SUMMARY
Intracellular microRNAs (miRNAs) are key regulators of gene expression. The role of extracellular miRNAs in neuronal activation and sensory behaviors are unknown. Here we report an unconventional role of extracellular miRNAs for rapid excitation of nociceptor neurons via toll-like receptor-7 (TLR7) and its coupling to TRPA1 ion channel. miRNA-let-7b induces rapid inward currents and action potentials in dorsal root ganglion (DRG) neurons. These responses require the GUUGUGU motif, only occur in neurons co-expressing TLR7 and TRPA1, and are abolished in mice lacking Tlr7 or Trpa1. Furthermore, let-7b induces TLR7/TRPA1-dependent single channel activities in DRG neurons and HEK293 cells over-expressing TLR7/TRPA1. Intraplantar injection of let-7b elicits rapid spontaneous pain via TLR7 and TRPA1. Finally, let-7b can be released from DRG neurons by neuronal activation, and let-7b inhibitor reduces formalin-induced TRPA1 currents and spontaneous pain. Thus, secreted extracellular miRNAs may serve as novelpain mediatorsvia activating TLR7 /TRPA1in nociceptor neurons.
Keywords: DRG neurons, microRNAs, Toll-like receptor 7, TRPA1
INTRODUCTION
MicroRNAs (miRNAs) are 18–25 nucleotide noncoding RNAs and bind the 3′ untranslated regions of mRNAs to regulate gene expression post-transcriptionally (Bartel, 2004). miRNAs have been implicated in various biological roles including neurodegeneration (Eacker et al., 2009). Extracellular miRNAs are also present in circulating blood and cerebrospinal fluid in disease conditions (Lehmann et al., 2012) and binds toll-like receptor-7 (TLR7) (Fabbri et al., 2012). TLR7 is activated by single-strand RNAs to initiate innate immune responses via transcriptional regulation (Takeuchi and Akira, 2010). The miRNA lethal-7 (let-7), first discovered in the nematode as a key developmental regulator (Reinhart et al., 2000), is abundant and highly conserved across species (Pasquinelli et al., 2000). Interestingly, let-7 has been detected in the cerebrospinal fluid of patients with Alzheimer’s disease (Lehmann et al., 2012). Extracellular application of let-7b induces apoptosis of cortical neurons via activation of neuronal TLR7 and myeloid differentiation factor 88 (MyD88)-dependent transcriptional regulation of TNF-α (Lehmann et al., 2012). miRNAs were also found in nociceptive primary sensory neurons (nociceptors) of dorsal root ganglion (DRG) to regulate pain and the expression of sodium channels (Zhao et al., 2010). However, the unique role of secreted extracellular miRNAs in modulating the activation and excitability of sensory neurons and sensory behaviors is virtually unknown.
Recently, we have shown that TLR7 is expressed by small-diameter DRG neurons (Liu et al., 2010) that are responsible for the sensations of pain and itch (Basbaum et al., 2009; Liu and Ji, 2013; Woolf and Ma, 2007). Furthermore, the synthetic TLR7 ligands imiquimod and loxoribine induced rapid inward currents and action potentials in DRG nociceptor neurons (Liu et al., 2010). However, the endogenous ligands of TLR7 remain to be identified. In this study, we evaluated whether extracellular miRNAs could serve as TLR7 agonists to rapidly activate DRG neurons and elicit pain and further determined functional coupling between TLR7 and ion channels.
RESULTS
Specific miRNAs induce inward currents in DRG neurons via TLR7 and TRPA1
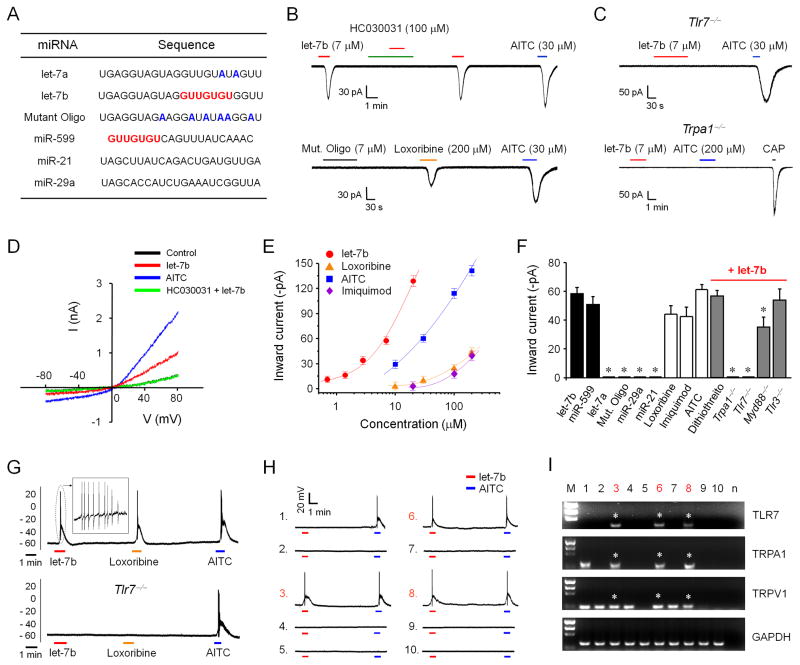
We first tested whether let-7b, which contains immune stimulatory GUUGUGU motif (Figure 1A) for the activation of TLR7 (Heil et al., 2004), would elicit electrophysiological responses in DRG neurons using patch-clamp recordings. Exposure of dissociated DRG neurons to let-7b, at the concentration of 7 μM (50 μg/ml), induced robust inward currents in small-diameter DRG neurons (<25 μm) of wild-type (WT) mice, and these currents were abolished in Tlr7−/− mice, indicating a TLR7-dependent action (Figures 1B and 1C). However, the mutant oligoribonucleotide (Mut. oligo) with reduced GU content (Figure 1A) (Lehmann et al., 2012) had no effects (Figures 1B).
Figure 1. let-7b induces inward currents and action potentials in small-sized DRG neurons via TLR7 and TRPA1.
(A) Sequences of miRNAs tested in this study. The GUUGUGU motif is underlined. In mutant oligonucleotides (Mut. Oligo) and let-7a, the mismatched nucleotides (compared with let-7b) are highlighted in blue. (B and C) Inward currents induced by let-7b, loxoribine, and AITC. Note that let-7b-induced inward currents are blocked by HC030031 and abolished in Tlr7−/− and Trpa1−/− mice. Mut. Oligo fails to evoke any currents. 15–25 neurons were recorded in each condition. (D) I/V curves elicited by let-7b (7 μM) and AITC (200 μM). n = 5 patches for each condition. let-7b and AITC elicit similar I/V curves with the reversal potential of 0 mV. let-7b-induced current is suppressed by HC030031 (100 μM). n = 5 neurons. (E) Dose-response curves showing the amplitudes of inward currents induced by let-7b, loxoribine, imiquimod, and AITC. n = 5–18 neurons. Note a left shift of the let-7b curve. (F) Summary of amplitudes of the inward currents under all the experimental conditions (n = 5–25 neurons). The concentrations are: 7 μM for let-7a, let-7b and Mutant Oligo, 8 μM for miR-599, 10 μM for miR-29a and miR21, 30 μM for AITC, 200 μM for loxoribine and imiquimod, and 5 mM for dithiothreitol. *P<0.05, compared to let-7b, n = 5–25 neurons. The results are mean ± SEM. (G) Action potentials induced by let-7b, loxoribine, and AITC in DRG neurons. n = 5–15 neurons. Note that let-7b-induced action potentials are eliminated in all 15 neurons from Tlr7−/− mice. (H and I) Current-clamp recording (H) and single-cell RT-PCR (I) in 10 small-sized DRG neurons. Note that let-7b induces action potentials only in TLR7-expressing neurons. Three neurons (#3, #6, and #8, highlighted in red) respond to let-7b (7 μM) with action potentials and also express Tlr7, Trpa1, and Trpv1 mRNAs (indicated with *). M, molecular weights; n, negative control. Asterisks indicate TLR7-positive neurons.
We next assed whether TRPA1, expressed by TRPV1-positive nociceptors (Liu et al., 2010; Story et al., 2003), is involved in miRNA-induced inward currents. We found all the let-7b-responsive neurons also responded to the TRPA1 agonist AITC (Jordt et al., 2004) (Figure 1B). Notably, let-7b-induced inward currents were completely blocked by the TRPA1 antagonist HC030031 (100 μM) and eliminated in Trpa1−/− mice (Figures 1 B and 1C). TLR7 agonist loxoribine (200 μM) also evoked inward currents (Figure 1B), which were abolished in Tlr7−/− and Trpa1−/− mice (data not shown).
I/V curve analysis further revealed that let-7b-evoked currents possess typical property of TRP channels: they are suppressed by HC030031 and have the reverse potential of 0 mV (Figure 1D). Dose-response curves of inward currents showed a left-shift of the let-7b-elicited curve, compare to that elicited by AITC, loxoribine, and imiquimod, suggesting that let-7b has the highest efficacy for TRPA1 activation. The lowest concentration of let-7b we tested to elicit inward current is 0.7 μM (Figure 1E). The amplitudes of inward currents induced by different miRNA and synthetic TLR7 ligands are summarized in Figure 1F.
We further assessed whether miRNA-induced neuronal activation is sequence-dependent. miR-599, which also contains the GUUGUGU motif (Figure 1A), elicited TLR7-dependent current (Figure S1A). By contrast, miR-29a and miR-21 (lacking the GUUGUGU motif, Figure 1A) had no effects (Figure 1F and Figure S1B). Of interest, let-7a (Figure 1A), a close family member of let-7b with a single nucleotide change in the GUUGUGU motif (GUUGUAU), failed to induce any inward current (Figure 1F and Figure S1C). Together, these results suggest that GU-rich core (GUUGUGU) is critically required for inducing miRNA-induced inward currents. A database search (http://www.mirbase.org/) indicated that there are only a few miRNAs of Homo sapiens and Mus musculus that contain the motif (Table S1).
let-7b induces action potentials in DRG neurons that co-express TLR7 and TRPA1
let-7b (7 μM) evoked marked action potentials in DRG neurons, which were completely blocked by HC030031 and eliminated in Tlr7−/−-deficient neurons (Figure 1G and Figure S2A). Loxoribine but not Mut. Oligo also induced action potentials in AITC-responsive neurons (Figure 1G, Figures S2A and S2B). These data suggest that miRNA let-7b also causes excitation of sensory neurons via activation of TLR7 and TRPA1.
To further define if TLR7 and TRPA1 are co-expressed in DRG neurons, we performed combined patch-clamp recording and single-cell PCR analysis (Liu et al., 2012a) in small-diameter DRG neurons. Around 30% of these neurons expressed TLR7, and all the TLR7-expressing neurons responded to let-7b and AITC with actions potentials (Figures 1H and 1I) and inward currents (Figures S3A–C). These results suggest a perfect match between the expression and function of TLR7 in DRG neurons. Double staining also confirmed co-localization of TLR7 and TRPA1 in DRG neurons (Figure S3D). Of note all the TLR7+ neurons contain TRPA1, which is expressed by the TRPV1+ DRG neurons (Figures 1H and 1I and Figure S3E), as previously reported (Story et al., 2003).
Intracellular signaling is not required for miRNA-induced activation of DRG neurons
MyD88 is an adaptor protein and critical for the conventional signaling of most TLRs including TLR7 and the transcription of inflammatory genes (Akira et al., 2006; Liu et al., 2012b). In Myd88 deficient DRG neurons, let-7b and AITC-induced inward currents were largely intact, although the amplitudes of currents were slightly reduced compared to that of WT neurons (Figure 1F and Figure S1D). Thus, MyD88 is not essentially required for the let-7b-induced currents. Moreover, exposure of DRG neurons to inhibitors of PKA, PKC, PLC, ERK, and G-proteins did not impair loxoribine-induced inward currents (Figure S1E), arguing against an involvement of intracellular signaling in the TLR7/TRPA1 interaction. Noxious compounds were shown to activate TRPA1 through covalent modification of cysteines (Macpherson et al., 2007). However, the reducing agent dithiothreitol (DTT) did not affect the let-7b-induced inward current (Figure S1F), suggesting a possible non-covalent interaction between TLR7 and TRPA1. Although TLR3 detects double-strand RNAs (Akira et al., 2006) and double-strand total RNAs from brain tissues induced inward currents in DRG neurons via TLR3 (Liu et al., 2012a), let-7b-induced inward currents are Tlr3-independent (Figure 1F).
let-7b induces TRPA1-mediated inward currents in HEK293 cells
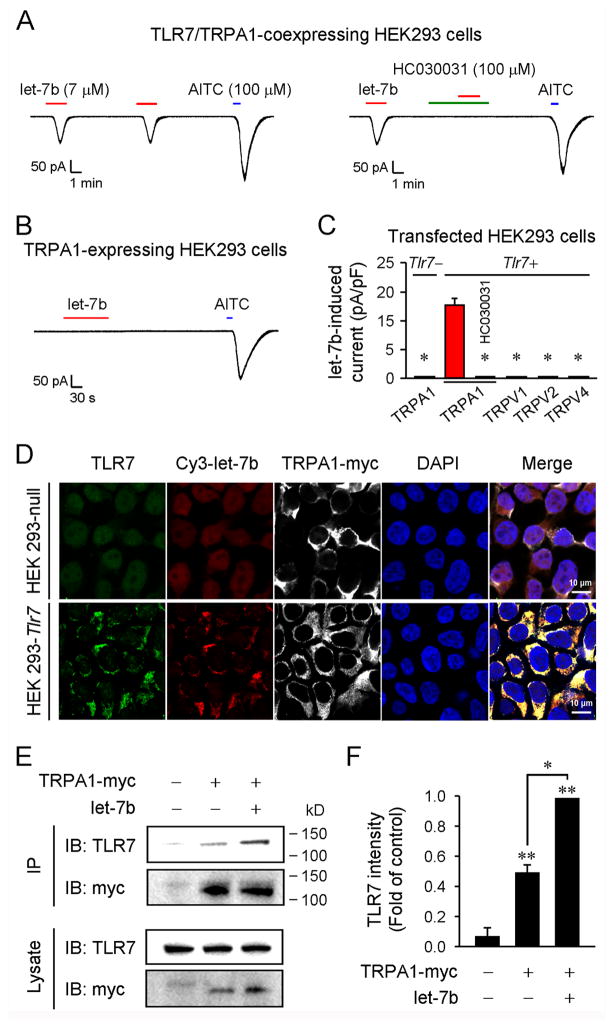
Can miRNA also activate TLR7 and TRPA1 in a heterologous cell system? To address this issue we tested the actions of miRNA in HEK293 cells over-expressing TLR7/TRPA1. let-7b evoked robust inward currents in these cells, which were completely blocked by HC030031 (Figure 2A). Notably, let-7b had no effect in HEK293 cells over-expressing TRPA1 alone (Figure 2B), confirming that TLR7 is essential for mediating the let-7b-induced currents in HEK293 cells. In contrast, TLR7 ligand failed to induce inward currents in HEK293 cells over-expressing TLR7/TRPV1, TLR7/TRPV2, or TLR7/TRPV4 (Figure 2C). Thus, TLR7 selectively interacts with TRPA1 (but not with TRPV1, TRPV2, and TRPV4) to generate TRPA1-mediated inward currents in a heterologous cell system.
Figure 2. let-7b binds TLR7, enhances TLR7/TRPA1 interaction, and induces TRPA1-mediated inward currents in HEK293 cells.
(A) Inward currents induced by let-7b (7 μM) and AITC (100 μM) in HEK293 cells over-expressing TLR7 and TRPA1. n = 15 cells. let-7b-induced current is blocked by HC030031 (100 μM) in HEK293 cells. n = 15 cells. (B) let-7b (7 μM) does not induce inward currents in HEK293 cells over-expressing TRPA1 alone. n = 15 cells. (C) Quantification of let-7b (7 μM)-induced inward currents in HEK293 cells transfected with Tlr7 and/or Trpa1, Trpv1, Trpv2, and Trpv4 cDNA. n = 15 cells. (D) Triple staining of Cy3-let-7b (Cy3), TLR7 (anti-TLR7), and TRPA1-myc (anti-myc) in Tlr7-transfected HEK293 cells and non-transfected Tlr7 null cells. The cell nuclei were labeled with DAPI. Scales, 10 μm. Note that cy3-let-7b only binds to TLR7-expressing cells. (E and F) Coimmunoprecipitation with anti-myc antibody shows TLR7 and TRPA1-myc bands from HEK293 cells co-expressing TLR7/TRPA1. Note that let-7b (7 μM, 5 min) increases the interaction of TLR7/TRPA1. F. Quantification of TLR7 bands. *P< 0.05, n = 3 separate cultures.
let-7b binds TLR7 and enhances TLR7/TRPA1 interaction in HEK293 cells
Next, we investigated whether fluorescence-labeled let-7b (Cy3-let-7b) can bind TLR7 in HEK293 cells. Immunocytochemistry showed surface and cytoplasm expression of TLR7 in Tlr7-transfected cells but not in Tlr7 null cells (Figure 2D). Notably, we only observed Cy3-let-7b binding on TLR7-expressing cells (Figure 2D). Triple staining of let-7b/TLR7/TRPA1 revealed co-localization of all three on the cell surface as well as in cytoplasm (Figure 2D), providing subcellular bases for TLR7/TRPA1 interaction. Co-immunoprecipitation with an anti-myc antibody in TLR7/TRPA1-myc-expressing HEK293 cells revealed both TRPA1-myc and TLR7 bands (Figures 2E and 2F), suggesting a biochemical interaction of TLR7 and TRPA1. Of interest, a brief stimulation with let-7b (7 μM, 5 min) significantly increased the interaction of TLR7/TRPA1, as indicated by increased intensity of TLR7 band (Figures 2E and 2F).
let-7b induces single channel activities of TRPA1 in DRG neurons and HEK293 cells
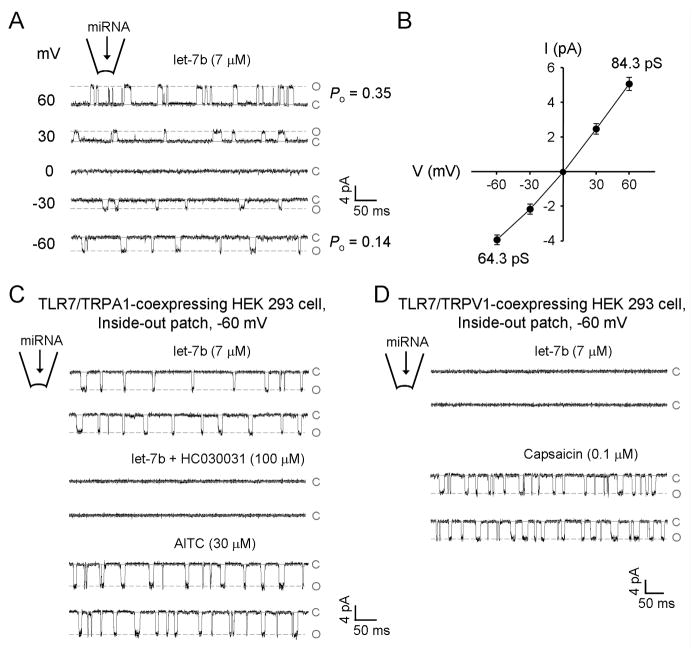
To further validate functional interaction of TLR7 and TRPA1 on the cell surface, we carried out single channel recordings in both DRG neurons and HEK293 cells. Inside-out patch recordings in DRG neurons showed that intra-pipette delivery of let-7b to the extracellular surface elicited voltage-dependent single-channel opening events, and the TRP channel property was validated by the I/V curve with the reversal potential = 0 mV (Figures 3A and 3B). Inside-out patch recordings in HEK293 cells over-expressing TLR7/TRPA1 also demonstrated that let-7b could elicit HC030031-senstive single channel activities (Figure 3C). Similarly, loxoribine induced single channel activities of TRPA1 (data not shown). In contrast, HEK293 cells over-expressing TLR7/TRPV1 only demonstrated single channel activities in response to capsaicin but not let-7b (Figure 3D). Together, these results suggest that let-7b could bind TLR7 on the extracellular surface to induce single channel activities of TRPA1.
Figure 3. let-7b induces single channel activities of TRPA1 in DRG neurons and heterologous HEK293 cells.
(A) Inside-out patch recordings in membrane excised from small-sized DRG neurons. let-7b, delivered through recording pipette, induces single channel currents. The probability of channel opening (Po) at +60 and −60 mV is 0.35 ± 0.07 and 0.14 ± 0.05, respectively. EGTA (1 mM) was included in the pipette solution to prevent desensitization and inactivation. c: closed state; o: open state. (B) I/V curve of let-7b-induced single channel activities in DRG neurons. The average single channel currents obtained in 1 min were plotted against voltages. (C) Inside-out patch recordings (−60 mV) in membrane excised from TLR7/TRPA1 over-expressing HEK293 cells. let-7b (7 μM, through recording pipette) induces single channel currents, which are blocked by HC030031. After washout, bath application of AITC (30 μM) also elicits single channel activities. (D) Single channel activities elicited by capsaicin (100 nM) but not by let-7b (7 μM) in inside-out patches from HEK293 cells over-expressing TLR7/TRPV1. n = 5 patches for each condition.
TLR7 is partially required for the function and surface expression of TRPA1 in DRG neurons
To further assess the interaction of TLR7 and TRPA1 in DRG neurons, we evaluated TRPA1 currents in WT and Tlr7−/− mice. Inside-out patch recordings in DRG neurons showed a significant reduction of TRPA1 single channel conductance and open probability in Tlr7-deficient neurons (Figures S4A and S4B). Immunocytochemistry also revealed a significant reduction of surface expression of TRPA1 in Tlr7-deficient DRG neurons (Figures S4C and S4D). As previously reported (Schmidt et al., 2009), AITC increased the surface expression of TRPA1 in WT mice. Both the basal and AITC-induced surface expression of TRPA1 were reduced in Tlr7−/− mice (Figures S4C and S4D). These data indicate that TLR7 can modulate both the activity and surface expression of TRPA1.
Endogenous let-7b release contributes to formalin-induced TRPA1 currents in DRG neurons
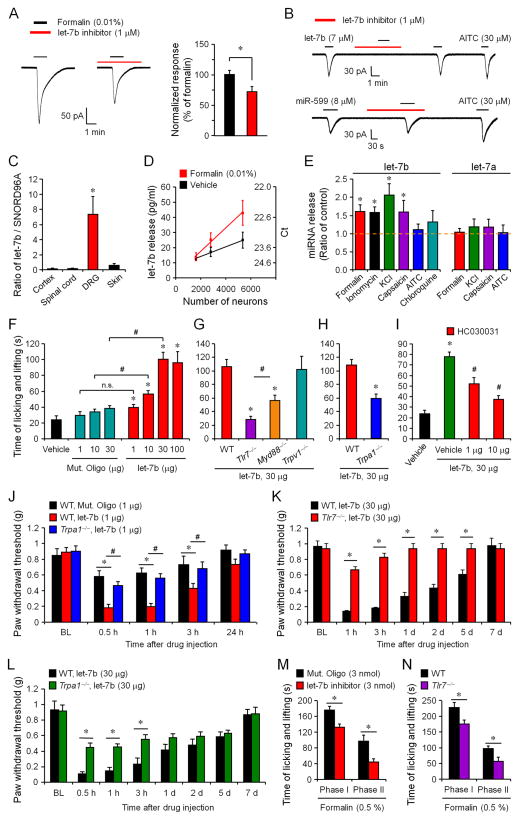
Does endogenous miRNA also play a role in modulating neuronal activity? As previously reported (McNamara et al., 2007), low concentration of formalin (0.01%) elicited TRPA1-mediated inward currents in DRG neurons (Figures 4A, S1G, and S1H). Intriguingly, the formalin-induced inward current was significantly inhibited by a let-7b inhibitor (1 μM, anti-mmu-let-7b-5p) (Figure 4A). As expected, let-7b inhibitor blocked the let-7b but not miR-599 induced currents (Figure 4B), validating a selective inhibition of let-7b.
Figure 4. Activity-dependent release of let-7b in DRG cultures and involvement of endogenous let-7b in TRPA1 function and inflammatory pain.
(A) Inhibition of formalin (0.01%)-induced inward currents by let-7b inhibitor (anti-mmu-let-7b-5p, 1 μM). Right, amplitude of formalin-induced currents after being normalized to control. *P<0.05, n = 12–15 neurons. (B) Blockade of let-7b- but not miR-599-induced currents in AITC-sensitive DRG neurons by let-7b inhibitor. n = 7–8 neurons. (C) let-7b expression levels in DRG, spinal cord, cortex, and skin tissues, assessed by quantitative RT-PCR and normalized to miRNA SNORD96A (a housekeeping miRNA). *P<0.05, compared to spinal cord, cortex, and skin, n = 3 mice. (D) let-7b release in DRG cultures at different neuronal densities before and after formalin treatment (0.01%, 30 min). let-7b levels in culture medium were assessed by quantitative RT-PCR and normalized to spike-in cel-miRNA-39. *P<0.05, two-way ANOVA, n = 3 cultures. (E) let-7b and let-7a release (fold of control) in DRG culture medium following 30 min treatment of formalin (0.01%), ionomycin (10 ng/ml), KCl (50 mM), capsaicin (1 μM), AITC (300 μM), or chloroquine (1 mM). *P<0.05, compared to control, n = 4–12 cultures. (F) let-7b-induced spontaneous pain at different doses. *P<0.05, vs. vehicle; #P<0.05, n.s., not significant. (G and H) Reduction of let-7b (30 μg)-induced spontaneous pain in Trpa1−/−, Tlr7−/−, and Myd88−/− mice but not in Trpv1−/− mice. *P < 0.05, vs. WT control; #P<0.05. (I) Dose-dependent inhibition of let-7b-evoked spontaneous pain by intraplantar HC030031 (1, 10 μg). *P<0.05, vs. let-7b vehicle (RNase free-water); #P<0.05, vs. HC030031 vehicle (5% DMSO, green bar). (J) Low-dose let-7b (1 μg) induced mechanical allodynia in WT mice and Trpa1−/− mice. *P<0.05; #P<0.05. (K and L) High-dose let-7b (30 μg) induced mechanical allodynia in WT mice and Tlr7−/− (K) and Trpa1−/− (L) mice. *P<0.05; #P<0.05. (M) Inhibition of formalin (0.5%) induced pain in Phase I (0–10 min) and Phase II (10–45 min) by intraplantar let-7b inhibitor (3 nmol). *P<0.05. (N) Formalin (0.5%) induced pain in Phase I (0–10 min) and Phase II (10–45 min) in WT and Tlr7−/− mice. *P<0.05. The data are mean ± SEM. n = 5–11 mice for behavioral studies in panels F–N.
let-7b is highly expressed in DRGs and released from DRG neurons by neuronal activity
To determine the expression of let-7b in different tissues, we employed quantitative RT-PCR developed by Qiagen (see Supplemental methods). Of interest let-7b levels in DRG tissue are much higher than that of spinal cord, cortex, and skin tissues (Figure 4C). To further quantify miRNA release in DRG cultures we introduced an exogenous miRNA, Caenorhabditis elegans miRNA-39 (cel-miRNA-39) as a spike-in control. The basal release of let-7b in DRG cultures is around 10–25 pg/ml (≈ 1.0 to 2.5 pM or 6x105-1.5x106 copies/μl, MW=7,133), depending on the density of the cultures (Figure 4D). This basal release is comparable to that of CGRP, a well-known pain mediator (Qin et al., 2008). Apparently, let-7b release in DRG cultures is much higher than that in CSF (5000 copy/μl)(Lehmann et al., 2012). Formalin (0.01%) induced a density-dependent increase in let-7b release (Figure 4D). Activation of C-nociceptors with capsaicin and neuronal depolarization with KCl also increased let-7b release, suggesting an activity-dependent release (Figure 4E). However, AITC and the pruritogen chloroquine had no significant effects, because these agents only activate a very small population of DRG neurons (Figure 4E). Moreover, let-7b release was induced by ionomycine, an inducer of intracellular Ca2+ increase (Figure 4E). The basal release of let-7a was also evident (≈ 40 pg/ml) in DRG cultures. However, let-7a release was not increased by formalin, KCl, and capsaicin (Figure 4E). It is suggested that different mechanisms are involved in the release of let-7a and let-7b. We also found a quick basal release of let-7b in hindpaw skin preparation: within 5 min the let-7b concentration reached to 3.0 ± 0.5 pM (n=6 mice).
Intraplantar injection of let-7b elicits pain in mice via TLR7 and TRPA1
Activation of small DRG neurons (nociceptors) is known to elicit pain (Basbaum et al., 2009; Caterina and Julius, 2001; Hucho and Levine, 2007; Woolf and Ma, 2007). TRPA1 is an ancient molecule for chemosensation (Kang et al., 2010) and activation of TRPA1 triggers pain in rodents (Bautista et al., 2006; Patapoutian et al., 2009; Story et al., 2003). We investigated if extracellular (intraplantar) delivery of miRNAs would induce pain-like behaviors in mice. Compared to Mut. Oligo, let-7b elicited rapid and transient (<5 min) spontaneous nocifensive pain in mice (Figure 4F), as revealed by licking and lifting behaviors of mice. These behaviors are dose-dependent, peaking at the dose of 30 μg (Figure 4F). Notably, let-7b-evoked nocifensive pain was abolished in Tlr7−/− mice, reduced in Trpa1−/− and Myd88−/− mice, but unaltered in Trpv1−/− mice (Figures 4F and 4G). Consistently, intraplantar pre-treatment of HC030031 dose-dependently suppressed let-7b-evoked spontaneous pain in wild-type mice (Figure 4I). Furthermore, at a low dose (1 μg, i.e. 9.3 μM in 15 μl), let-7b induced persistent (> 3 h) and TRPA1-dependent mechanical allodynia (Figure 4J). Strikingly, at the dose of 30 μg, let-7b induced long-lasting (> 5 d) mechanical allodynia, which was reduced in Tlr7−/− and Trpa1−/− mice (Figures 4K and 4L), as well as in Myd88−/− but not in Trpv1−/− mice (data not shown). Intraplantar pre-treatment of let-7b inhibitor reduced low-dose formalin (0.5%) induced spontaneous pain in both phases (Figure 4M), suggesting an essential role of endogenous let-7b in inflammatory pain. Low-dose formalin (0.5%)-induced inflammatory pain was also reduced in Tlr7−/− mice (Figure 4N). Thus, extracellular let-7b is both sufficient and required for inducing inflammatory pain via the activation of TLR7 and TRPA1.
DISCUSSION
We have identified a previously unrecognized action of extracellular miRNAs (e.g., let-7b) for direct activation and excitation of nociceptor neurons. let-7b induced rapid (within a minute) inward currents and actions potentials, and these effects require TLR7 but not TLR3. We also demonstrated that let-7b binds TLR7 on the surface of TLR7-expressing HEK293 cells. Notably, let-7b is more potent than the synthetic TLR7 agonists loxoribine and imiquimod in inducing inward currents in DRG neurons, and is also sufficient to induce TLR7-dependent inward currents in heterologous HEK293 cells. We found that miRNA-induced neuronal activation is sequence-dependent and requires the GUUGUGU motif. Thus, miR-599 but not miR-29a and miR-21 evoked TLR7-dependent inward currents. Strikingly, let-7a, a close family member of let-7b failed to induce any inward current, although both were shown to induce TLR7 and MyD88-dependent TNF-α release in immune cells (Lehmann et al., 2012). Thus, the extracellular actions of miRNAs are highly selective.
Another important finding of this study is we identified a novel functional interaction of TLR7 and TRPA1 in both DRG neurons and HEK293 cells. First, TLR7 is completely co-localized with TRPA1 in small TRPV1-expressing DRG neurons. Second, let-7b-induced inward currents (with reverse potential of 0 mV) and action potentials were abolished in Trpa1-deficient DRG neurons and blocked by the TRPA1 antagonist HC030031. In contrast, let-7b failed to evoke inward currents in HEK293 cells over-expressing TLR7/TRPV1, TLR7/TRPV2, and TLR7/TRPV4, supporting a selective interaction of TLR7 with TRPA1. Third, TLR7 and TRPA1 can be co-immunoprecipitated in HEK293 cells, and this biochemical interaction of TLR7/TRPA1 was enhanced in response to let-7b stimulation. Fourth, inside-out recordings in DRG neurons and HEK293 cells demonstrated that let-7b was sufficient to induce single channel activities of TRPA1 in these cells, supporting a functional interaction of TLR7/TRPA1 on the extracellular surface. Fifth, intracellular signaling (e.g., MyD88, PKA, PKC, PLC, and MAPK signaling) is dispensable for this interaction. Finally, TRPA1 single channel activities and surface expression were reduced in Tlr7-deficinet DRG neurons. Although all these lines of evidence support a functional interaction of TLR7 and TRPA1, further studies are needed to investigate protein-protein interaction between TLR7 and TRPA1.
Activation of TRPA1 in peripheral terminals of nociceptive neurons is known to evoke pain (Bautista et al., 2006; Patapoutian et al., 2009; Story et al., 2003). Intraplantar injection of let-7b also elicited rapid nocifensive pain via TLR7 and TRPA1 but not TRPV1. Moreover, let-7b induced persistent mechanical allodynia through TLR7 and TRPA1. Of interest let-7b is highly enriched in DRG tissues and endogenous let-7b can be released from DRG neurons in an activity-dependent manner. Although our findings point to an unconventional signaling of TLR7 via TRPA1, we should not rule out the role of MyD88, especially in the maintenance phase of persistent pain. We should also point out the discrepancy between the let-7b concentrations for eliciting physiological effects (μM) and the let-7b concentrations detected in DRG culture medium (pM). Such discrepancy was also found for CGRP (pM vs. μM) (Qin et al., 2008), a well-known pain mediator. Given the facts that (1) RNAs are generally unstable and (2) in vivo local concentrations of let-7b near axons should be much higher than that in culture medium, let-7b may reach physiological concentrations (μM) after tissue injury to activate TLR7 and TRPA1. Importantly, let-7b inhibitor significantly reduced formalin-induced inflammatory pain.
In summary, we have demonstrated an unconventional extracellular role of specific miRNAs with the GUUGUGU motif (e.g., let-7b) for direct activation and excitation of nociceptive sensory neurons to evoke pain via TLR7 and TRPA1. Thus, secreted miRNAs may represent a new class of pain mediators. Secreted let-7b was implicated as a biomarker for disease (Lehmann et al., 2012). Also, let-7b changes in DRGs and circulation were found in chronic pain conditions (Orlova et al., 2011; von et al., 2011; Zhao et al., 2010). Future studies are necessary to determine the tissue origins of miRNAs (e.g., let-7b) detected in circulation.
EXPERIMENTAL PROCEDURES
See a detailed description in the Supplemental Experimental Procedures.
Reagents
We purchased miRNAs from Integrated DNA Technologies, fluorescence-conjugated siRNA (Cy3-let-7b) from Sigma, and let-7b inhibitor from Qiagen.
Animals
Knockout mice lacking Tlr7, Tlr3, Trpa1, Trpv1, and Myd88 and corresponding WT control mice (B6129PF2/J background for Trpa1; C57BL/6 background for Tlr7, Tlr3, Trpv1, and Myd88) were obtained from Jackson Laboratories. All the knockout mice were viable and showed no developmental defects. Young mice (4–5 weeks) were used for electrophysiological studies in DRG neurons. Adult male mice (8–10 weeks) of knockout mice and corresponding wild-type control mice, as well as CD1 mice were used for behavioral and pharmacological studies. All the animal procedures were approved by the Institutional Animal Care & Use Committee of Duke University.
Whole-cell and inside-out single channel recordings
Whole-cell patch clamp recordings in DRG neurons (small sizes, <25 μm) and HEK293 cells were performed at room temperature using an Axopatch-200B amplifier. To record inward currents, voltage clamp was conducted at holding membrane potential of −70 mV. Inside-out patch clamp recordings were also performed at room temperature.
Pain behavior test
Intraplantar injection (10 μl) was made by a 27G needle. miRNA-evoked spontaneous pain was assessed by measuring the time (seconds) mice spent on licking, lifting, or flinching the affected paw in 5 min. All the behaviors were tested blindly.
Statistical analyses
All data were expressed as mean ± SEM and analyzed using student’s t-test (two groups) or One-Way ANOVA followed by post-hoc Bonferroni test. The criterion for statistical significance was P<0.05.
Supplementary Material
Acknowledgments
We thank Dr. Ardem Patapoutian for providing TRPA1 antibody, Dr. Sun Wook Hwang for providing Trpa1, Trpv1, Trpv2, and Trpv4 constructors, and Dr. Jorg Grandl for critical reading of the manuscript. This study was supported by NIH R01 grants DE17794, DE22743, and NS67686 to RRJ and NIH R21 grant NS82985 to ZZX. TB was supported by fellowships (PBLAP3-123417 and PA00P3-134165) from Switzerland.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, two supplemental tables, and four Supplemental figures.
None of the authors of this manuscript have a financial interested related to this work.
All the authors have no financial interests in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kalin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012a;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012b;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465:1671–1685. doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, Schwartzman RJ, Ajit SK. MicroRNA modulation in complex regional pain syndrome. J Transl Med. 2011;9:195. doi: 10.1186/1479-5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- von SD, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, Zhang L, Hu H, Kotnis S, Bingham B, Liu W, Whiteside GT, Samad TA, Kennedy JD, Ajit SK. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS ONE. 2011;6:e17670. doi: 10.1371/journal.pone.0017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhao J, Lee MC, Momin A, Cendan CM, Shepherd ST, Baker MD, Asante C, Bee L, Bethry A, Perkins JR, Nassar MA, Abrahamsen B, Dickenson A, Cobb BS, Merkenschlager M, Wood JN. Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci. 2010;30:10860–10871. doi: 10.1523/JNEUROSCI.1980-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.