Abstract
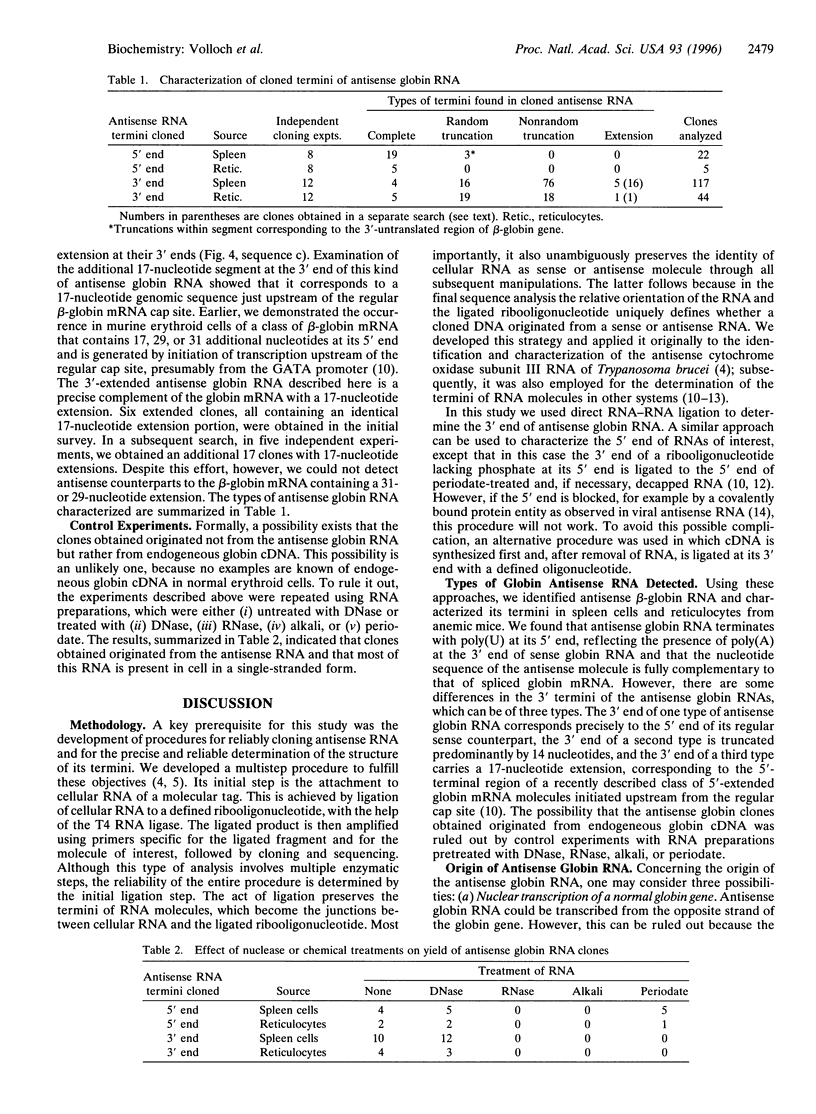
The aim of the experiments described in this paper was to test for the presence of antisense globin RNA in mouse erythroid tissues and, if found, to characterize these molecules. The present study made use of a multistep procedure in which a molecular tag is attached to cellular RNA by ligation with a defined ribooligonucleotide. The act of ligation preserves the termini of RNA molecules, which become the junctions between cellular RNAs and the ligated ribooligonucleotide. It also unambiguously preserves the identity of cellular RNA as a sense or antisense molecule through all subsequent manipulations. Using this approach, we identified and characterized antisense beta-globin RNA in erythroid spleen cells and reticulocytes from anemic mice. We show in this paper that the antisense globin RNA is fully complementary to spliced globin mRNA, indicative of the template/transcript relationship. It terminates at the 5' end with a uridylate stretch, reflecting the presence of poly(A) at the 3' end of the sense globin mRNA. With respect to the structure of their 3' termini, antisense globin RNA can be divided into three categories: full-size molecules corresponding precisely to globin mRNA, truncated molecules lacking predominantly 14 3'-terminal nucleotides, and extended antisense RNA containing 17 additional 3'-terminal nucleotides. The full-size antisense globin RNA contains two 14-nt-long complementary sequences within its 3'-terminal segment corresponding to the 5'-untranslated region of globin mRNA. This, together with the nature of the predominant truncation, suggests a mechanism by which antisense RNA might give rise to new sense-strand globin mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A. Replication of the genomic RNA of a positive-strand RNA animal virus from negative-sense transcripts. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12443–12447. doi: 10.1073/pnas.91.26.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Volloch Z., Aviv H. Messenger RNA population analysis during erythroid differentiation: a kinetical approach. J Mol Biol. 1977 Feb 25;110(2):191–203. doi: 10.1016/s0022-2836(77)80068-5. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Byrnes J. J., Jurmark B. S., So A. G. Reticulocyte RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3400–3404. doi: 10.1073/pnas.70.12.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont-Racine M., Bertrand E., Pictet R., Grange T. A highly sensitive method for mapping the 5' termini of mRNAs. Nucleic Acids Res. 1993 Apr 11;21(7):1683–1684. doi: 10.1093/nar/21.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen A. K., Peffley D. M. The length of 5'-untranslated leader sequences influences distribution of 3-hydroxy-3-methylglutaryl-coenzyme A reductase mRNA in polysomes: effects of lovastatin, oxysterols, and mevalonate. Arch Biochem Biophys. 1995 Oct 1;322(2):475–485. doi: 10.1006/abbi.1995.1491. [DOI] [PubMed] [Google Scholar]
- Hill J. R., Morris D. R. Cell-specific translation of S-adenosylmethionine decarboxylase mRNA. Regulation by the 5' transcript leader. J Biol Chem. 1992 Oct 25;267(30):21886–21893. [PubMed] [Google Scholar]
- Koonin E. V. Similarities in RNA helicases. Nature. 1991 Jul 25;352(6333):290–290. doi: 10.1038/352290c0. [DOI] [PubMed] [Google Scholar]
- Liu X., Gorovsky M. A. Mapping the 5' and 3' ends of Tetrahymena thermophila mRNAs using RNA ligase mediated amplification of cDNA ends (RLM-RACE). Nucleic Acids Res. 1993 Oct 25;21(21):4954–4960. doi: 10.1093/nar/21.21.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallie R. Characterization of the extreme 5' ends of RNA molecules by RNA ligation-PCR. PCR Methods Appl. 1993 Aug;3(1):54–56. doi: 10.1101/gr.3.1.54. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Strauss J. H. Replication strategies of the single stranded RNA viruses of eukaryotes. Curr Top Microbiol Immunol. 1983;105:1–98. doi: 10.1007/978-3-642-69159-1_1. [DOI] [PubMed] [Google Scholar]
- Troutt A. B., McHeyzer-Williams M. G., Pulendran B., Nossal G. J. Ligation-anchored PCR: a simple amplification technique with single-sided specificity. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9823–9825. doi: 10.1073/pnas.89.20.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V. Z., Schweitzer B., Rits S. Evolutionarily conserved elements in the 5' untranslated region of beta globin mRNA mediate site-specific priming of a unique hairpin structure during cDNA synthesis. Nucleic Acids Res. 1994 Dec 11;22(24):5302–5309. doi: 10.1093/nar/22.24.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V. Cytoplasmic synthesis of globin RNA in differentiated murine erythroleukemia cells: possible involvement of RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1208–1212. doi: 10.1073/pnas.83.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Schweitzer B., Rits S. Inhibition of pre-mRNA splicing by antisense RNA in vitro: effect of RNA containing sequences complementary to introns. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1600–1605. doi: 10.1016/0006-291x(91)91757-4. [DOI] [PubMed] [Google Scholar]
- Volloch V., Schweitzer B., Rits S. Ligation-mediated amplification of RNA from murine erythroid cells reveals a novel class of beta globin mRNA with an extended 5'-untranslated region. Nucleic Acids Res. 1994 Jul 11;22(13):2507–2511. doi: 10.1093/nar/22.13.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Schweitzer B., Rits S. Synthesis of globin RNA in enucleated differentiating murine erythroleukemia cells. J Cell Biol. 1987 Jul;105(1):137–143. doi: 10.1083/jcb.105.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Schweitzer B., Rits S. Uncoupling of the synthesis of edited and unedited COIII RNA in Trypanosoma brucei. Nature. 1990 Feb 1;343(6257):482–484. doi: 10.1038/343482a0. [DOI] [PubMed] [Google Scholar]
- Volloch V., Schweitzer B., Zhang X., Rits S. Identification of negative-strand complements to cytochrome oxidase subunit III RNA in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10671–10675. doi: 10.1073/pnas.88.23.10671. [DOI] [PMC free article] [PubMed] [Google Scholar]





