Abstract
Hypothalamic tuberoinfundibular dopamine (TIDA) neurons remain unaffected in Parkinson disease (PD) while there is significant degeneration of midbrain nigrostriatal dopamine (NSDA) neurons. A similar pattern of susceptibility is observed following acute exposure to the neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and the resistance of TIDA neurons to MPTP is associated with increased expression of parkin and ubiquitin carboxy-terminal hydrolase L-1 (UCHL-1). In the present study, the response of TIDA and NSDA neurons to acute MPTP administration following chronic MPTP exposure was examined. Mice were treated with ten injections of either MPTP (20 mg/kg; s.c.; every 3.5 days) or saline vehicle (10 ml/kg; s.c.; every 3.5 days). Following a 21 day recovery period, chronic saline- and MPTP-treated mice received an additional injection of either saline (10 ml/kg; s.c.) or MPTP (20 mg/kg; s.c.) and were sacrificed 24 h later. NSDA neurons displayed significant axon terminal degeneration (as reflected by decreases in DA, tyrosine hydroxylase (TH) and DA transporter concentrations in the striatum) as well as loss of TH-immunoreactive (IR) neurons in the substantia nigra (SN) following MPTP, whereas TIDA neurons revealed no overt axon terminal pathology or loss of TH-IR cell bodies. NSDA neuronal pathology was associated with transient decreases in concentrations of parkin and UCHL-1 protein in the SN, which returned to normal levels by 21 days following cessation of chronic neurotoxicant exposure. Resistance of TIDA neurons to MPTP toxicity was correlated with a transient increase in UCHL-1 and a sustained elevation in parkin in the arcuate nucleus. TIDA neurons represent a DA neuron population with a unique and inherent ability to adapt to acute and chronic toxicant administration with a sustained elevation of the neuroprotective protein parkin. The correlation between the ability to increase parkin and UCHL-1 expression and the resistance of DA neurons to neurotoxicant exposure is consistent with a functional link between these features and an underlying differential susceptibility to toxicant-associated neurodegeneration.
Keywords: Parkin, UCHL1, MPTP, Tuberoinfundibular, Recovery, Parkinson disease
1. Introduction
Parkinson disease (PD) is one of the most common neurode-generative disorders with hallmark symptoms including tremor at rest, bradykinesia, muscle rigidity and postural disturbances (Braak et al., 2003; Fahn, 2003; Shahed and Jankovic, 2007). NSDA neurons, with cell bodies in the substantia nigra (SN) and axon terminals in the striatum (ST), are a key component of the extrapyramidal motor system. The degeneration of NSDA neurons and the corresponding loss of DA innervation to the ST underlies the motor impairment observed in PD (Braak and Braak, 2000; Braak et al., 2003).
Although many cell types are affected in PD, central DA neurons show the most profound degree of cell loss (Braak et al., 2003; Ahlskog, 2004; Sulzer, 2007). However, PD does not affect all DA neuronal systems to the same extent. For example, while NSDA neurons show severe degeneration in PD, tuberoinfundibular dopamine (TIDA) neurons of the hypothalamus remain unaffected (Braak and Braak, 2000; Braak et al., 2003; Ahlskog, 2004; Langston and Forno, 2004). TIDA neurons originate in the arcuate nucleus (ARC) and terminate adjacent to the hypophysial portal vessels in the median eminence (ME). Reuptake of DA in TIDA neurons is mediated by low affinity, high volume transport as well as by the DA transporter (DAT), albeit to a lesser extent than NSDA neurons (Demarest and Moore, 1979; Revay et al., 1996; DeMaria et al., 2000; Lookingland and Moore, 2005). Despite these differences in DA reuptake mechanisms, the cellular machinery responsible for DA synthesis, storage, release and catabolism are similar in both NSDA and TIDA neurons.
The differential susceptibility of TIDA and NSDA neurons to PD-associated cellular pathology has also been observed in neurotoxicant-based animal models of PD (Willis and Donnan, 1987; Mogi et al., 1988; Sundstrum et al., 1990; Behrouz et al., 2007; Benskey et al., 2012). Despite inherent limitations, neurotoxicant-based models of PD accurately recapitulate many of the key molecular pathological features associated with PD, including mitochondrial dysfunction, oxidative stress, impairment of ubiquitin proteasome pathway (UPP) function, activation of programmed cell death, and neuroinflammation (Zang and Misra, 1993; Jackson-Lewis et al., 1995; Petroske et al., 2001; Dauer and Przedborski, 2003; Hoglinger et al., 2003; Przedborski et al., 2004; Fornai et al., 2005; Chan et al., 2006). The mitochondrial Complex I inhibitor, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyradine (MPTP) is the most widely used neurotoxicant that consistently produces predictable loss of NSDA soma and axon terminals, similar to that found in PD. While administration of MPTP severely damages NSDA neurons, TIDA neurons are resistant to both acute and chronic exposure (Behrouz et al., 2007; Benskey et al., 2012).
Previous studies have found that TIDA neurons fully recover axon terminal DA stores within 24 h following a single administration of MPTP, while NSDA neurons show sustained depletion of DA stores for up to 72 h post-MPTP (Behrouz et al., 2007; Benskey et al., 2012). Differential expression of the PD-associated proteins, parkin and ubiquitin carboxy-terminal hydrolase L-1 (UCHL-1) is associated with the differential susceptibility of TIDA and NSDA neurons to acute MPTP toxicity (Behrouz et al., 2007; Benskey et al., 2012). Up-regulation of both parkin and UCHL-1 is observed in the ARC within 24 h following MPTP, while there is a slight decrease in parkin and UCHL1 concentrations in the SN. The differential expression of parkin is dose-dependent, with parkin expression increasing in the ARC and decreasing in SN with incremental doses of MPTP (Behrouz et al., 2007; Benskey et al., 2012). TIDA resistance to MPTP appears to reflect an intrinsic capability to cope with cytotoxic stress, a neuronal phenotype that may rely on the compensatory up-regulation of neuroprotective proteins such as parkin and UCHL-1. The finding that recovery of TIDA neurons from acute MPTP administration is dependent upon protein synthesis is consistent with this hypothesis (Benskey et al., 2012).
In past studies our group has utilized an acute, single injection of MPTP in order to identify early events following MPTP administration, which differ between TIDA and NSDA neurons (Behrouz et al., 2007; Benskey et al., 2012). However, the appearance of NSDA cellular pathology in PD most likely does not result from a single event but, more plausibly, from the culmination of multiple deleterious events working in combination over time. Similarly, prolonged toxicant exposure likely entails a multitude of cytotoxic events, which may differ from a simple one time exposure. Accordingly, investigations that aim to identify mechanisms unique to TIDA neuronal recovery must also consider the ability of these neurons to recover from prolonged exposure, in addition to how previous cytotoxicity affects the response profile to acute toxicity. Accordingly, the purpose of the current study was to determine whether chronic MPTP exposure results in TIDA neuronal degeneration or renders TIDA neurons susceptible to subsequent neurotoxicant exposure. Further, the ability of TIDA neurons to elicit, as well as maintain, the compensatory up-regulation of parkin and UCHL-1 following various MPTP dosing paradigms was examined to determine if enhanced expression of these proteins is a transient or a sustained event.
2. Materials and methods
2.1. Animals
All experiments were conducted in 8–10 week old male C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, MA). Animals were randomly assigned to treatment groups, and housed two to four per cage, maintained in a light-controlled (12 h light/dark cycle; lights on 06:00 h), temperature-controlled (22 ± 1 °C) room, with food and tap water provided ad libitum. The Michigan State University Institutional Animal Care & Use Committee approved all experiments using live animals (AUF 01/08-123-00).
2.2. Drug preparation
MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (Sigma–Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline to yield a final concentration of 2.0 mg/ml.
Probenecid
p-(dipropylsulfamoyl)benzoic acid (Sigma–Aldrich) was dissolved in 0.1 N NaOH, which was stabilized with 1 M Tris–HCl to a pH of 7.4 and final volume adjusted with ddH2O (Petroske et al., 2001; Meredith et al., 2008).
2.3. Drug administration
Mice (n = 16/group) were treated with MPTP using an amended version of a previously described chronic administration regimen (Petroske et al., 2001; Meredith et al., 2008). As depicted in Fig. 1, animals received ten MPTP (20 mg/kg; s.c.; every 3.5 days) or saline (10 ml/kg; s.c.; every 3.5 days) injections over the course of 35 days. All animals received injections of probenecid (250 mg/kg; i.p.) 30 min prior to chronic MPTP or saline administration. Twenty-one days following the last chronic MPTP or saline injection, mice received a single injection of either MPTP (20 mg/kg; s.c.) or saline (10 ml/kg; s.c.) and were sacrificed 24 h later. MPTP and probenecid were freshly prepared on each injection day. Probenecid is an organic cation transporter inhibitor used to decrease excretion of the active MPTP metabolite, MPP+, thereby maintaining brain levels of the neurotoxicant during the 3.5-day injection interval. It has previously been shown that probenecid does not interfere with neurochemical indices of DA axon terminal integrity used in the present study (Petroske et al., 2001; Meredith et al., 2008).
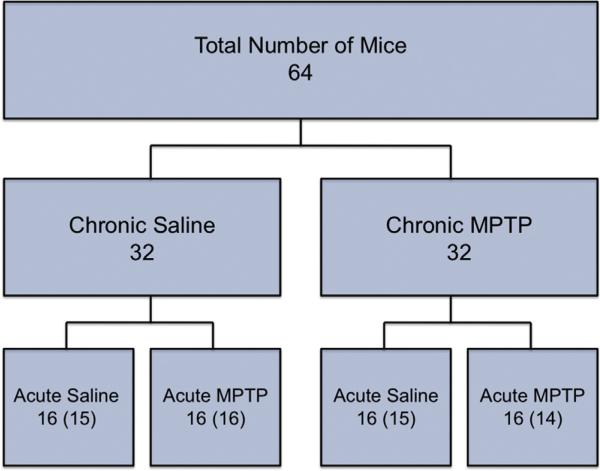
Fig. 1.
Experimental design and group assignment. Mice (64 total) were randomly assigned to each treatment group. Thirty-two mice were treated with chronic saline or chronic MPTP. Following 35 days of treatment, mice in the chronic saline and MPTP groups were divided and assigned randomly to receive an acute saline or MPTP injection 21 days after the last chronic treatment. Numbers represent the planned distribution and numbers in parentheses indicate actual numbers used following attrition.
2.4. Tissue preparation
For neurochemical and Western blot analyses, mice (n = 10/group) were sacrificed by decapitation and brains were rapidly removed and placed on an ice-cooled glass stage. Under a dissecting microscope the ME was collected using forceps and the remaining brain was quickly frozen on dry ice. Consecutive frozen coronal sections (500 mm) were prepared throughout the rostrocaudal extent of the regions containing DA neuronal subpopulations of interest using a cryostat set at –10 °C (CTD-Model Harris, International Equipment Co., Needham, MA) and the regions of interest were microdissected using a modification of the method described previously (Palkovits, 1973; Palkovits and Brownstein, 1983). These tissue samples were used for neuro-chemical and Western blotting and were processed according to the appropriate protocols described below.
For immunohistochemical analysis of TH-immunoreactive (IR) neurons, mice (n = 4–6/group) were deeply anesthetized with a ketamine:xylazine cocktail (26.6 mg/kg:4 mg/kg; s.c.). Once fully anesthetized (showing no withdrawal reflexes), mice were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were removed, post fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose.
Coronal sections (35 μm) through the entire rostrocaudal axis of the brain were prepared with a cryostat (–19 °C) using Multiblock™ processing (Neuroscience Associates, Knoxville, TN). Immunohistochemistry was performed on free floating sections using a primary rabbit anti-TH antibody (AB152, Millipore), followed by a biotin-conjugated, goat anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove, PA). Bound peroxidase was visualized with 0.05% 3,3′-diaminobenzidine tetrahydrochloride with 0.01% hydrogen peroxide using an ABC Elite kit (Vector Laboratories, Burlingame, VT). Sections were counter-stained with Cresyl Violet to determine if decreases in THIR cell counts correspond with loss of Nissl stained cells.
2.5. Neurochemical analyses
Microdissected brain tissue samples were placed into cold tissue buffer (0.1 M phosphate–citrate buffer pH 2.5) and sonicated with three consecutive 1 s bursts (Heat Systems Ultrasonics, Plainview, NY). Protein was pelleted by centrifugation at 12,000 × g (Beckman Coulter Microfuge, Palo Alto, CA) for 1 min. The content of DA in supernatants was determined with high pressure liquid chromatography coupled with electrochemical detection (HPLC-ED) using a Waters 515 HPLC pump (Waters Corporation, Milford, MA) and an ESA Coulochem 5100A electrochemical detector with an oxidation potential of +0.4 V. DA content was quantified by comparing the peak heights of each sample to the peak heights of standards.
Tissue pellets were re-suspended in 1 N NaOH and assayed for protein using the bicinchonic acid (BCA) protein assay (Walker, 1994). To correct for differences in sample size the DA content was normalized to the amount of protein in each sample and expressed as a concentration in ng DA per mg protein.
2.6. Western blot analyses
Microdissected brain samples were sonicated in cold homogenization buffer (TBS containing 1% SDS, 0.1 mM PMSF, 1 mM DTT with Complete Mini Protease Inhibitor Cocktail Tablets, Roche Diagnostics, Mannheim, Germany) pH 7.4, and centrifuged (10,000 × g, 10 min). The supernatants containing total cytoplasmic protein were removed and placed into fresh microcentrifuge tubes. The supernatants were assayed for protein content using the BCA protein method (Walker, 1994). Protein (10–15 μg) from each sample was run on polyacrylamide gels and transferred to 0.45 μm FL-PVDF membrane (Millipore, Pittsburgh, MA, USA) by electrophoresis and reacted with 1:1000 rabbit anti-parkin (Cell Signaling), 1:2000 rabbit anti-tyrosine hydroxylase (Millipore), 1:1000 rat anti DA transporter (Millipore), 1:800 rabbit anti-UCHL1 (Cell Signaling), 1:1000 mouse anti-β-actin (Cell Signaling) and 1:5000 mouse anti GAPDH (Sigma) primary antibodies overnight at 4 °C and incubated with IR Dye 800-conjugated goat anti-rabbit, 800-goat anti-rat and 680-conjugated goat anti-mouse (Li-Cor Biosciences) secondary antibodies (1:15,000 dilution in blocking buffer) for 1 h at room temperature.
Membranes were washed and bound antibodies were visualized with the Odyssey infrared imager (Li-Cor Biosciences). The density of each band was quantified by measuring the infrared absorbance using the Odyssey infrared imager and Odyssey software (Version 3.0, Li-Cor Biosciences). Relative density was obtained by normalizing the band density of target proteins to that of the control protein, used to account for variations in loading of samples onto the gel. GAPDH or β-actin were used as the control proteins, and their detection and visualization was linear. Expression levels of GAPDH or β-actin were similar amongst the compared brain regions regardless of treatment. Each FL-PVDF membrane contained representative samples from all experimental conditions.
2.7. Unbiased-stereological cell counting of TH positive neurons
Unbiased stereological counting of TH-IR cells was performed as previously described (Behrouz et al., 2007). In brief, using Stereo-Investigator software (Version 4.03 Microbrightfield, Inc. 2000), sections were viewed on a screen at low magnification (4×) and the SN and ARC were delineated through the rostrocaudal extent of the respective nuclei. For estimates of neuron every sixth section was sampled. TH-IR neurons were counted using the Optical Fractionator method. Approximately 7–10 sections per animal were needed to count the entire SN, whereas 5–7 sections per animals were needed to count the ARC. Counting TH or Nissl stained cells was performed using a 40× objective. Nissl counts were performed to determine if loss of TH cell numbers corresponded to cell loss or merely loss of TH immunoreactivity. The coefficient of error for each estimate was calculated and was less than 0.1 (Gundersen, m = 1) (Gundersen and Jensen, 2011).
2.8. Statistical analyses
Power analyses were conducted to determine sample size required for each experiment based on an α of 0.05 for all planned comparisons and the expected standard error of measurement for each endpoint. For neurochemical endpoints, a sample size of 8 per group yields a power of 0.85 to detect a 15% difference between saline and MPTP treatment groups. For protein expression endpoints, a sample size of 10 per group yields a power of 0.80 to detect a 23% difference between saline and MPTP treatment groups. For cell count endpoints, a sample size of 4 per group yields a power of 0.94 to detect a 15% difference between saline and MPTP groups. The experimenter was blind to all experimental conditions during data collection and analysis. Two-way ANOVA was used to detect statistical significance between two or more groups when there were two independent variables in the study. A p-value of less than or equal to 0.05 was considered statistically significant. If the ANOVA revealed an interaction of statistical significance, post hoc analysis was followed by between group comparisons using Tukey's test.
3. Results
3.1. The effects of MPTP on TH and DAT concentrations in the striatum: confirmation of successful neurotoxicant administration
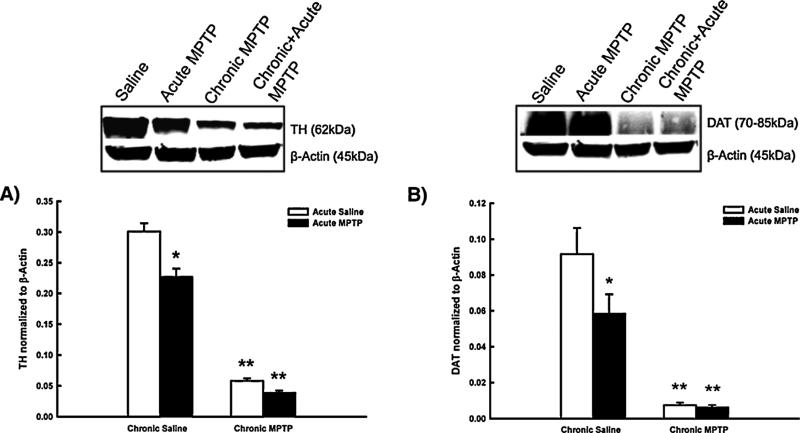
In order to confirm successful neurotoxicant administration in all MPTP treatment paradigms, concentrations of TH and DA transporter (DAT) in the ST were quantified using Western blot analyses. Striatal TH concentrations decreased to approximately 80% of saline treated controls following an acute injection of MPTP (Fig. 2, Panel A). Striatal TH concentrations are further reduced to approximately 20% of saline treated animals following chronic MPTP administration. Acute MPTP administration 21 days following the completion of the chronic MPTP regimen produced no further decrease in ST TH concentrations beyond that observed with the chronic regimen alone.
Fig. 2.
The effects of MPTP on TH and DAT concentrations in the ST: confirmation of successful neurotoxicant administration. Male C57BL/6J mice (n = 10/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were killed by decapitation 24 h later. TH and DAT concentrations were determined by Western blotting and normalized to β-actin. Columns represent mean TH or DAT concentrations + 1SEM. *TH or DAT concentrations significantly different (p < 0.05) from saline-treated controls. **TH or DAT concentrations significantly different (p < 0.05) from those of single-MPTP treated animals and saline treated controls. Representative blots from all groups are shown above graphs.
In addition to monitoring changes in TH concentrations in the ST, changes in DAT following MPTP were also monitored in order to serve as an additional validating index of NSDA axon terminal density and positive control of MPTP administration. Similar to the pattern of TH loss, DAT concentrations are reduced to approximately 70% of saline treated animals following acute MPTP exposure (Fig. 2, Panel B). Chronic MPTP administration reduces ST DAT concentrations to approximately 10% of saline treated animals, and the acute injection of MPTP following completion of the chronic regimen had no further effect on DAT concentrations in the ST.
3.2. The effects of MPTP on DA concentrations, DA metabolism and DA turnover in axon terminals of NSDA and TIDA neurons
Previous reports have shown that “PD-susceptible” NSDA neurons exhibit a profound and sustained loss of axon terminal DA stores following acute MPTP exposure, whereas “PD-resistant” TIDA neurons recover terminal DA within 24 h (Benskey et al., 2012). The ability of TIDA neurons to recover from acute MPTP administration following previous chronic toxicant exposure has not been examined. Fig. 3 shows the neurochemical response profiles of NSDA and TIDA neurons following MPTP. Acute exposure to MPTP, results in an approximately 50% reduction of DA concentrations in the ST compared to saline-treated control animals. Chronic MPTP treatment decreases ST DA to less than 10% of control animals and acute MPTP following the chronic regimen had no significant effect beyond that of the chronic regimen alone (Fig. 3, Panel A). In contrast, 24 h following an acute MPTP exposure, DA concentrations in the ME are not different than that of controls (Fig. 3, Panel B). ME DA concentrations were not significantly decreased following the chronic MPTP regimen, nor after acute MPTP administration following chronic exposure to MPTP (Fig. 3, Panel B).
Fig. 3.
The effects of MPTP on DA concentrations in axon terminals of NSDA and TIDA neurons. Male C57BL/6J mice (n = 6/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were sacrificed 24 h later. DA concentrations are expressed as ng/mg protein. Columns represent mean DA concentrations + 1SEM. *DA concentrations significantly different (p < 0.05) from those of saline treated controls. **DA concentrations significantly different (p < 0.05) from those of single-MPTP treated animals and saline treated controls.
Chronic MPTP as well as chronic + acute MPTP administration resulted in a decrease in the DA metabolite DOPAC in the ST (Fig. 4, Panel A). Further, all MPTP dosing regimens examined produced an increase in DA turnover within the ST, as indexed by the ratio of DOPAC to DA, indicative of increased activity of remaining NSDA neurons (Fig. 4, Panel B). There was no change in the concentrations of DOPAC or the turnover of DA in the ME (Fig. 4, Panels C and D). Nor was there a change in norepinephrine concentrations in the ME between vehicle (24.1 ± 2.1 ng/mg protein), acute MPTP (31.5 ± 4.2 ng/mg protein), chronic MPTP (24.1 ± 5.5 ng/mg protein) or chronic + acute MPTP (29.0 ± 2.45 ng/mg protein) treated animals.
Fig. 4.
The effects of MPTP on DA metabolism and turnover in axon terminals of NSDA and TIDA neurons. Male C57BL/6J mice (n = 6/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were sacrificed 24 h later. DOPAC concentrations are expressed as ng/mg protein. DA turnover is represented by the ratio of the DA metabolite DOPAC to DA. Columns represent mean DOPAC concentrations or DOPAC/DA ratio + 1SEM. *DOPAC concentrations or DOPAC/DA ratios significantly different (p < 0.05) from those of saline treated controls.
3.3. The effects of MPTP on TH-IR cell numbers in the SN and ARC
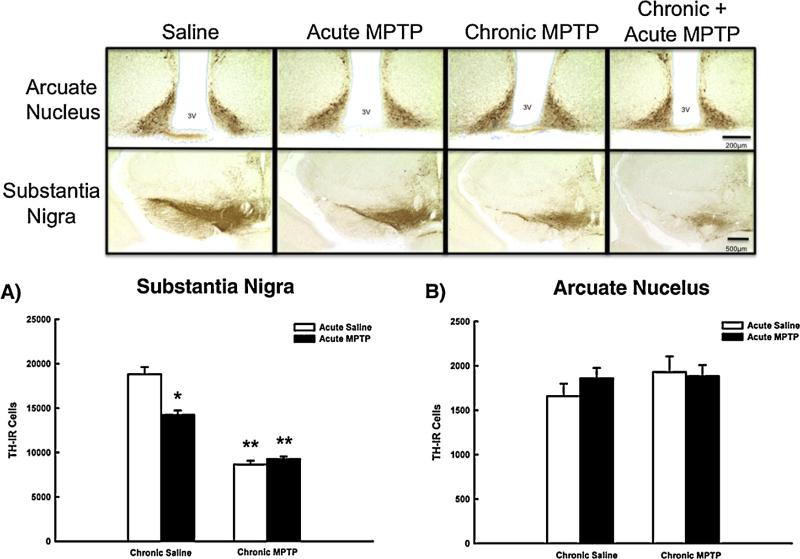
TIDA neurons recover terminal DA concentrations following an acute administration of MPTP (Benskey et al., 2012), but the effect of acute MPTP administration alone, or acute MPTP following previous chronic MPTP exposure on the number of TH-IR cells in the ARC is not known. Accordingly, TH-IR cells of the SN and ARC were quantified using unbiased stereology following MPTP. The number of TH-IR cells in the SN was decreased to approximately 80% of vehicle-treated controls following acute MPTP administration (Fig. 5, Panel A). Despite decreased TH-IR cell numbers following acute MPTP, there were no differences in the number of Nissl stained cells in the SN of vehicle (19,575 ± 1163) and acute MPTP-treated mice (18,874 ± 523). Chronic MPTP exposure further decreases TH-IR cell numbers in the SN to approximately half that of saline treated controls, and this was associated with a concomitant decrease in the number of Nissl stained cells (9549 ± 527). The acute single injection of MPTP in animals receiving prior chronic MPTP had no further effect on TH-IR or Nissl (9020 ± 364) cell numbers in the SN beyond that of the chronic paradigm alone. There was no significant change in TH-IR cell number within the ARC following any of the dosing regimes examined (Fig. 5, Panel B).
Fig. 5.
The effects of MPTP on TH-IR cell numbers in the SN and ARC. Male C57BL/6J mice (n = 6/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were perfused 24 h later. Numbers of TH-IR cells were estimated using unbiased stereology. Columns represent mean TH-IR cell numbers + 1SEM. *TH-IR cell numbers significantly different (p < 0.05) from saline-treated controls. **TH-IR cell numbers significantly different (p < 0.05) from single MPTP-treated animals and saline-treated controls. Representative photomicrographs of TH-IR cells in the SN and ARC surrounding the third ventricle (3V) are shown above graphs.
3.4. Changes in protein concentrations in the SN and ARC following MPTP administration
Recent studies have shown that recovery of TIDA neurons from a single dose of MPTP is correlated with an acute increase in the expression of the PD-associated genes, UCHL-1 and parkin in the ARC (Benskey et al., 2012). Conversely, following a single dose of MPTP, these same proteins show a decrease in the cell body regions of the highly susceptible NSDA neurons. If an increase in these proteins was serving a neuroprotective role, then the same differential expression pattern should be observed following the chronic MPTP paradigms used in the current study.
Fig. 6 shows changes in UCHL-1 protein concentrations in the SN and ARC following the various MPTP treatment regimens. UCHL-1 protein concentrations in the SN are decreased 24 h following an single injection of MPTP (Fig. 6, Panel A). Twenty-one days following the completion of the chronic MPTP regimen UCHL-1 protein concentrations are no longer different than those of control animals. However, acute MPTP administration in animals with prior chronic MPTP exposure reduces UCHL-1 concentrations in the SN (Fig. 6, Panel A).
Fig. 6.
Changes in UCHL-1 protein concentrations in the SN and ARC following MPTP administration. Male C57BL/6J mice (n = 10/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were killed by decapitation 24 h later. UCHL-1 concentrations were determined by Western blotting and normalized to GAPDH. Columns represent mean UCHL-1 concentrations + 1SEM. *UCHL-1 concentrations significantly different (p < 0.05) from saline-treated controls. Representative blots from all groups are shown above graphs.
Changes in UCHL-1 protein concentrations in the ARC following MPTP treatment are shown in Fig. 6, Panel B. As has been previously reported, UCHL-1 protein concentrations are increased 24 h following an acute injection of MPTP (Benskey et al., 2012). Twenty-one days following completion of the chronic MPTP regimen, UCHL-1 concentrations in the ARC are not different from saline treated animals; however, acute MPTP administration in animals receiving prior chronic MPTP exposure increases UCHL-1 protein concentrations as compared to vehicle-treated controls.
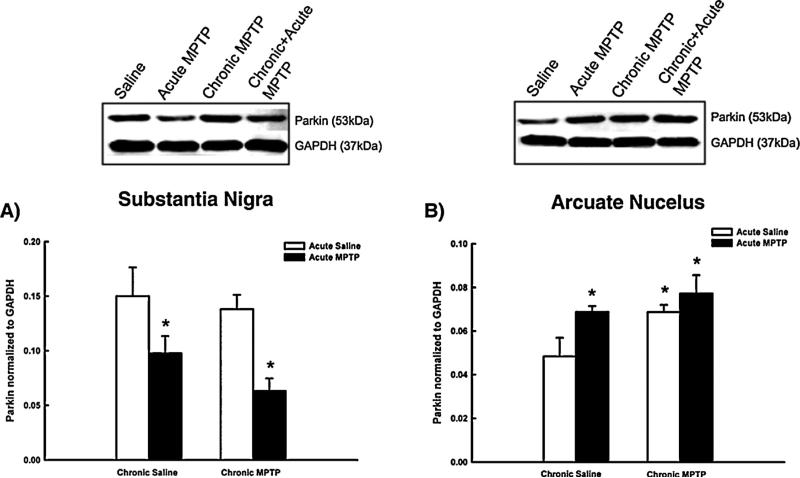
Fig. 7 depicts changes in parkin protein expression in the SN and ARC following the acute, chronic and chronic + acute MPTP dosing paradigms. Fig. 7, Panel A shows that parkin protein concentrations in the SN are reduced from control levels 24 h following acute MPTP administration. Parkin protein concentrations return to control levels 21 days following the completion of the chronic MPTP paradigm. Acute MPTP administration in animals receiving prior chronic MPTP exposure reduced parkin protein concentrations as compared to vehicle-treated controls.
Fig. 7.
Changes in parkin protein concentrations in the SN and ARC following MPTP administration. Male C57BL/6J mice (n = 10/group) were chronically treated with MPTP (10× 20 mg/kg; s.c.) or saline (10× 10 ml/kg; s.c.) over the course of 35 days. 21 days following the last chronic injection mice received a single additional injection of MPTP (20 mg/kg; s.c., black columns) or saline (10 ml/kg; s.c., white columns) and were killed by decapitation 24 h later. Parkin concentrations were determined by Western blotting and normalized to GAPDH. Columns represent mean parkin concentrations + 1SEM. *Parkin concentrations significantly different (p < 0.05) from saline-treated controls. Representative blots from all groups are shown above graphs.
Fig. 7, Panel B replicates previous findings, showing parkin protein concentrations in the ARC to be increased 24 h following acute MPTP administration, demonstrating the reproducibility of this unique response to toxicant-induced injury. The increase in parkin protein in the ARC is sustained for 21 days following completion of the chronic MPTP regimen. Acute MPTP administration in animals receiving prior chronic MPTP exposure did not increase parkin concentrations beyond the elevated levels observed in animals receiving the chronic MPTP regimen alone.
4. Discussion
The demise of NSDA neurons observed in PD is generally believed to result from a combination of several cellular dysfunctions, culminating in irreversible cytotoxicity. Environmental toxicant exposure, NSDA cellular structure, mitochondrial dysfunction, UPP impairment, cytosolic DA oxidation and the loss of protective proteins are factors which are posited to work in concert, resulting in NSDA cell loss (Greenamyre et al., 1999; Betarbet et al., 2000; McNaught and Jenner, 2001; Priyadarshi et al., 2001; Sulzer, 2007). Due to the complexity of the interactions that contribute to NSDA cellular pathology, investigations aimed at translating mechanisms unique to TIDA neuronal recovery into therapeutics must also consider the ability of TIDA neurons to recover from several modalities of cytotoxicity.
The majority of the key cellular dysfunctions thought to contribute to NSDA cell death in PD are recapitulated by MPTP, however the specific mechanism and severity of toxicity is dependent upon the dosing paradigm used. A single exposure to MPTP will predominantly result in transient dysfunction within the dopaminergic axon terminal including reduction in vesicular DA, ATP loss and free radical formation (Di Monte et al., 1986; Hasegawa et al., 1990; Jackson-Lewis et al., 1995; Lotharius and O'Malley, 2000; Chan et al., 2006; Scotcher et al., 2006). Chronic MPTP exposure has been shown to result in relatively sustained DA axon terminal loss, motor abnormalities, as well as necrotic and apoptotic like cell death (Jackson-Lewis et al., 1995; Tatton and Kish, 1997; Lotharius and O'Malley, 2000; Petroske et al., 2001).
In the present study the ability of TIDA neurons to recover from cytotoxicity produced by different MPTP dosing paradigms was examined. In addition to investigating the effects of acute and chronic MPTP administration alone, mice received an acute MPTP administration 21 days following chronic MPTP exposure. The use of a second insult superimposed on a chronic injury was a strategy employed to determine if a repeated MPTP-induced injury sensitizes TIDA neurons to subsequent toxicant-induced injury and whether the compensatory increase in protein expression (i.e. parkin and UCHL-1 expression) found following an acute injury persists after prolonged MPTP-induced toxicity. The results from these studies show that TIDA neurons are highly resistant to the DA-associated toxicity and do not suffer the loss of protective proteins that are thought to play a role in the demise of NSDA neurons.
The active metabolite of MPTP, MPP+, is taken up into DA axon terminals by DAT and causes a retrograde mechanism of toxicity in NSDA neurons. As such, indices of DA axon terminals in the ST (TH and DAT protein) were measured as a positive control of successful neurotoxicant administration. NSDA neurons exhibit drastic reductions in concentrations of DAT and TH in the ST, confirming successful MPTP administration. Concomitant with these changes is a severe depletion of striatal DA stores. In contrast, TIDA neurons showed no change in axon terminal DA concentrations following acute or chronic toxicant administration, nor did repeated MPTP exposure sensitize TIDA neurons to subsequent insult.
The differential ability of TIDA and NSDA neurons to recover axon terminal DA homeostasis may be due to their different cytoarchitecture and/or regulatory mechanisms. Unlike prototypical DA neurons, TIDA neurons do not form classic synapses but instead terminate near capillaries within the hypophysial portal system. TIDA neurons lack D2 autoreceptors and have lower concentrations of DAT as compared to NSDA neurons (Moore and Lookingland, 1995; Timmerman et al., 1995). Following entry into DA axon terminals, MPP+ has a high affinity for the vesicular monoamine transporter, being transported into synaptic vesicles and displacing stored DA into the cytosol (Moriyama et al., 1993; Lotharius and O'Malley, 2000). Within NSDA neurons, purged vesicular DA can diffuse from neurons into the synapse, activating D2 autoreceptors, thereby inhibiting DA synthesis and release. Additionally, DA in the synaptic cleft can be transported back into the axon terminal by DAT, further inhibiting DA synthesis through end product inhibition. In contrast, due to lower levels of DAT, loss of released DA into the hypophysial portal blood, and the lack of D2 autoreceptors, the reuptake and inhibition of DA synthesis is severely reduced in TIDA neurons (Timmerman et al., 1995). In this situation TIDA neurons would be able to reconstitute normal DA synthesis and repackaging of cytosolic DA into vesicles more rapidly than NSDA neurons. It is possible that the rapid ability of TIDA neurons to clear cytosolic DA (whether it be into vesicles or into portal circulation) may contribute to their observed resistance to the same forms of toxicity that severely injures NSDA neurons.
NSDA neurons, which exhibit terminal DA dysfunction, also exhibited loss of TH-IR cell bodies in the SN in a manner that was consistent with the severity of the dosing regimen. In contrast, there was no cell loss observed in the ARC following any of the MPTP dosing regimens examined. It should be noted that the loss of TH-IR cells in the SN following a single injection of MPTP most likely does not reflect actual cell death, but rather a reduction in viable TH protein within the SN. Animals in this group were sacrificed 24 h following the single injection, and are thus in the active phase of MPTP-induced neurodegeneration. During this phase, which extends up to 4 days following MPTP administration, counts of TH-IR cells are consistently lower than counts of Nissl stained neurons (Jackson-Lewis et al., 1995). MPTP causes transient disappearance of TH in the absence of actual cell loss in several different cell types including NSDA neurons, cultured embryonic neurons, as well as retinal cells (Sanchez-Ramos et al., 1986; Tatton et al., 1990; Jackson-Lewis et al., 1995; Sanchez-Ramos et al., 2006). As such, the decrease in the number of observable TH-IR cells in the SN following a single injection of MPTP reflects loss of TH within these cells as opposed to dopaminergic cell death. In accordance with this idea, there was no change in Nissl counts within the SN between mice receiving a single injection of MPTP and saline treated control animals.
Unlike NSDA neurons, TIDA neurons showed no decrease in THIR cell numbers within the active phase of MPTP-induced neurodegeneration. This may indicate that the resistance of TIDA neurons to MPTP may simply be due to extrinsic factors, which protect these neurons from MPTP exposure, such as reduced bioactivation of MPTP or limited MPP+ entry to TIDA neurons. However, recent studies have found that MPP+ concentrations within the ME are actually 5 fold higher than that found in the ST following acute MPTP exposure (Benskey et al., 2012). Further, TIDA neurons are also resistant to the lipophilic toxicant, rotenone, which does not require bio-activation or transporter presence for entry into the cell. Finally, TIDA neurons show an initial depletion of axon terminal DA 4 h post-MPTP administration, demonstrating that the toxicant does indeed gain entry and cause initial cytotoxicity within these neurons (Behrouz et al., 2007). Accordingly, the lack of an active phase MPTP-induced loss of TH in TIDA neurons is likely due to an intrinsic capability to maintain a suitable environment for proper protein structure and function.
A deficit in protective proteins is also thought to play a role in the cellular pathology of NSDA neurons in the PD brain. Two such proteins that have been implicated in PD-pathogenesis, UCHL-1 and parkin, were differentially altered in the ARC and SN following MPTP administration in the current study. UCHL-1 concentrations were transiently decreased in the SN and transiently increased in the ARC following MPTP exposure. Previous in vitro studies using mouse neuroblastoma cells have shown that UCHL-1 concentrations decreases during times of maximal oxidative stress, followed by a compensatory increase during recovery (Shen et al., 2006). In agreement, in vivo studies have shown that UCHL-1 concentrations in the ARC decrease 4 h post-MPTP, a time when MPP+ concentrations are highest in TIDA neurons and the MPP+-induced oxidative stress is likely to be maximal (Benskey et al., 2012). This initial decrease in UCHL-1 is followed by a compensatory increase in UCHL-1 protein, which is consistent with initial injury followed by rapid recovery. Similarly, in the present study UCHL-1 protein concentrations are increased in the cell body region of TIDA neurons 24 h following acute MPTP administration, a time when these neurons have fully recovered DA homeostasis. Conversely, UCHL-1 concentrations remain low for up to 24 h in NSDA neurons, which also display sustained DA depletion and are notoriously ill suited to deal with oxidative stress (Hung and Lee, 1998; Jenner, 2003). Taken together, these data are consistent with the hypothesis that TIDA neurons have a high intrinsic capacity to quell increases in oxidative stress and thus maintain a homeostatic cellular environment. The high correlation between the ability of DA neurons to increase UCHL-1 and the maintenance of axon terminal DA suggest UCHL-1 (like parkin) may play a role in this process.
In support of the idea that TIDA and NSDA neurons have a differential ability to cope with oxidative stress is the differential expression profile of parkin in the SN and ARC following MPTP. Transient decreases in parkin concentrations were observed in the SN for up to 24 h following the last injection of MPTP but returned to basal levels following the 21-day recovery period. Similar to the decrease in UCHL-1, the transient decrease in parkin concentrations in the SN within 24 h of MPTP exposure is potentially mediated by increases in oxidative and nitrosative stress within NSDA neurons. Exposure to oxidative, nitrosative and proteolytic stressors causes a shift in parkin solubility and subcellular localization, modifying parkin to an insoluble form (Wang et al., 2005a). MPTP administration is known to cause a large increase in oxidative and nitrosative stress (Sriram et al., 1997; Cookson et al., 2003; Chung et al., 2004; Yao et al., 2004; Przedborski and Ischiropoulos, 2005; Sriram et al., 2005; Wang et al., 2005b; Yokoyama et al., 2008a, 2008b). Thus, it is possible that the MPTP-induced decrease in parkin concentrations in the SN represents a shift in the soluble pool of parkin toward the insoluble pool mediated by oxidative and nitrosative stress. Indeed, parkin mRNA does not decrease in the SN following exposure to MPTP (Benskey et al., 2012), which is consistent with a post-translational change in parkin within NSDA neurons rather than a decrease in parkin expression.
In stark contrast to the transient changes in parkin concentrations within the SN, the increase in parkin expression in the ARC following MPTP exposure is robust, reproducible and sustained. Parkin concentrations are observed to be increased within 24 h following the final injection of MPTP, but also following a 21 day recovery period. However, there appears to be a ceiling effect in the ability of MPTP to elicit an increase in parkin expression, as the additional acute MPTP administration in mice treated chronically with MPTP yielded no further increases of parkin concentrations in the ARC. Parkin mRNA increases following a single injection of MPTP (Behrouz et al., 2007), yet it is not known whether this compensatory increase in gene expression continues throughout the course of repeated toxicant exposure, or if parkin mRNA and/or protein degradation is inhibited. More detailed studies are needed to address these issues. However, it is known that there is a long-lasting change in the expression profile of the neuroprotective protein parkin in TIDA neurons following repeated exposure to MPTP and that this phenotype is distinct from that of susceptible NSDA neurons. The ability of TIDA neurons to elicit such a long lasting adaptation is likely a major determinant in their resistance to chronic toxicant exposure.
The data presented herein reveal a pattern of susceptibility in both loss of axon terminal DA concentrations and TH-IR cell numbers to MPTP toxicity that is highly correlated with the presence or absence of the PD-associated proteins parkin and UCHL-1. Although previous studies have shown that parkin does increase specifically within DA neurons of the ARC (Benskey et al., 2012), the possibility that parkin also increases in non-DA neurons or glial cells in the same brain region cannot be excluded and may be contributory. Although likely, it is not known if the observed increase in UCHL-1 concentrations occurs within TIDA neurons themselves, or in other surrounding neurons or glia. UCHL-1 is known to be an important component of the UPP that responds to oxidative stress, while parkin specifically seems to play a pivotal role in dopaminergic homeostasis, displaying an impressively wide range of neuroprotective benefits including protection against oxidative stress, proteosomal dysfunction, and regulation of mitochondrial quality control (Petrucelli et al., 2002; Jiang et al., 2004; Narendra et al., 2008; Poole et al., 2008). Parkin up-regulation, whether endogenously or exogenously, protects from rotenone, paraquat, 6-hydroxydopamine, alpha synuclein and most apropos to the current study, MPTP toxicity (Wang et al., 2005a; Yamada et al., 2005; Vercammen et al., 2006; Manfredsson et al., 2007; Yasuda et al., 2011). Further, the compensatory increase in the PD-associated proteins parkin and UCHL-1 following MPTP seems to be specific, as expression of other PD related genes (alpha synuclein, DJ-1, Pink1 and LRRK2) do not change (Benskey et al., 2012). Parkin and UCHl-1 are both components of the UPP, suggesting that the ability to maintain a homeostatic environment for proper protein function plays a role in the ability of DA neurons to recover from toxicity.
In conclusion, the findings that parkin and UCHL-1 are decreased in the soma regions of the highly susceptible NSDA neurons and increased in the soma regions of the highly resilient TIDA neurons, supports the idea that the loss of protective proteins is a contributing factor to the demise of DA neurons. Further, it appears that TIDA neurons specifically, are highly resilient due to their ability to alter expression of the neuroprotective protein parkin, an ability that is not only rapid but can also be sustained. Although the data presented herein is not causational, there remains a very high correlation between the presence of the proteins parkin and UCHL-1 and the ability of DA neurons to recover from MPTP toxicity. More detailed studies are needed in order to confirm that either parkin and/or UCHL-1 are necessary and sufficient to protect DA neurons from MPTP. Nonetheless, the sustained adaptation of increased parkin expression and the maintained resistance to repeated toxicant exposure makes TIDA neurons an attractive platform for dissecting mechanisms which may be aimed to halt NSDA cellular pathology in the context of PD.
Acknowledgement
This work was supported by the National Institute of Health grant 1R01 NS065338-01A2.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Ahlskog JE. Challenging conventional wisdom: the etiologic role of dopamine oxidative stress in Parkinson's disease. Mov Disord. 2004;20:271–82. doi: 10.1002/mds.20362. [DOI] [PubMed] [Google Scholar]
- Behrouz B, Drolet RE, Sayed ZA, Lookingland KJ, Goudreau JL. Unique responses to mitochondrial complex I inhibition in tuberoinfundibular dopamine neurons may impart resistance to toxic insult. Neuroscience. 2007;147:592–8. doi: 10.1016/j.neuroscience.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey M, Behrouz B, Sunryd J, Pappas SS, Baek SH, Huebner M, et al. Recovery of hypothalamic tuberoinfundibular dopamine neurons from acute toxicant exposure is dependent upon protein synthesis and associated with an increase in parkin and ubiquitin carboxy-terminal hydrolase-L1 expression. Neurotoxicology. 2012;33:321–31. doi: 10.1016/j.neuro.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000:247. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rub U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chan P, DeLanney LE, Irwin I, Langston JW, Monte D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem. 2006;57:348–51. doi: 10.1111/j.1471-4159.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Chung KKK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–31. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O'Farrell C, Schlossmacher M, Farrer MJ. RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet. 2003;12:2957–65. doi: 10.1093/hmg/ddg328. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Demarest K, Moore K. Lack of a high affinity transport system for dopamine in the median eminence and posterior pituitary. Brain Res. 1979;171:545–51. doi: 10.1016/0006-8993(79)91060-6. [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Nagy GM, Lerant AA, Fekete MIE, Levenson CW, Freeman ME. Dopamine transporters participate in the physiological regulation of prolactin. Endocrinology. 2000;141:366–74. doi: 10.1210/endo.141.1.7281. [DOI] [PubMed] [Google Scholar]
- Di Monte D, Jewell SA, Ekstrum G, Sandy MS, Smith MT. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridine (MPP+) cause rapid ATP depletion in isolated hepatocytes. Biochem Biophys Res Commun. 1986;137:310–5. doi: 10.1016/0006-291x(86)91211-8. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, et al. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin–proteasome system and α-synuclein. Proc Natl Acad Sci USA. 2005;102:3413–8. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson's disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 2011;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Takeshige K, Oishi T, Murai Y, Minakami S. 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun. 1990;170:1049–55. doi: 10.1016/0006-291x(90)90498-c. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Carrard G, Michel PP, Medja F, Lombes A, Ruberg M, et al. Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson's disease. J Neurochem. 2003;86:1297–307. doi: 10.1046/j.1471-4159.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EHY. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 1998;24:76–84. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–54. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol. 2004;3:129–33. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]
- Lookingland KJ, Moore KE. Handbook of chemical neuroanatomy. Vol. 21. Elsevier; Philadelphia: 2005. Chapter VIII: Functional neuroanatomy of hypothalamic dopaminergic neuroendocrine systems. pp. 435–523. [Google Scholar]
- Lotharius J, O'Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. J Biol Chem. 2000;275:38581–8. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS, Mandel RJ. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp Neurol. 2007;207:289–301. doi: 10.1016/j.expneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci Lett. 2001;297:191–4. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14:S112–5. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kojima K, Kiuchi K, Nagatsu T. Effects of systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine to mice on tyrosine hydroxylase l-3,4-dihydroxyphenylalanine decarboxylase, dopamine beta-hydroxylase, and mono-amine oxidase activities in the striatum and hypothalamus. J Neurochem. 1988;50:1053–6. doi: 10.1111/j.1471-4159.1988.tb10572.x. [DOI] [PubMed] [Google Scholar]
- Moore KE, Lookingland K. Psychopharmacology: the fourth generation of progress. Springer; New York, Heidelberg: 1995. Dopaminergic neuronal systems in the hypothalamus. pp. 245–56. [Google Scholar]
- Moriyama Y, Amakatsu K, Futai M. Uptake of the neurotoxin, 4-methylphenylpyridinium, into chromaffin granules and synaptic vesicles: a proton gradient drives its uptake through monoamine transporter. Arch Biochem Biophys. 1993;305:271–7. doi: 10.1006/abbi.1993.1422. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–50. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Brain microdissection techniques. Vol. 2. John Wiley and Sons; Chichester: 1983. Microdissection of brain areas by the punch technique. pp. 1–36. [Google Scholar]
- Petroske E, Meredith G, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O'Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, et al. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–19. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ Res. 2001;86:122–7. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson's disease. Antioxid Redox Signal. 2005;7:685–93. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–9. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Revay R, Vaughan R, Grant S, Kuhar MJ. Dopamine transporter immunohistochemistry in median eminence, amygdala, and other areas of the rat brain. Synapse. 1996;22:93–9. doi: 10.1002/(SICI)1098-2396(199602)22:2<93::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Barrett JN, Goldstein M, Weiner WJ, Hefti F. 1-Methyl-4-phenylpyridinium (MPP+) but not 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) selectively destroys dopaminergic neurons in cultures of dissociated rat mesencephalic neurons. Neurosci Lett. 1986;72:215–20. doi: 10.1016/0304-3940(86)90083-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Michel P, Weiner WJ, Hefti F. Selective destruction of cultured dopaminergic neurons from fetal rat mesencephalon by 1-methyl-4-phenylpyridinium: cytochemical and morphological evidence. J Neurochem. 2006;50:1934–44. doi: 10.1111/j.1471-4159.1988.tb02500.x. [DOI] [PubMed] [Google Scholar]
- Scotcher KP, Irwin I, DeLanney LE, Langston JW, Monte D. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. J Neurochem. 2006;54:1295–301. doi: 10.1111/j.1471-4159.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Shahed J, Jankovic J. Vol. 83. Elsevier; Philadelphia: 2007. Motor symptoms in Parkinson's disease. Handbook of clinical neurology; pp. 329–42. [DOI] [PubMed] [Google Scholar]
- Shen H, Sikorska M, Leblanc J, Walker P, Liu Q. Oxidative stress regulated expression of ubiquitin carboxyl-terminal hydrolase-L1: role in cell survival. Apoptosis. 2006;11:1049–59. doi: 10.1007/s10495-006-6303-8. [DOI] [PubMed] [Google Scholar]
- Sriram K, Pai KS, Boyd MR, Ravindranath V. Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res. 1997;749:44–52. doi: 10.1016/s0006-8993(96)01271-1. [DOI] [PubMed] [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KKK, Wong E, Lim KL, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–86. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–50. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sundstrum E, Fredriksson A, Archer T. Chronic neurochemical and behavioral changes in MPTP-lesioned C57BL/6 mice: a model for Parkinson's disease. Brain Res. 1990;528:181–8. doi: 10.1016/0006-8993(90)91656-2. [DOI] [PubMed] [Google Scholar]
- Tatton N, Kish S. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Kwan MM, Verrier MC, Seniuk NA, Theriault E. MPTP produces reversible disappearance of tyrosine hydroxylase-containing retinal amacrine cells. Brain Res. 1990;527:21–31. doi: 10.1016/0006-8993(90)91056-m. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Deinum M, Westerink B, Schuiling G. Lack of evidence for dopamine autoreceptors in the mediobasal hypothalamus: a microdialysis study in awake rats. Neurosci Lett. 1995;195:113–6. doi: 10.1016/0304-3940(95)11794-w. [DOI] [PubMed] [Google Scholar]
- Vercammen L, Van der Perren A, Vaudano E, Gijsbers R, Debyser Z, Van den Haute C, et al. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson's disease. Mol Ther. 2006;14:716–23. doi: 10.1016/j.ymthe.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- Wang C, Ko HS, Thomas B, Tsang F, Chew KCM, Tay SP, et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet. 2005a;14:3885–97. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- Wang C, Tan JMM, Ho MWL, Zaiden N, Wong SH, Chew CLC, et al. Alterations in the solubility and intracellular localization of parkin by several familial Parkinson's disease-linked point mutations. J Neurochem. 2005b;93:422–31. doi: 10.1111/j.1471-4159.2005.03023.x. [DOI] [PubMed] [Google Scholar]
- Willis GL, Donnan GA. Histochemical, biochemical and behavioural consequences of MPTP treatment in C-57 black mice. Brain Res. 1987;402:269–74. doi: 10.1016/0006-8993(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mizuno Y, Mochizuki H. Parkin gene therapy for a-synucleinopathy: a rat model of Parkinson's disease. Hum Gene Ther. 2005;16:262–70. doi: 10.1089/hum.2005.16.262. [DOI] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101:10810–4. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Hayakawa H, Nihira T, Ren YR, Nakata Y, Nagai M, et al. Parkin-mediated protection of dopaminergic neurons in a chronic MPTP-minipump mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70:686–97. doi: 10.1097/NEN.0b013e3182269ecd. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Kuroiwa H, Yano R, Araki T. Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson's disease. Neurol Sci. 2008a;29:293–301. doi: 10.1007/s10072-008-0986-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Takagi S, Watanabe Y, Kato H, Araki T. Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice. J Neural Transm. 2008b;115:831–42. doi: 10.1007/s00702-008-0019-6. [DOI] [PubMed] [Google Scholar]
- Zang LY, Misra H. Generation of reactive oxygen species during the monoamine oxidase-catalyzed oxidation of the neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Biol Chem. 1993;268:16504–12. [PubMed] [Google Scholar]