Abstract
Background
We evaluated a two stage ovarian cancer screening strategy that incorporates change of CA 125 over time and age to estimate risk of ovarian cancer. Women with high risk scores were referred for transvaginal ultrasound (TVS).
Methods
A single-arm, prospective study of post-menopausal women was conducted. Participants underwent an annual CA 125 blood test. Based on the Risk of Ovarian Cancer Algorithm (ROCA) result, women were triaged to next annual CA 125 (low risk), repeat CA 125 in three months (intermediate risk), or TVS and referral to a gynecologic oncologist (high risk).
Results
4051 women participated over 11 years. The average annual rate of referral to a CA125 in three months was 5.8%, and the average annual referral rate to TVS and review by a gynecologic oncologist was 0.9%. Ten women underwent surgery based on TVS, with four invasive ovarian cancers (one Stage 1A, two Stage 1C and one Stage IIB), two ovarian tumors of low malignant potential (both Stage 1A), one endometrial cancer (Stage 1), and three benign ovarian tumors, providing a positive predictive value of 40% (95% CI 12.2%, 73.8%) for detecting invasive ovarian cancer. The specificity was 99.9% (95% CI 99.7%, 100%). All four women with invasive ovarian cancer were enrolled in the study for at least three years with low risk, annual CA 125 values prior to a rising CA 125.
Conclusions
ROCA followed by TVS demonstrated excellent specificity and PPV in a population of U.S. women at average risk for ovarian cancer.
Keywords: Ovarian cancer screening, CA125, Cancer screening
Introduction
Despite advances in treatment, ovarian cancer remains a highly lethal disease. Some 70% of women with ovarian cancer present at late stage, when long term cure rates are less than 30%. When caught at an early stage, survival rates are as high as 75–90%. At present, however, there are no proven strategies for the early detection for ovarian cancer.
One of the challenges to developing an effective screening strategy for women in the general population has been the requirement of a very high specificity. Unlike breast cancer screening, in which a biopsy can be performed for definitive diagnosis, ovarian cancer screening requires invasive surgery and removal of the ovaries in order to make a definitive diagnosis. Therefore, any screening strategy for ovarian cancer must minimize false positives in order to decrease the number of unnecessary operations. In postmenopausal women in the United States, the prevalence of ovarian cancer is only 1 in 2500. Given this prevalence, a screening strategy must not only exhibit a high sensitivity of greater than 75% for asymptomatic early stage disease, but also a very high specificity of greater than 99.6% to achieve a positive predictive value (PPV) of 10%, i.e., ten operations for each case of ovarian cancer detected,1 a generally accepted limit for balancing risk with benefit among practitioners and advocates.
Prior studies have demonstrated that using a fixed cutpoint of CA125 or an abnormal transvaginal ultrasound (TVS) as a first line test does not have sufficient specificity to achieve a PPV of 10%. In the Prostate, Lung, Colon and Ovary (PLCO) trial involving 37,500 postmenopausal women, for example, abnormal CA125 (>35 U/mL) produced a PPV of 3.7% and TVS only 1%.2 Both sensitivity and specificity can, however, be improved when a rising CA 125, often within a normal range, is used to prompt TVS.3–7 Rising CA125 values are associated with progressive growth of ovarian cancer, whereas stable CA125 values, even when elevated, are associated with benign conditions. A computer algorithm, called the Risk of Ovarian Cancer Algorithm (ROCA), was developed using data from multiple prospective longitudinal screening trials of postmenopausal women at normal risk for developing ovarian cancer and a statistical model describing the change-point CA125 serial profiles in women subsequently diagnosed with ovarian cancer and the generally flat CA125 serial profiles in all other women. Serial CA125 values from women on that study who developed ovarian cancer as well as those from women who did not develop ovarian cancer were used to develop an algorithm to quantify the risk of a given woman having a change-point (due to ovarian cancer). ROCA measures the relative closeness of the woman’s longitudinal CA125 pattern to the change-point profile in ovarian cancer cases from previous trials compared with the flat profiles seen in all other women without ovarian cancer in previous trials.4,5 In a small fraction of women whose ROCA score is sufficiently high, TVS is performed as a second step. The purpose of this study was to determine if this two-step strategy has sufficiently high specificity and PPV for screening postmenopausal women at general population risk in the United States.
Methods
This study was conducted at the University of Texas MD Anderson Cancer Center, Houston TX; Women’s Hospital, Houston TX; Women and Infants Hospital, Providence, RI; Baylor University Medical Center, Dallas, TX; the John Stoddard Cancer Center, Des Moines, IA; Family Practice, the University of Texas Health Science Center, Houston, TX; and the Geffen Cancer Center and Research Institute, Vero Beach, FL. This study was approved by the Institutional Review Board at all seven study sites. This study is registered with ClinicalTrials.gov, number NCT00539162.
Women were eligible to participate if they: 1) were between 50–74 years old; 2) were post-menopausal, defined as ≥ 12 months of amenorrhea; 3) retained at least one ovary; and 4) could provide written informed consent. Patients were excluded if they had: 1) a prior bilateral oophorectomy; 2) an active malignancy other than breast cancer within the past five years; 3) a previous history of ovarian cancer; or 4) a family history of one or more 1st or 2nd degree relative with breast or ovarian cancer. When relatives had breast cancer, at least one had to have been diagnosed pre-menopause to exclude participation. Patients receiving hormonal adjuvant therapy for breast cancer were eligible provided that they had no evidence of recurrence for at least one year after primary chemotherapy.
ROCA was originally developed using data from prospective screening trials for postmenopausal women that included over 22,000 women in the United Kingdom and over 5000 women in Sweden. Statistical analysis of these data indicated most women without ovarian cancer had a flat CA125 profile - a baseline level individual to each woman, around which her CA125 levels fluctuated. In contrast, women with incident cases of ovarian cancer had a baseline level followed by a sharp increase in CA125 values significantly above her baseline, called a change-point CA125 profile, which could not be explained by background CA125 fluctuations. These thousands of profiles form the basis for the ROCA calculation that determines a woman’s risk of having ovarian cancer at that timepoint.4,5 For each new woman in the study, the probability calculation of ovarian cancer begins with incidence based on her age, and increases the closer her profile is to the change-point profiles compared to the flat profiles. ROCA is re-calculated after every additional CA125 value. This systematic method is more efficient than using a single cut-point (e.g. 35 U/mL), or an ad-hoc rule of thumb, as it incorporates all sources of signal (baseline CA125 level, doubling time) and noise (variation of CA125 baseline levels, variation of baseline levels between women, variation between cases of doubling times) to obtain the most efficient signal/noise ratio, maximizing sensitivity for any level of specificity. The clinical recommendations for follow up are based on the ROCA risk score. If a patient’s ROCA risk of ovarian cancer score is less than 1 in 2000 (called “normal risk”), the recommendation is for the woman to return for a repeat CA125 in one year. For a ROCA risk between 1 in 2000 to 1 in 500 (“intermediate risk”), the recommendation is to return for a repeat CA125 in three months. For a ROCA risk of greater than 1 in 500 (“elevated risk”), the recommendation is for a transvaginal ultrasound and referral to a gynecologic oncologist. After each additional CA125 value, ROCA is re-calculated and a new recommendation is made.
After obtaining informed consent, all patients underwent a baseline CA125 screen and completed a medical history questionnaire. Blood samples were transported on ice and serum was separated and frozen at − 80° C on the same day. Samples were shipped on dry ice and thawed promptly before assays were performed. All CA125 II assays were performed at MD Anderson Cancer Center using the Roche platform. Values were transmitted to the data coordinating center at Massachusetts General Hospital, where the ROCA was applied and the patient’s risk was calculated.
The ROCA score was communicated each time to the participant’s recruitment site. Investigators at each study site communicated the risk scores and screening recommendations to the patients. For patients with an elevated risk score, transvaginal ultrasound was performed at each of the study sites by an attending radiologist. Using standardized criteria, ovarian morphology was assessed and was considered abnormal if there were cystic and solid areas (complex). Single, thin-walled anechoic cysts with no septa or papillary projections were considered normal.
In addition to the transvaginal ultrasound, women with elevated risk scores also had a consultation with a gynecologic oncologist. The decision for surgery was determined by the gynecologic oncologist. Operative and pathology notes were transmitted to the lead site to confirm diagnosis. Central pathology review was conducted at the lead site by a gynecologic pathologist (MTD) to confirm stage, grade and histology.
Specificity (fraction of those patients without ovarian cancer who did not undergo surgery) and positive predictive value (fraction that had ovarian cancer, of those who underwent surgery) were calculated. Annual rates of referral for each of the screening options were calculated. Exact confidence limits were calculated, based on Fisher’s exact test for the binomial distribution. The power of this trial will provide precise confidence limits for estimating specificity and an important lower bound on the positive predictive value. A much larger definitive trial is required to provide accurate estimates of sensitivity. Our study was originally designed to draw blood from 1600 to 2300 women per year, yielding 14,602 woman-years of observation (2,300 prevalent years and 12,302 incident years). With an annual incidence of ovarian cancer in this postmenopausal population of 45/100,000, we expect 8 ovarian cancer cases, enabling us to establish an important 95% confidence interval lower bound for the positive predictive value (PPV) of 11% assuming similar experience to Jacobs’ pilot of 6,734 women using ROCA with a PPV of 23%. The definition of specificity for an annual screening program is the proportion of true negatives who test negative per year. Because a gold standard test is not available, we estimate apparent specificity with a defined follow-up time of one year. Let D be the number of screening episodes for which a subject has not had a diagnosis of ovarian cancer within one year from the beginning of the screening episode. Let E be the number of screening episodes for which a subject does not undergo surgery. For an estimated specificity of 98% and D=13,036 screening episodes, we expect E to be 12,776, with 261 women referred to ultrasound, which would give a narrow 95% confidence interval for specificity of (97.76%, 98.24%). This confidence interval clearly excludes 97% specificity, so the sample size is sufficient for obtaining precise estimates of specificity. Given that only 8 cases of ovarian cancer are expected, the lack of a gold standard for 13,036 episodes will have little effect on our estimate of specificity.
Results
From 2001–2011, a total of 4060 women were enrolled during the 11 year period. Nine women were ineligible due to age restrictions. The final study sample consisted of 4051 participants. The total number of screen years was 16,832 years, with an average number of 4.2 screen years per woman. The median age was 59 years (range 50 – 74 years) (Table 1). Eighty-two percent of the women were white, 10% were African American, 5% were Hispanic, 3% were Asian and <1% answered “other”. Eighty-seven percent of the study population had ever been pregnant, with a median of two pregnancies. Eighty percent had used oral contraceptives for a median of 48 months, and 61% had ever used hormone replacement therapy with median duration of 60 months. Fourteen percent of study participants had a personal history of breast cancer.
Table 1.
Baseline characteristics of study population (N=4051)
| Characteristics at enrollment | Median | Range |
|---|---|---|
| Age, years | 59 | 50–74 |
| Duration of HRT use in months | 60 | 0–624 |
| Duration of OCP use in months | 48 | 0–480 |
|
| ||
| No. | % | |
|
| ||
| Ethnicity | ||
| White | 3315 | 82 |
| African American | 406 | 10 |
| Hispanic | 187 | 5 |
| Asian | 104 | 3 |
| Other | 39 | 1 |
| Ever pregnant | 3168 | 87 |
| Ever use OCP | 2966 | 80 |
| Ever use HRT | 2255 | 61 |
| Hysterectomy | 777 | 21 |
| Personal history of breast cancer | 470 | 14 |
Abbreviations: HRT, hormone replacement therapy; OCP, oral contraceptive pill.
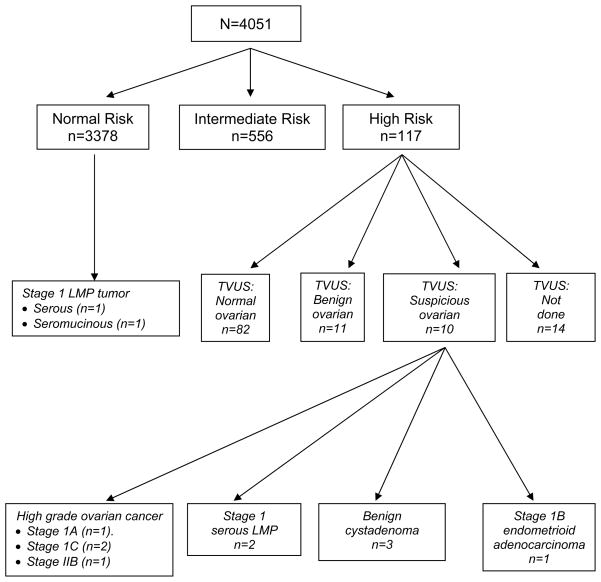
The average annual rates and the overall rates for participants being triaged into the normal risk group (which would require return in one year for a CA125), the intermediate risk group (which would require women to repeat their CA125 in three months), and high risk group (which would require TVS and referral to a gynecologic oncologist) are presented in Table 2. The average annual rate for triage to the normal risk group was 93.3%, for the intermediate risk group was 5.8%, and for the high risk group was 0.9%. Over the 11 year period, 83.4% of participants remained in the normal risk category and only had to return for an annual CA125. In addition, 13.7% (n=556) over the 11 year period had to repeat a CA125 in three months. Finally, 2.9% (n=117) were high risk by ROCA. Figure 1 summarizes the outcomes for the study population. Patients were classified into normal risk, intermediate risk, or high risk groups based upon their most acute ROCA increase in calculation during their participation in the study.
Table 2.
Screening rates for risk groups
| Normal risk1 | Intermediate risk2 | High risk3 | |
|---|---|---|---|
| Average annual rate | 93.3% | 5.8% | 0.9% |
| Overall rate | 83.4% | 13.7% | 2.9% |
Normal risk, return in 1 year for CA 125
Intermediate risk, repeat CA 125 in 3 months
High risk, transvaginal ultrasound and referral to gynecologic oncologist
Figure 1.
Overall flow diagram for participants through December 1, 2011 showing the number of patients by most acute ROCA category.
TVS = transvaginal ultrasound
Of the 117 women who were triaged to undergo a TVS and referral to a gynecologic oncologist, 82 women had a normal TVS, 11 had benign ovarian findings, ten had suspicious ovarian findings and 14 women did not have a TVS done. Of the 14 women who did not have a TVS: i) four had recurrence of a previously diagnosed cancer; ii) six patients declined; iii) one patient was unable to undergo TVS due to vaginal stenosis but instead had a transabdominal ultrasound; and iv) three were not performed based on the judgment of the physician. Excluding the four patients with recurrent cancer, nine of the remaining ten women who did not have a TVS did have repeat CA125 measurements three months later which decreased to below the triggering CA125 values, and the tenth woman underwent a TVS off study which was normal.
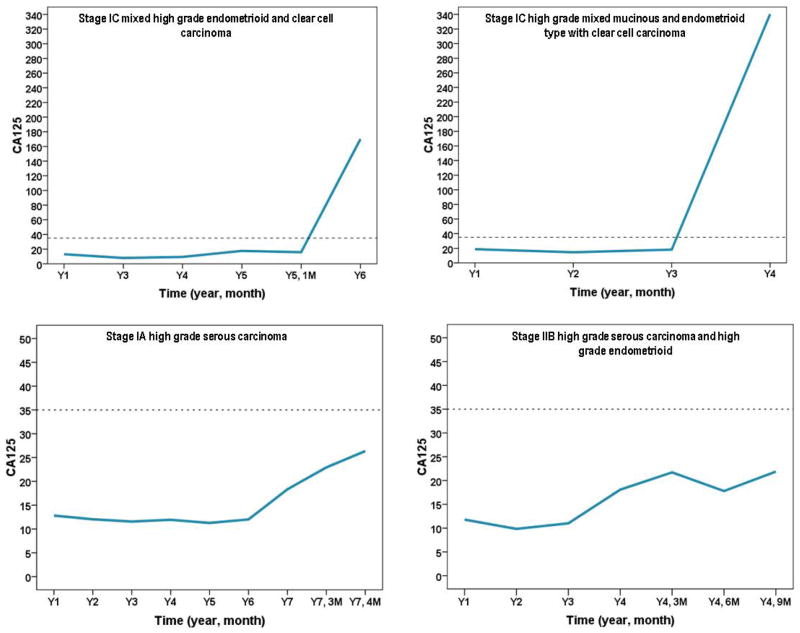
All ten with suspicious ovarian findings on TVS underwent surgery (Table 3). Three patients had benign cystadenomas, two patients had Stage I ovarian serous tumors of low malignant potential (LMP), four patients had early stage high grade invasive ovarian cancers, and one patient was ultimately found to have endometrial cancer. In Figure 2, the CA125 values are plotted over time for the four high grade invasive ovarian cancers. Interestingly, in all four of the invasive cases, women had normal, low risk ROCA scores and had been returning for annual CA125 tests for at least three years prior to the elevated CA 125 values. All four invasive cancers were early stage high grade ovarian cancers.
Table 3.
Study-directed surgeries
| No. | Age at enrollment | CA 125 | TVS | Symptoms | Findings at surgery | ||
|---|---|---|---|---|---|---|---|
| Baseline | Triage to “high risk” | # of annual tests | |||||
| 1 | 68 | 29 | 74 | 1 | Abnormal | No | Stage 1 serous LMP in background of papillary serous adenofibroma |
| 2 | 64 | 9 | 15 | 6 | Abnormal | No | Stage 1 serous LMP |
| 3 | 63 | 12 | 24 | 2 | Abnormal | No | Cystadenoma, mixed mucinous and serous components |
| 4 | 55 | 12 | 22 | 3 | Abnormal | No | Stage IIB high grade serous carcinoma and focal high grade endometrioid carcinoma |
| 5 | 53 | 13 | 18 170 |
3 | #1:Normal #2:Abnormal |
GI | Stage IC endometrioid adenocarcinoma and clear cell carcinoma |
| 6 | 69 | 75 | 75 | 0 | Abnormal | No | Serous cystadenofibroma |
| 7 | 65 | 19 | 340 | 3 | Abnormal | No | Stage IC mixed mucinous and endometrioid type with clear cell carcinoma |
| 8 | 60 | 45 | 59 | 0 | Abnormal | No | 1st surgery: no ovarian disease; 2nd surgery: Stage IB grade 1 endometrial cancer (endometrioid adenocarcinoma) |
| 9 | 60 | 13.4 | 30.5 | 8 | Abnormal | No | Serous cystadenofibroma |
| 10 | 56 | 12.82 | 22.9 | 6 | Abnormal | No | Stage lA high grade serous carcinoma |
TVS, transvaginal ultrasound LMP, low malignant potential
Figure 2.
CA125 values over time for invasive ovarian cancers.
(a) Stage 1C mixed grade endometrioid and clear cell carcinoma
(b) Stage IC high grade mixed mucinous and endometrioid type with clear cell carcinoma
(c) Stage lA high grade serous carcinoma
(d) Stage IIB high grade serous carcinoma and high grade endometrioid
Patient #4 had CA125 values at baseline, Year 2, and Year 3 of 12 IU/ml, 10 IU/ml, and 11 IU/ml, respectively (Table 3 and Figure 2d). When the CA125 rose to 18 IU/ml in Year 4, the ROCA triaged her as “intermediate risk” and a three month repeat CA125 was recommended. The three month CA125 was 22 IU/ml, and the ROCA triaged her as “high risk” and recommended a TVS and referral to a gynecologic oncologist. She was completely asymptomatic. Her TVS revealed a complex irregularly shaped ovarian cyst with no septations, measuring 2.5 x 2.4 x 2.8 centimeters. The gynecologic oncologist and the patient chose to repeat the TVS in 3 months rather than proceed directly to surgery. A follow-up TVS three months later demonstrated a larger cyst measuring 4.6 x 4.0 x 3.6 centimeters with increased complexity. The CA125 at that time was 21.8 IU/ml. The patient underwent surgery and was found to have a Stage IIB high grade serous ovarian cancer. She received adjuvant carboplatin and paclitaxel chemotherapy and is currently without evidence of disease 42 months after her diagnosis. Patient #5 had CA125 measurements at baseline, Year 3 (she missed Year 2), and Year 4 of 13 IU/ml, 8 IU/ml, and 8 IU/ml, respectively (Table 3 and Figure 2a). At Year 5, her CA125 rose to 18 IU/ml and the ROCA triaged her as “high risk”. The patient had vague abdominal discomfort. Her TVS was normal, and the decision with the consulting gynecologic oncologist was for an immediate CT scan, which was negative. As a result, the recommendation by the gynecologic oncologist was a repeat CA125 and TVS in three months. At that time, the patient also underwent a full work-up by a gastroenterologist that included an upper gastrointestinal series. She was diagnosed with H. Pylori and placed on antibiotics with improvement. The patient missed her three month follow-up for ROCA, and six months later returned for CA125 which was now 170 IU/ml. The TVS at that time demonstrated a two centimeter complex ovarian mass. The patient underwent surgery and was found to have a Stage IC high grade endometrioid and clear cell ovarian cancer. She received adjuvant carboplatin and paclitaxel chemotherapy and is currently without evidence of disease 28 months after her diagnosis. Patient #7 had baseline, Year 2, and Year 3 CA125 measurements of 19 IU/ml, 15 IU/ml, and 18 IU/ml, respectively (Table 3 and Figure 2c). In Year 4, her CA 125 was 340 IU/ml and ROCA triaged her as “high risk”. Her TVS demonstrated a complex 5.9 x 6.3 x 4.0 centimeter ovarian mass and she underwent surgery that revealed a Stage 1C high grade mixed endometrioid, mucinous, and clear cell ovarian cancer. She received adjuvant carboplatin and paclitaxel chemotherapy and is currently without evidence of disease 42 months after diagnosis. Patient # 10 had six years of annual CA125 values between 11.3 IU/ml and 12.8 IU/ml. In Year 7 her CA125 increased to 18.3 IU/ml and ROCA triaged her as “intermediate risk” and to repeat a CA125 in three months. The CA125 at the three month follow-up was 22.9 IU/ml and ROCA triaged her to “high risk”. Her TVS revealed a 8.7 x 6.3 x 6.1 centimeter complex septated cystic mass. She underwent a total laparoscopic bilateral salpingo-oophorectomy and was found to have a Stage 1A high grade serous ovarian cancer. The patient declined chemotherapy and will be followed closely with CA125 levels monitored every three months. She is currently NED four months from her diagnosis.
Patient #8 had a baseline CA125 of 45 IU/ml, which then elevated to 59 IU/ml 3 months later. ROCA triaged her as “high risk” (Table 3). The patient underwent TVS 2 months after her second CA125 (5 months after her baseline CA125). The TVS demonstrated a right simple ovarian cyst and moderate ascites. The uterus was well visualized with a heterogeneous appearance. The endometrial thickness was 3mm. One month later, she underwent a laparoscopic bilateral salpingo-oophorectomy. Biopsies of a sigmoid nodule and epiploica were performed. The endocervical canal was dilated in order to accommodate a uterine manipulator. All pathology was negative for malignancy. Several weeks after her surgery, the patient reported vaginal bleeding. An endometrial biopsy revealed a grade 1 endometrial cancer. The patient underwent total laparoscopic hysterectomy and staging procedure three months after her laparoscopic salpingo-oophorectomy. The final pathology revealed a grade 1 endometrial adenocarcinoma, negative lymph nodes and 52% myometrial invasion. The patient was diagnosed with Stage 1B endometrial cancer and is currently without evidence of disease 24 months from her diagnosis.
Two women were found to have Stage I ovarian serous LMP tumors. Patient #1’s TVS demonstrated an 11.8 x 6.5 x 4.7 centimeter complex pelvic mass. Patient #2’s TVS demonstrated a 9.6 x 7.0 centimeter complex pelvic mass. Both patients underwent surgery and are currently alive and NED. The remaining three women who underwent study-directed surgeries were found to have benign cystadenomas.
The specificity for the two stage screening strategy was 99.9% (95% CI 99.7%, 100%). The positive predictive value (PPV) for identifying an invasive ovarian cancer was 40% (95% CI 12.2%, 73.8%). The sample size calculations for this study aimed to achieve a minimum lower bound of 11% for the PPV and a confidence interval for specificity that rules out values of 97% or less. Both of these goals were exceeded by this study. This means that no more than two to three operations were required to detect one case of invasive ovarian cancer. If LMP tumors were included, the specificity for the two stage screening approach was 99.9% (95% CI, 99.7, 100%) and the PPV was 60% (95% CI, 26.2%, 87.8%). The study was not powered to detect sensitivity, however, we know that no cases of invasive ovarian cancer were missed. The health status (changes to participant’s medical or cancer history) of participants was updated at screening appointments and through follow-up letters and phone calls with participants one year following their last blood test. The last patient accrued on December 31, 2011. Since then a full year of follow-up has occurred and no cases of invasive ovarian cancer were reported during this time period. However, two additional LMP cases were not detected by the ROCA algorithm.
Discussion
This single-arm prospective ovarian cancer screening study in postmenopausal women demonstrates that the two-step screening strategy using CA125 and ROCA calculation, followed by referral of a small number of women to transvaginal ultrasound and consultation with a gynecologic oncologist, achieves high specificity with very few false positive results. Given the relatively low prevalence of ovarian cancer in the general population, a screening strategy that minimizes unnecessary operations due to false positive values is crucial.
Interestingly, the specificity in this U.S. trial (99.9%) is very similar to the specificity reported in the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) at the initial screen, 99.9%.7 In addition, the positive predictive value (PPV) in the UKCTOCS initial screen was 35.1% and in our study was 40%, which greatly exceeds the minimum clinical benchmark of 10%. Even after observing 6 invasive ovarian cancers when 8 were expected (within Poisson variation), the 95% confidence interval’s lower bound on PPV is 12%, exceeding the 10% lower acceptable limit the study was powered to achieve. The PPV in our study and in the UKCTOCS initial screen demonstrates that no more than two to three operations were needed to detect one case of invasive ovarian cancer. This contrasts with results of an arm in the UKCTOCS trial that utilized annual TVS as the primary screen and without CA125 where 36 operations were required to diagnose each case of ovarian cancer. Annual TVS detected a large number of benign pelvic masses that still prompted surgery. As CA125 rarely continuously rises in the presence of benign disease, use of a two stage strategy based on serial CA125s essentially ignores the benign disease and improves specificity. Positive predictive value is improved dramatically with this strategy compared to the 3.7% observed with a single abnormal value of CA125 in the PLCO trial. Our study and the UKCTOCS highlight the critical importance of how a biomarker is utilized.
Importantly, this study also provides insight into incident cases that may be detected using the ROCA two-stage screening strategy, suggesting that serial CA125 may be adequately sensitive to detect early stage disease in a fraction of cases. The UKCTOCS study showed 47% of prevalent invasive cases (16 of 34) were Stage 1 or II however data on incident cases from that trial have not yet been reported. In our study, all four of the incident invasive cancers were Stage 1 or II. Data from our study suggests that incident cases detected through ROCA are likely to be early stage cancers. This contrasts with the current presentation of ovarian cancer in which over 75% of women are diagnosed with Stage III or IV disease.
Greater sensitivity may relate to the detection of rising CA125 within the normal range, observed in two of our four invasive cases (Figure 2). In addition, patients with an increase relative to their own baseline were recalled in three months, rather than in one year. Both factors may explain why early stage disease was detected in our trial and a stage shift was found in the UKCTOCS trial, whereas there was no stage shift or survival advantage in the PLCO.2
We found that the majority of women who participated in the study only had to return on an annual basis for a repeat CA125. Our average annual rate of normal risk ROCA calculation was 93.3%, which is very similar to the 90.9% rate reported in the UKCTOCS initial screen. In addition, we had similar rates for our intermediate risk ROCA category, an average rate of 8.6% in the UK versus 5.8% in this US study. Finally, we have similarly low rates of women being referred for transvaginal ultrasound and gynecologic oncology consultation, with a 0.5% in the UKCTOCS initial screen and 0.9% in our study.7 Thus, using this strategy for ovarian cancer screening in the general postmenopausal population should be cost-effective, as the majority of women would only need to return on an annual basis for a CA125. Less than 1% of women would need to proceed on to undergoing a transvaginal ultrasound and referral to a physician.
While this strategy for ovarian cancer screening in postmenopausal women demonstrates excellent specificity, it is not practice-changing at this time. More definitive data, including sensitivity and the effect of this strategy on decreasing mortality from ovarian cancer is required. Currently other biomarkers such as HE4, CA72.4 and MMP7 are being studied alone and in combination to evaluate whether they potentially increase sensitivity without decreasing specificity. In the meantime, results of the UKCTOCS study, a large 200,000 woman, prospective, randomized study designed to address sensitivity and mortality from ovarian cancer, will likely be available by 2015. In our study, ROCA identified early stage incident cases with excellent specificity and highlights the potential for implementing effective strategies for the early detection of ovarian cancer in postmenopausal women.
Supplementary Material
Acknowledgments
This study was supported by funds from the MD Anderson SPORE in Ovarian Cancer NCI P50 CA83639, the Bioinformatics Shared Resources of the MD Anderson CCSG NCI P30 CA16672, the National Foundation for Cancer Research, philanthropic support from Golfers Against Cancer, the Tracey Jo Wilson Foundation, the Mossy Foundation, the Norton family, and Stuart and Gaye Lynn Zarrow. We would like to thank Concepcion Teodoro and Joseph Celestino for their technical support.
This study was approved by the IRB at each of the participating institutions. All subjects provided written informed consent prior to participating in the study.
Footnotes
Conflicts of Interest
Steven Skates is a co-inventor of the Risk of Ovarian Cancer Algorithm, patent number US5800347, which Massachusetts General Hospital has licensed. Deepak Bedi is an unpaid consultant and an unpaid member of the speaker’s bureau for Philips Healthcare. Richard Moore has received research funding from Fujirebio Diagnostics Inc and Abbott Diagnostics Inc.
Robert C Bast Jr. receives royalties for the discovery of CA 125 from Fujirebio Diagnostics Inc and serves on the advisory board for Vermillion. None of the other authors declared any conflicts of interest.
Authors’ Contributions: KHL, SS, DB, HF, and RCB contributed to the design of the study.
KHL, SS, MH, DB, TB, LL, RM, CG, SH, WN, OA, JG, HF, RCB were involved with study implementation. KHL, SS, MH, DB, TB, LL, RM, CG, SH, WN, OA, JG, and MTD participated in data collection. KHL, SS, MH, LL, RM, SH, WN, OA, JG, MTD, CCS, NH ensured the integrity of the data. KHL, SS, MTD, CCS, and NH were involved with interpretation of study results. SS, CCS, NH, and RCB contributed to the statistical analysis. KHL, SS, MTD, CCS, NH, and RCB assisted in drafting the manuscript, figures, and tables. KHL, SS, MH, DB, TB, LL, RM, CG, SH, WH, OA, MTD, CCS, MH, and RCB reviewed and approved the final version of the manuscript. KHL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Skates SJ, Xu FJ, Yu YH, et al. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer. 1995;76(S10 suppl):2004–10. doi: 10.1002/1097-0142(19951115)76:10+<2004::aid-cncr2820761317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Buys SS, Partridge E, Greene MH, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–9. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs IJ, Skates S, Davies AP, et al. Risk of diagnosis of ovarian cancer after raised serum CA125 concentration: a prospective cohort study. BMJ. 1996;313:1355. doi: 10.1136/bmj.313.7069.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skates SJ, Pauler DK, Jacobs IJ. Screening Based on the Risk of Cancer Calculation From Bayesian Hierarchical Changepoint and Mixture Models of Longitudinal Markers. J Am Stat Assoc. 2001;96:429–39. [Google Scholar]
- 5.Skates SJ, Menon U, MacDonald N, et al. Calculation of the Risk of Ovarian Cancer From Serial CA-125 Values for Preclinical Detection in Postmenopausal Women. J Clin Oncol. 2003;21:206–10. doi: 10.1200/JCO.2003.02.955. [DOI] [PubMed] [Google Scholar]
- 6.Menon U, Skates SJ, Lewis S, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005;23:7919–26. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 7.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–40. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.