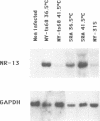
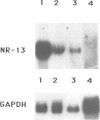
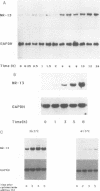
Abstract
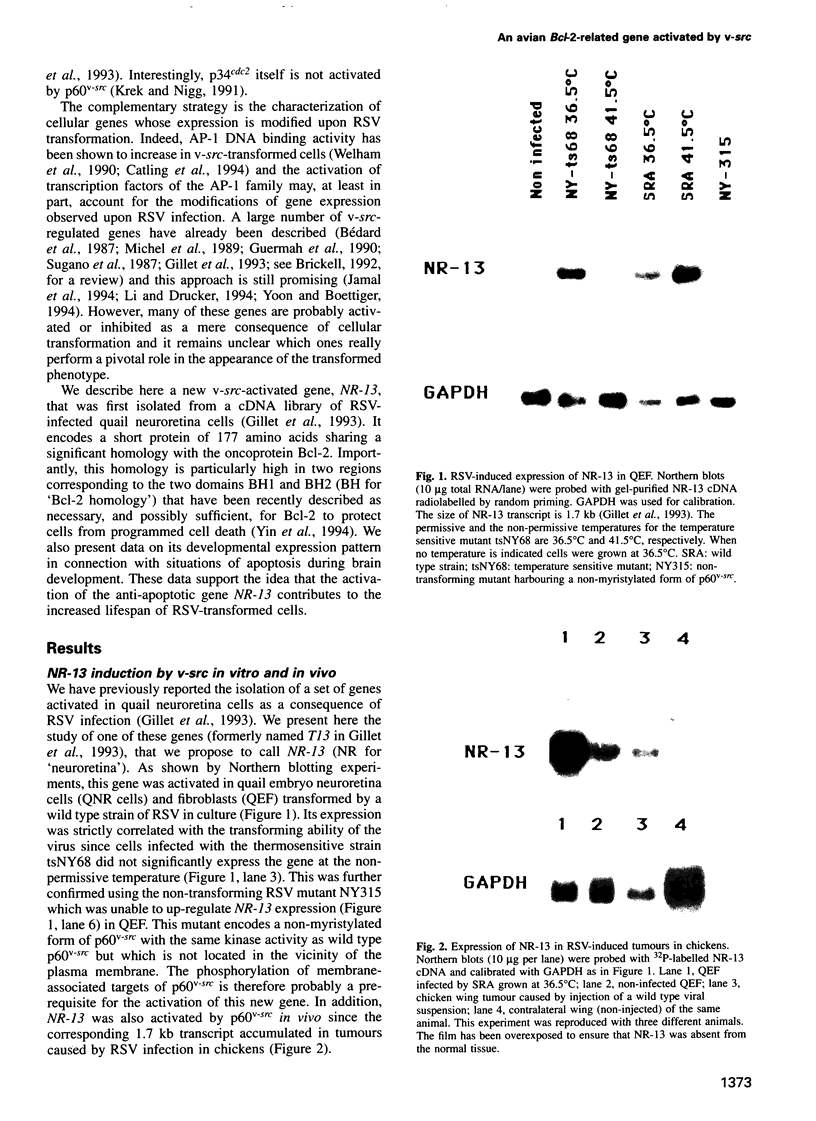
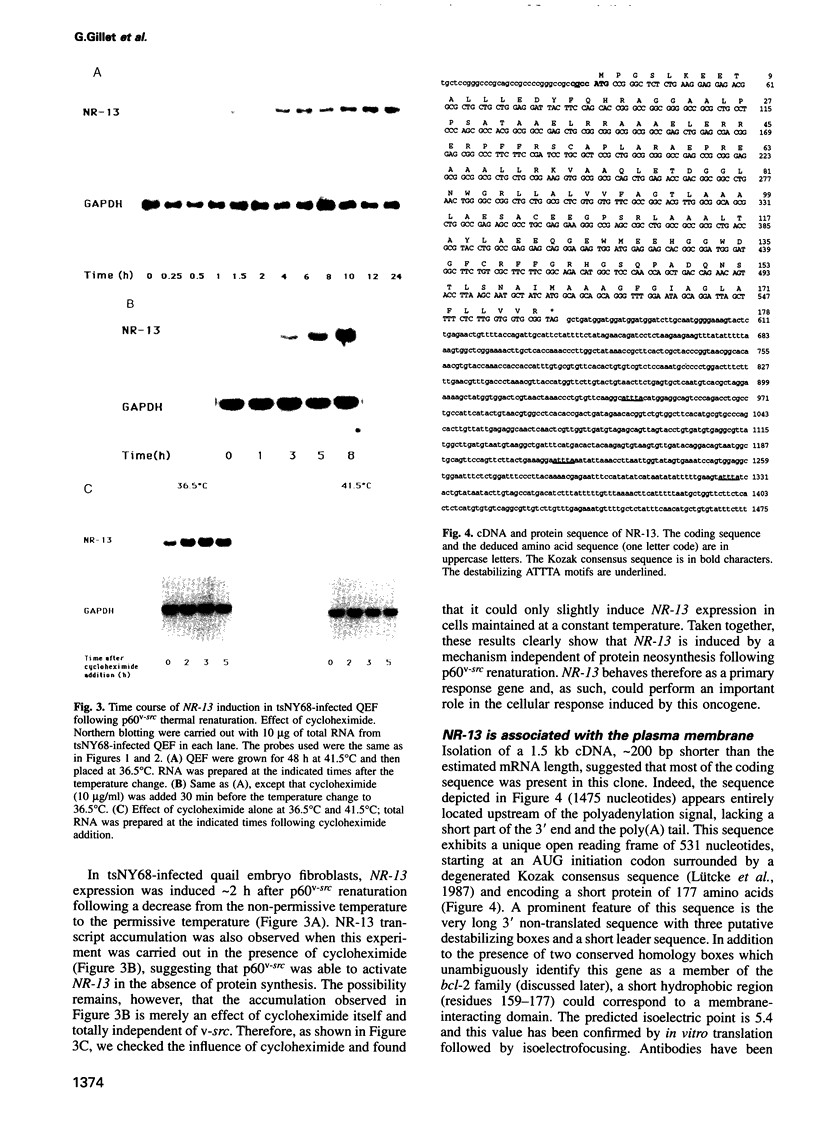
The oncoprotein p60v-src encoded by the Rous sarcoma virus (RSV) genome is the prototype of non-receptor tyrosine kinases. More than 50 targets of p60v-src have been described to date. However, the precise mechanisms of RSV transformation remain to be elucidated. Here, we present the study of a new v-src-activated gene, NR-13, which encodes a protein identified as a new member of the Bcl-2 family. This protein is localized in the membrane with a pattern already observed with Bcl-2. In quail embryos, this gene is mainly expressed in neural and muscular tissues. Its expression is dramatically down-regulated after embryonic day 7 (E7) in the optic tectum. To evaluate a possible role for NR-13 in the control of apoptotic processes in this particular brain area, in situ hybridization and DNA ladder fractionation studies were performed to correlate NR-13 expression with typical situations of apoptosis during brain development. Our results support the idea that RSV could activate anti-apoptotic functions of the host cell resulting in an increase of their lifespan, which could be particularly relevant to tumour formation.
Full text
PDF
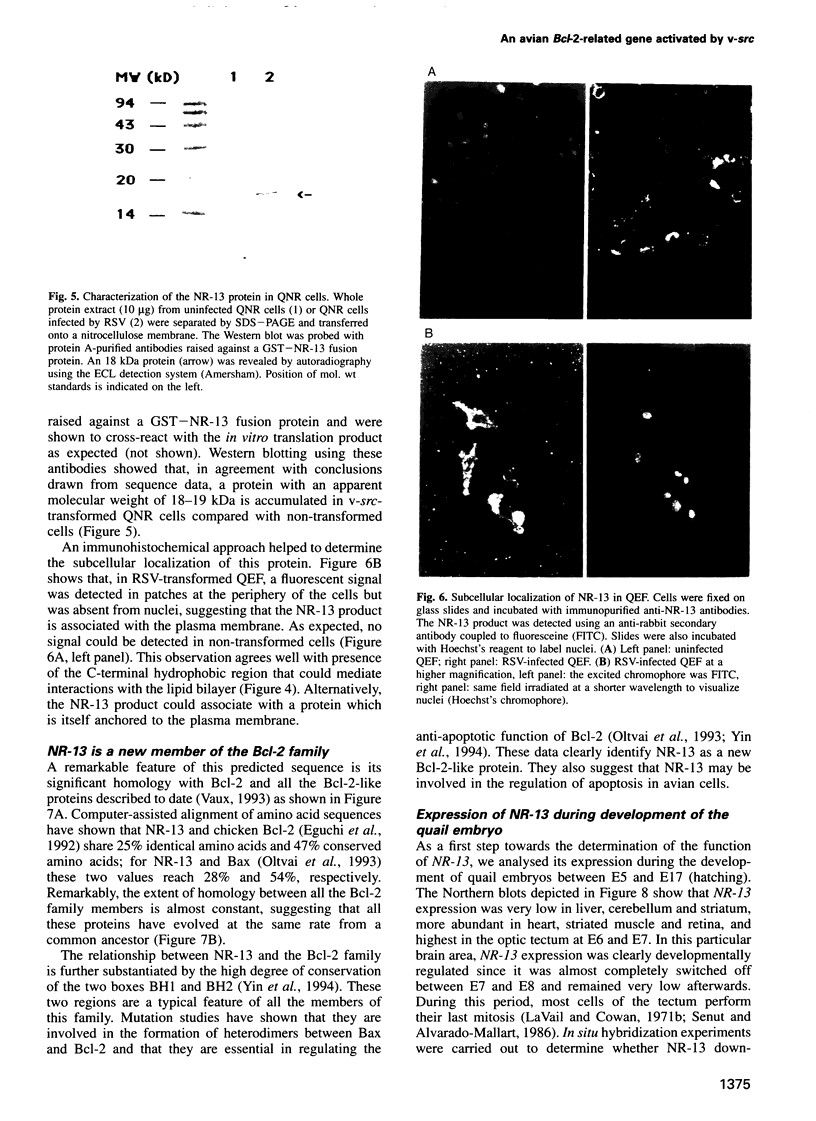
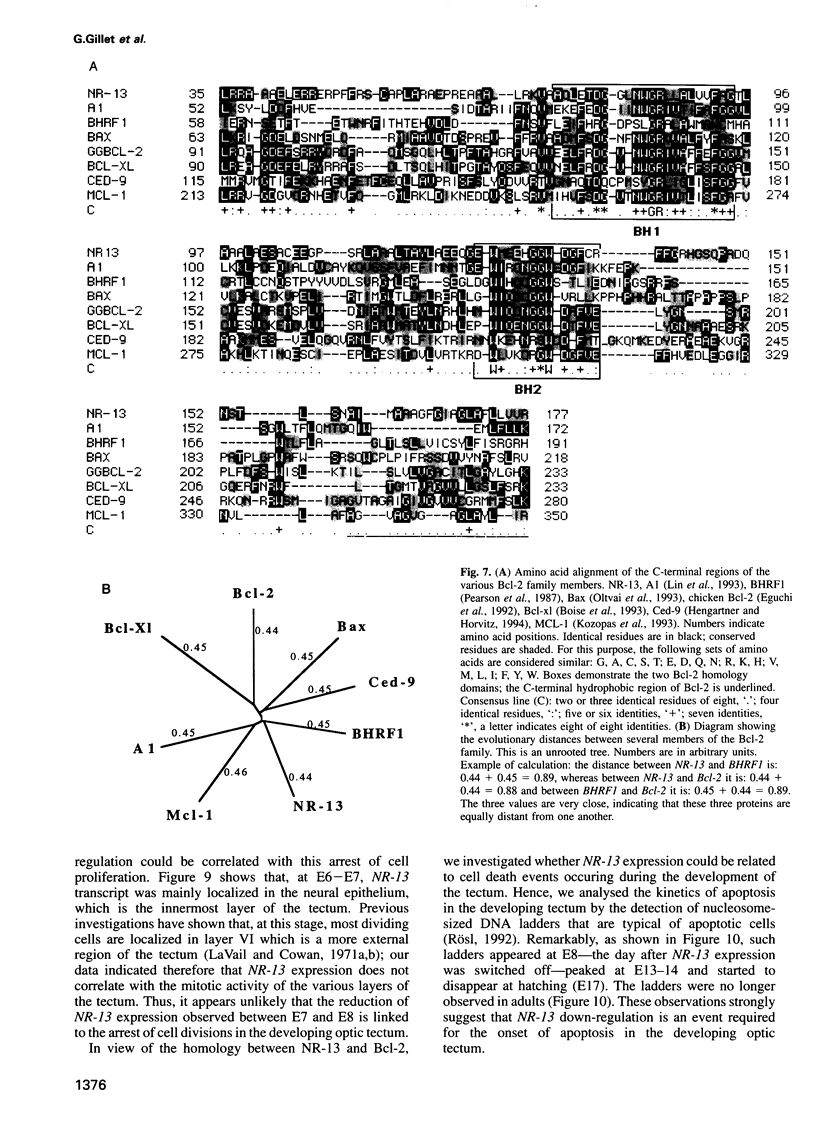
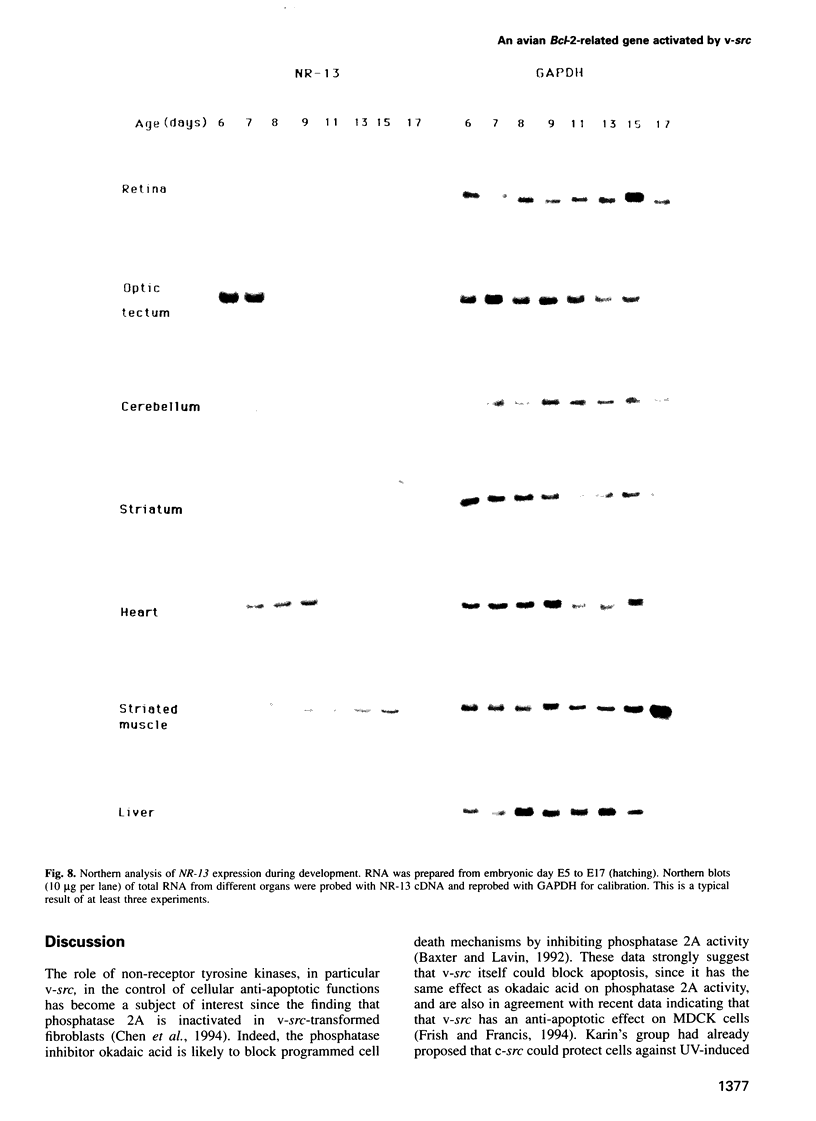
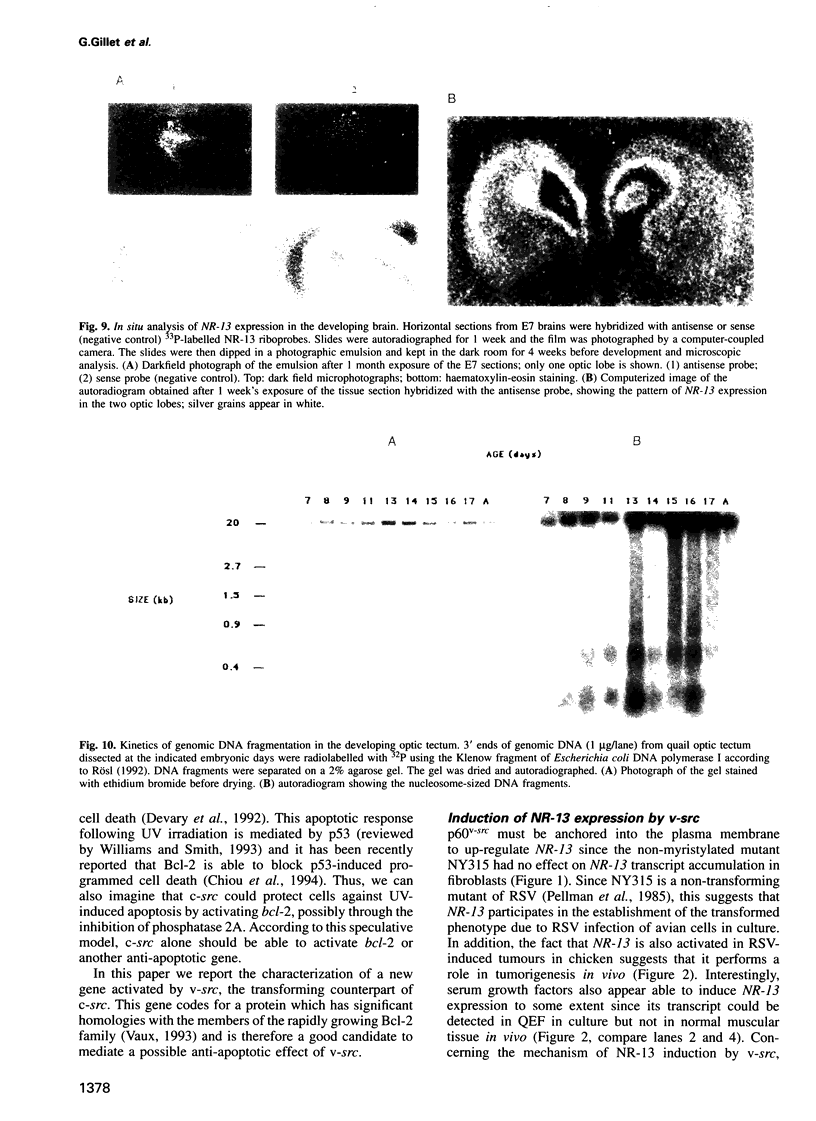



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter G. D., Lavin M. F. Specific protein dephosphorylation in apoptosis induced by ionizing radiation and heat shock in human lymphoid tumor lines. J Immunol. 1992 Mar 15;148(6):1949–1954. [PubMed] [Google Scholar]
- Bedard P. A., Alcorta D., Simmons D. L., Luk K. C., Erikson R. L. Constitutive expression of a gene encoding a polypeptide homologous to biologically active human platelet protein in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6715–6719. doi: 10.1073/pnas.84.19.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Borzillo G. V., Endo K., Tsujimoto Y. Bcl-2 confers growth and survival advantage to interleukin 7-dependent early pre-B cells which become factor independent by a multistep process in culture. Oncogene. 1992 May;7(5):869–876. [PubMed] [Google Scholar]
- Brickell P. M. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit Rev Oncog. 1992;3(4):401–446. [PubMed] [Google Scholar]
- Catling A. D., Fincham V. J., Frame M. C., Haefner B., Wyke J. A. Mutations in v-Src SH3 and catalytic domains that jointly confer temperature-sensitive transformation with minimal temperature-dependent changes in cellular tyrosine phosphorylation. J Virol. 1994 Jul;68(7):4392–4399. doi: 10.1128/jvi.68.7.4392-4399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Parsons S., Brautigan D. L. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem. 1994 Mar 18;269(11):7957–7962. [PubMed] [Google Scholar]
- Chiou S. K., Rao L., White E. Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol. 1994 Apr;14(4):2556–2563. doi: 10.1128/mcb.14.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Ewert D. L., Tsujimoto Y. Isolation and characterization of the chicken bcl-2 gene: expression in a variety of tissues including lymphoid and neuronal organs in adult and embryo. Nucleic Acids Res. 1992 Aug 25;20(16):4187–4192. doi: 10.1093/nar/20.16.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994 Feb;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet G., Michel D., Crisanti P., Guérin M., Herault Y., Pessac B., Calothy G., Brun G., Volovitch M. Serum factors and v-src control two complementary mitogenic pathways in quail neuroretinal cells in culture. Oncogene. 1993 Mar;8(3):565–574. [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guermah M., Gillet G., Michel D., Laugier D., Brun G., Calothy G. Down regulation by p60v-src of genes specifically expressed and developmentally regulated in postmitotic quail neuroretina cells. Mol Cell Biol. 1990 Jul;10(7):3584–3590. doi: 10.1128/mcb.10.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M. O., Horvitz H. R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994 Feb 25;76(4):665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol Cell Biol. 1990 Apr;10(4):1307–1318. doi: 10.1128/mcb.10.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A. R., Elmore M. A., Kellie S. Differential tyrosine-specific phosphorylation of integrin in Rous sarcoma virus transformed cells with differing transformed phenotypes. Oncogene. 1990 Sep;5(9):1349–1357. [PubMed] [Google Scholar]
- Jamal H. H., Cano-Gauci D. F., Buick R. N., Filmus J. Activated ras and src induce CD44 overexpression in rat intestinal epithelial cells. Oncogene. 1994 Feb;9(2):417–423. [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kaech S., Schnierle B., Wyss A., Ballmer-Hofer K. Myristylation and amino-terminal phosphorylation are required for activation of pp60c-src during mitosis. Oncogene. 1993 Mar;8(3):575–581. [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991 Feb;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971 May 21;28(3):391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971 May 21;28(3):421–441. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X., Drucker D. J. Parathyroid hormone-related peptide is a downstream target for ras and src activation. J Biol Chem. 1994 Mar 4;269(9):6263–6266. [PubMed] [Google Scholar]
- Lin E. Y., Orlofsky A., Berger M. S., Prystowsky M. B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993 Aug 15;151(4):1979–1988. [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Merino R., Ding L., Veis D. J., Korsmeyer S. J., Nuñez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994 Feb 1;13(3):683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D., Gillet G., Volovitch M., Pessac B., Calothy G., Brun G. Expression of a novel gene encoding a 51.5 kD precursor protein is induced by different retroviral oncogenes in quail neuroretinal cells. Oncogene Res. 1989;4(2):127–136. [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- O'Brien M. C., Fukui Y., Hanafusa H. Activation of the proto-oncogene p60c-src by point mutations in the SH2 domain. Mol Cell Biol. 1990 Jun;10(6):2855–2862. doi: 10.1128/mcb.10.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Luka J., Petti L., Sample J., Birkenbach M., Braun D., Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987 Sep;160(1):151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rösl F. A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 1992 Oct 11;20(19):5243–5243. doi: 10.1093/nar/20.19.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe H., Knudsen B., Okada M., Nada S., Nakagawa H., Hanafusa H. Molecular cloning and expression of chicken C-terminal Src kinase: lack of stable association with c-Src protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2190–2194. doi: 10.1073/pnas.89.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Senut M. C., Alvarado-Mallart R. M. Development of the retinotectal system in normal quail embryos: cytoarchitectonic development and optic fiber innervation. Brain Res. 1986 Sep;394(1):123–140. doi: 10.1016/0165-3806(86)90088-x. [DOI] [PubMed] [Google Scholar]
- Sternberg D. W., Scholz G., Fukui Y., Hanafusa H. Activation of a histone H1 kinase by tyrosine phosphorylation in v-src-transformed fibroblasts. EMBO J. 1993 Jan;12(1):323–330. doi: 10.1002/j.1460-2075.1993.tb05660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Taylor S. J., Shalloway D. The cell cycle and c-Src. Curr Opin Genet Dev. 1993 Feb;3(1):26–34. doi: 10.1016/s0959-437x(05)80337-5. [DOI] [PubMed] [Google Scholar]
- Vaux D. L. A boom time for necrobiology. Curr Biol. 1993 Dec 1;3(12):877–878. doi: 10.1016/0960-9822(93)90223-b. [DOI] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A., Lang A., Wyke A. W. Mitogenesis induced by pp60v-src is not accompanied by increased expression of immediate early response genes. Oncogene. 1990 Feb;5(2):161–169. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wood K. A., Dipasquale B., Youle R. J. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993 Oct;11(4):621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994 May 26;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- Yoon H., Boettiger D. Expression of v-src alters the expression of myogenic regulatory factor genes. Oncogene. 1994 Mar;9(3):801–807. [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992 Sep 24;359(6393):336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- den Hertog J., Pals C. E., Peppelenbosch M. P., Tertoolen L. G., de Laat S. W., Kruijer W. Receptor protein tyrosine phosphatase alpha activates pp60c-src and is involved in neuronal differentiation. EMBO J. 1993 Oct;12(10):3789–3798. doi: 10.1002/j.1460-2075.1993.tb06057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]