phase, when both estradiol and progesterone are elevated, the stepwise regression analyses suggest that the elevated luteal phase CI reactivity does not result from elevated sex steroid concentrations, but rather appears to be driven by menstrual cycle increases in EPI reactivity (which was also greater in the luteal than the early follicular phase). While EPI reactivity also predicted CI reactivity in the early and late follicular phases, the amount of variance explained in CI reactivity was substantially lower. The effect of EPI on CI reactivity is consistent with the well-documented effects of EPI on hemodynamic responses (B. Levy et al., 1997; B. Levy, Perez, Perny, Thivilier, & Gerard, 2011), and likely occurs through EPI’s stimulation of myocardial β1 adrenergic receptors, which mediate both inotropic and chronotropic activities of the myocardium to increase CI.
Similarly for VRI reactivity, the stepwise regression analyses did not support an independent role of either estradiol or progesterone in driving luteal phase-related changes in VRI reactivity. Rather, greater luteal phase EPI reactivity and baseline NE concentrations predicted the vasodilatory responses to stress seen in the luteal phase. However, the independent modulatory role of catecholamines was weaker for VRI than CI. This is consistent with the results of McFetridge & Sherwood (2000), who observed exaggerated luteal-phase decreases in VR in the absence of increased catecholamine reactivity, though that study was limited by the use of a between-subjects design. Thus, other mechanisms not directly related to absolute catecholamine concentrations may alter VRI reactivity in the luteal phase. One possibility relates to the α-adrenoceptors. Catecholamine-induced stimulation of vascular α-adrenoceptors mediates vasoconstrictive responses (Ahlquist, 1976). Alpha 2-adrenoceptor sensitivity has been found to be reduced in the luteal phase of the menstrual cycle, at least in White women, though the mechanisms through which this occurs are unknown (Freedman & Girgis, 2000). If this mechanism were at play, although EPI release may increase in the luteal phase, its α adrenoceptor-mediated vasoconstrictive effects may be lessened while its β-adreneroceptor-mediated vasodilatory effects remain intact, thereby resulting in a net vasodilatory tone. Future studies testing the effect of adrenergic receptor blockade on stress reactivity throughout the menstrual cycle could help clarify this issue.
Of note, a menstrual cycle phase effect was found for the speech task but not the math task. Though participants’ neuroendocrine and cardiovascular responses were in the same direction for both tasks (Table 2), reactivity was greatest during the speech. The fact that the math task was modified to involve an addition rather than a subtraction exercise, making the task easier than the traditional TSST, may be one explanation for the reduced physiological responses during the math task. The nature of the tasks themselves may also be important: traditionally, speech stressors have been viewed as more gender relevant for women, while mental arithmetic stressors may be more gender relevant for men (Darnall & Suarez, 2009). Thus, the potency and nature of the stressor may unveil a menstrual cycle effect on stress reactivity, as suggested by previous research using stressors varying greatly in intensity (Kajantie & Phillips, 2006). The current study addresses several gaps in the literature on menstrual cycle and stress reactivity. It used a within-subjects design and is the only laboratory-based study examining stress reactivity at three key times throughout the menstrual cycle, thereby examining menstrual cycle effects during three distinct hormonal milieus. Finally, our simultaneous measurement of both hemodynamic and neuroendocrine reactivity in the same individuals allowed for the advancement of our current understanding of neuroendocrine and hormonal mechanisms contributing to myocardial and vascular stress reactivity in women across the reproductive cycle.
Table 2.
Mean (SD) baseline cardiovascular and neuroendocrine measures as a function of menstrual cycle phase.
| Early Follicular | Late Follicular | Luteal | |
|---|---|---|---|
| SBP (mmHg) | 110.6 (10.4) | 109.6 (10.8) | 111.3 (10.5) |
| DBP (mmHg) | 65.5 (7.7) | 63.7 (8.1) | 64.6 (6.8) |
| HR (bpm) | 69.0* (9.4) | 68.2# (10.4) | 71.2*# (8.1) |
| CI (l/min) | 4.2 (1.2) | 4.3 (1.1) | 4.4 (1.4) |
| SVI (ml/beat per M2) | 62.7 (19.0) | 64.8 (18.0) | 62.4 (18.8) |
| VRI (dyne s cm−5 M2) | 515.0* (167.6) | 484.6 (146.0) | 484.9* (126.6) |
| Epinephrine (pg/ml) | 10.7 (8.0) | 11.1 (7.1) | 11.4 (8.4) |
| Norepinephrine (pg/ml) | 172.8 (64.4) | 172.3 (68.6) | 190.8 (71.3) |
| Cortisol (pg/ml) | 6.8 (2.3) | 6.9 (2.3) | 6.2 (2.5) |
| ACTH (pg/ml) | 21.4 (10.3) | 21.4 (11.2) | 19.2 (6.5) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SVI, stroke volume index; VRI, vascular resistance index; ACTH, adrenocorticotropic hormone;
Note: Values in the same row with the same superscript (* or #) are significantly different from each other at the p<.05 level
Despite these strengths, the current study should be interpreted in light of its limitations. The absence of stress or cycle effects on the HPA-axis measures may have resulted from: 1) our choice to conduct all test sessions in the morning when circadian effects on the HPA axis may override stress and cycle effects (Kudielka, Schommer, Hellhammer, & Kirschbaum, 2004); 2), the absence of a measure of free (biologically available) cortisol, which may have obscured our ability to observe a menstrual cycle influence on cortisol since estradiol influences corticotropin binding globulin (CBG) levels (Kirschbaum et al., 1999); and 3) since cortisol and ACTH were measured only once post-task, this may have limited our ability to capture the dynamic activation of the HPA axis during and following mental stress. It should also be acknowledged that the relatively young and narrow age range of the study participants may limit the generalizability of the current findings to other reproductive phases when estradiol and progesterone are particularly dynamic (e.g., puberty and during the menopause transition). Additionally, because we did not assess subjective mood during the TSST, we could not assess the effects of subjective responses to stress on physiologic stress reactivity. Lastly, because half of the sample was exposed to pain testing prior to mental stress testing, the possibility exists that there may have been carryover effects of pain-induced stress reactivity that could have influenced stress reactivity to the TSST. However, because the order of pain versus stress testing was held constant for each subject there is no reason to believe that the menstrual cycle effects on stress reactivity would have been confounded by the order of events. Moreover, the 15-minute recovery between pain and stress testing was imposed to minimize carry over effects.
In conclusion, while this study showed that myocardial and vascular determinants of BP reactivity vary with menstrual cycle phase, it also suggests that these hemodynamic determinants are more directly influenced by EPI reactivity to stress than by endogenous sex hormones. By and large, cardiovascular stress reactivity in these medically and psychologically healthy women is remarkably stable throughout the menstrual cycle. The hemodynamic shifts in CI and VRI reactivity that were observed across phases may represent an adaptive mechanism aimed at keeping BP reactivity relatively constant. Taken together, the hemodynamic response profile observed during the luteal phase of the menstrual cycle, of enhanced CI reactivity but greater vasodilation to preserve stable BP reactivity, a profile seen in groups at lower risk for CVD (Allen et al., 1993; Girdler et al., 1990; Light et al., 1993; Sherwood et al., 2010; Treiber et al., 2000), may be one mechanism by which the female sex hormones confer cardio protection in premenopausal women. This is not to say, however, that there may not be subgroups of women for whom hemodynamic mechanisms fail to maintain stable BP reactivity across the menstrual cycle. Future studies examining menstrual cycle influences on cardiovascular and neuroendocrine reactivity in women at higher risk for CVD, such as women who suffer from obesity or who have a strong family history of CVD, are warranted.
Table 3.
Mean (SD) reactivity during mental stress as a function of menstrual cycle phase.
| Early Follicular | Late Follicular | Luteal | |
|---|---|---|---|
| SBP (mmHg) | |||
| Speech | 22.0 (9.7) | 23.3 (9.3) | 22.3 (9.3) |
| Math | 15.5 (8.2) | 15.8 (8.6) | 15.0 (8.5) |
| DBP (mmHg) | |||
| Speech | 16.3 (7.2) | 16.6 (6.6) | 14.8 (5.3) |
| Math | 11.2 (5.6) | 11.6 (5.0) | 11.1 (5.8) |
| HR (bpm) | |||
| Speech | 17.4 (8.7) | 18.9 (8.8) | 19.2 (10.0) |
| Math | 11.6 (5.7) | 12.7 (7.1) | 12.3 (6.4) |
| CI (l/min) | |||
| Speech | 1.1* (0.9) | 1.2 (1.0) | 1.4* (1.0) |
| Math | 0.6 (0.7) | 0.6 (0.7) | 0.6 (0.7) |
| SVI (ml/beat per M2) | |||
| Speech | −0.7 (10.3) | −1.6 (10.1) | 1.3 (10.8) |
| Math | −2.4 (7.4) | −4.0 (7.7) | −2.6 (7.9) |
| VRI (dyne s cm−5 M2) | |||
| Speech | −10.0 (113.2) | 0.8* (86.0) | −24.9* (91.2) |
| Math | 12.6 (85.7) | 16.2 (87.3) | 13.6 (77.1) |
| Epinephrine (pg/ml) | |||
| Speech | 11.7*& (13.8) | 17.7* (26.2) | 17.9& (23.2) |
| Math | 6.1 (10.1) | 5.7 (10.3) | 5.8 (9.8) |
| Norepinephrine (pg/ml) | |||
| Speech | 31.1 (44.4) | 37.8 (45.2) | 41.1 (46.8) |
| Math | 17.6 (46.5) | 17.2 (53.4) | 27.3 (42.7) |
| Cortisol (pg/ml) | 0.2 (2.4) | 0.0 (2.5) | 0.1 (2.2) |
| ACTH (pg/ml) | 1.4 (6.2) | 1.6 (7.1) | 1.0 (4.8) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SVI, stroke volume index; VRI, vascular resistance index; ACTH, adrenocorticotropic hormone;
p<.01,
p<.05.
Note: Values in the same row with the same superscript (* or &) are significantly different from each other
Acknowledgements
This research was supported by NIH grant RO1-DA013705, CTSA grant UL1RR025747 and NIH grant T32-MH093315. Dr. Gordon is also the recipient of a Postdoctoral Fellowship of the Fonds de la Recherche en Santé du Québec (FRSQ).
References
- Abplanalp JM, Livingston L, Rose RM, Sandwisch D. Cortisol and growth hormone responses to psychological stress during the menstrual cycle. Psychosomatic Medicine. 1977;39(3):158–177. doi: 10.1097/00006842-197705000-00002. [DOI] [PubMed] [Google Scholar]
- Ahlquist RP. Present state of alpha- and beta-adrenergic drugs I. The adrenergic receptor. American Heart Journal. 1976;92(5):661–664. doi: 10.1016/s0002-8703(76)80086-5. [DOI] [PubMed] [Google Scholar]
- Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosomatic Medicine. 1993;55(6):505–517. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Burleson MH, Malarkey WB, Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, Berntson GG. Postmenopausal hormone replacement: effects on autonomic, neuroendocrine, and immune reactivity to brief psychological stressors. Psychosomatic Medicine. 1998;60(1):17–25. doi: 10.1097/00006842-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Chrousos GP, Gold PW. Catecholamine effects upon rat hypothalamic corticotropin-releasing hormone secretion in vitro. Journal of Clinical Investigation. 1988;82(3):839–846. doi: 10.1172/JCI113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. [Retrieved October 27, 2012];Women and Heart Disease Fact Sheet. 2009 [Google Scholar]
- Chapa IC, Mifflin S, Haywood JR, Hinojosa-Laborde C. Cardiovascular responses to NMDA in the paraventricular nucleus in male and female rats. Society of Neuroscience. Abstract. 1995;21:638. [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47(3):550–559. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen NJ, Schultz-Larsen K. Resting venous plasma adrenalin in 70-year-old men correlated positively to survival in a population study: the significance of the physical working capacity. Journal of Internal Medicine. 1994;235(3):229–232. doi: 10.1111/j.1365-2796.1994.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Collins A, Eneroth P, Landgren BM. Psychoneuroendocrine stress responses and mood as related to the menstrual cycle. Psychosomatic Medicine. 1985;47(6):512–527. doi: 10.1097/00006842-198511000-00002. [DOI] [PubMed] [Google Scholar]
- Darnall BD, Suarez EC. Sex and gender in psychoneuroimmunology research: past, present and future. Brain, Behavior, and Immunity. 2009;23:595–604. doi: 10.1016/j.bbi.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio G, Velardo A, Zizzo G, Avogaro A, Cipolli C, Della Casa L. Effect of estradiol on the sympathoadrenal response to mental stress in normal men. Journal of Clinical Endocrinology and Metabolism. 1994;79(3):836–840. doi: 10.1210/jcem.79.3.8077370. [DOI] [PubMed] [Google Scholar]
- Del Rio G, Verlardo A, Zizzo G, Marrama P, Della Casa L. Sex differences in catecholamine response to clonidine. International Journal of Obesity Related Metabolic Disorders. 1993;17(8):465–469. [PubMed] [Google Scholar]
- Del Rio G, Velardo A, Menozzi R, Zizzo G, Tavernari V, Venneri MG, Marrama P, Petraglia F. Acute estradiol and progesterone administration reduced cardiovascular and catecholamine responses to mental stress in menopausal women. Neuroendocrinology. 1998;67:269–274. doi: 10.1159/000054322. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Ziegler ME. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation. 1991;83(4) Suppl:II36–II42. [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, Bukhari AR, Martinez-Arrieta R, Tresguerres JA, Ramos JA. Effects of estrogens and progesterone on the catecholaminergic activity of the adrenal medulla in female rats. Life Science. 1988;42(9):1019–1028. doi: 10.1016/0024-3205(88)90432-8. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular alpha-adrenergic responsiveness. Hypertension. 2000;35(3):795–799. doi: 10.1161/01.hyp.35.3.795. [DOI] [PubMed] [Google Scholar]
- Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Annals of Internal Medicine. 1992;117(10):831–836. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosomatic Medicine. 2005;67(4):553–560. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Hinderliter AL, Wells EC, Sherwood A, Grewen KM, Light KC. Transdermal versus oral estrogen therapy in postmenopausal smokers: hemodynamic and endothelial effects. Obstetrics & Gynecology. 2004;103(1):169–180. doi: 10.1097/01.AOG.0000103998.48122.0b. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Light KC. Hemodynamic stress responses in men and women examined as a function of female menstrual cycle phase. International Journal of Psychophysiology. 1994;17(3):233–248. doi: 10.1016/0167-8760(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychology. 1993;12(3):180–192. doi: 10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosomatic Medicine. 2003;65(5):849–856. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosomatic Medicine. 1990;52(5):571–591. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Annals of Internal Medicine. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Ditto B, Lavoie KL, Pelletier R, Campbell TS, Arsenault A, Bacon SL. The effect of major depression on post-exercise cardiovascular recovery. Psychophysiology. 2011;48(11):1605–1610. doi: 10.1111/j.1469-8986.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- Gravetter FJ, Forzano L-AB. Research Methods for the Behavioral Sciences. 4th ed. Belmont, CA: Linda Schreiber-Ganster; 2011. [Google Scholar]
- Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clinical and Experimental Pharmacology and Physiology. 1999;26(2):122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE. Age at natural menopause and risk of cardiovascular disease. Archives of Internal Medicine. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. Journal of Clinical Epidemiology. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kasprowicz AL, Manuck SB, Malkoff SB, Krantz DS. Individual differences in behaviorally evoked cardiovascular response: temporal stability and hemodynamic patterning. Psychophysiology. 1990;27(6):605–619. doi: 10.1111/j.1469-8986.1990.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57(1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Mechlin B, Girdler SS. Menstrual cycle phase does not influence gender differences in experimental pain sensitivity. European Journal of Pain. 2010;14(1):77–82. doi: 10.1016/j.ejpain.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. Journal of Clinical Endocrinology and Metabolism. 1999;84(2):606–610. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Annals of Internal Medicine. 1991;114(5):345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Levy B, Bollaert PE, Charpentier C, Nace L, Audibert G, Bauer P. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Medicine. 1997;23(3):282–287. doi: 10.1007/s001340050329. [DOI] [PubMed] [Google Scholar]
- Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Critical Care Medicine. 2011;39(3):450–455. doi: 10.1097/CCM.0b013e3181ffe0eb. [DOI] [PubMed] [Google Scholar]
- Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Annals of Internal Medicine. 1988;108(1):7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- Light KC, Hinderliter AL, West SG, Grewen KM, Steege JF, Sherwood A. Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. Journal of Hypertension. 2001;19(2):269–278. doi: 10.1097/00004872-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Health Psychology. 1993;12(5):354–365. doi: 10.1037//0278-6133.12.5.354. [DOI] [PubMed] [Google Scholar]
- Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. American Journal of Obstetrics & Gynecology. 1992;167(6):1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- Lustyk MK, Douglas HA, Shilling EA, Woods NF. Hemodynamic and psychological responses to laboratory stressors in women: Assessing the roles of menstrual cycle phase, premenstrual symptomatology, and sleep characteristics. International Journal of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustyk MK, Olson KC, Gerrish WG, Holder A, Widman L. Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biological Psychology. 2010;83(2):84–92. doi: 10.1016/j.biopsycho.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Manhem K, Jern C, Pilhall M, Shanks G, Jern S. Haemodynamic responses to psychosocial stress during the menstrual cycle. Clinical Science (London) 1991;81(1):17–22. doi: 10.1042/cs0810017. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110(1):74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- McFetridge JA, Sherwood A. Hemodynamic and sympathetic nervous system responses to stress during the menstrual cycle. AACN Clinical Issues. 2000;11(2):158–167. doi: 10.1097/00044067-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Pines A, Fisman EZ, Levo Y, Averbuch M, Lidor A, Drory Y. The effects of hormone replacement therapy in normal postmenopausal women: measurements of Doppler-derived parameters of aortic flow. American Journal of Obstetrics & Gynecology. 1991;164(3):806–812. doi: 10.1016/0002-9378(91)90520-2. [DOI] [PubMed] [Google Scholar]
- Polefrone JM, Manuck SB. Effects of menstrual phase and parental history of hypertension on cardiovascular response to cognitive challenge. Psychosomatic Medicine. 1988;50(1):23–36. doi: 10.1097/00006842-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Pollard TM, Pearce KL, Rousham EK, Schwartz JE. Do blood pressure and heart rate responses to perceived stress vary according to endogenous estrogen level in women? American Journal of Physical Anthropology. 2007;132(1):151–157. doi: 10.1002/ajpa.20468. [DOI] [PubMed] [Google Scholar]
- Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284(5):H1800–H1807. doi: 10.1152/ajpheart.00866.2002. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.3 User’s Guide. Cary, NC: SAS Publishing; 2011. [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Park SB, Hughes JW, Blumenthal JA, Hinderliter A, Trivedi R. Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause. 2010;17(2):403–409. doi: 10.1097/gme.0b013e3181b9b061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Turner JR, Light KC, Blumenthal JA. Temporal stability of the hemodynamics of cardiovascular reactivity. International Journal of Psychophysiology. 1990;10(1):95–98. doi: 10.1016/0167-8760(90)90050-n. [DOI] [PubMed] [Google Scholar]
- Sita A, Miller SB. Estradiol, progesterone and cardiovascular response to stress. Psychoneuroendocrinology. 1996;21(3):339–346. doi: 10.1016/0306-4530(95)00053-4. [DOI] [PubMed] [Google Scholar]
- Stoney CM, Langer AW, Gelling PD. The effects of menstrual cycle phase on cardiovascular and pulmonary responses to behavioral and exercise stress. Psychophysiology. 1986;23(4):393–402. doi: 10.1111/j.1469-8986.1986.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Stoney CM, Owens JF, Matthews KA, Davis MC, Caggiula A. Influences of the normal menstrual cycle on physiologic functioning during behavioral stress. Psychophysiology. 1990;27(2):125–135. doi: 10.1111/j.1469-8986.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosomatic Medicine. 1991;53(2):185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Jackson RW, Davis H, Pollock JS, Kapuku G, Mensah GA. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension. 2000;35(3):722–725. doi: 10.1161/01.hyp.35.3.722. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Research. 1983;270(2):363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39(4):255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Archives of Clinical Neuropsychology. 2006;21(1):53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Warner R. Applied Statistics: From Bivariate Through Multivariate Techniques. Thousand Oaks, CA: SAGE Publications Inc.; 2012. [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. Journal of Clinical Endocrinology and Metabolism. 1971;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular Health Risk Management. 2005;1(4):291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105(11):1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]