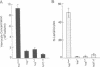Abstract
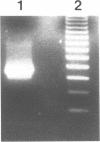
Proteins of the Jak family of non-receptor kinases play important roles in mammalian hematopoietic signal transduction. They mediate the cellular response to a wide range of cytokines and growth factors. A dominant mutation in a Drosophila Jak kinase, hopscotchTumorous-lethal (hopTum-l), causes hematopoietic defects. Here we conduct a molecular analysis of hopTum-l. We demonstrate that the hopTum-l hematopoietic phenotype is caused by a single amino acid substitution of glycine to glutamic acid at residue 341. We generate a true revertant of the hopTum-l mutation, in which both the molecular lesion and the mutant hematopoietic phenotype revert back to wild type. We also examine the effects of the G341E substitution in transgenic flies. The results indicate that a mutant Jak kinase can cause leukemia-like abnormalities.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. M., Lux A., Steller H., Krieger M. Macrophages in Drosophila embryos and L2 cells exhibit scavenger receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10375–10379. doi: 10.1073/pnas.89.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- BURNET B., SANG J. H. PHYSIOLOGICAL GENETICS OF MELANOTIC TUMORS IN DROSOPHILA MELANOGASTER. II. Genetics. 1964 Feb;49:223–235. doi: 10.1093/genetics/49.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binari R., Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994 Feb 1;8(3):300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Brasier A. R., Ron D., Tate J. E., Habener J. F. A family of constitutive C/EBP-like DNA binding proteins attenuate the IL-1 alpha induced, NF kappa B mediated trans-activation of the angiotensinogen gene acute-phase response element. EMBO J. 1990 Dec;9(12):3933–3944. doi: 10.1002/j.1460-2075.1990.tb07614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling A. D., Fincham V. J., Frame M. C., Haefner B., Wyke J. A. Mutations in v-Src SH3 and catalytic domains that jointly confer temperature-sensitive transformation with minimal temperature-dependent changes in cellular tyrosine phosphorylation. J Virol. 1994 Jul;68(7):4392–4399. doi: 10.1128/jvi.68.7.4392-4399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin H. O., Hanratty W. P. Characterization of a unique lethal tumorous mutation in Drosophila. Mol Gen Genet. 1976 Mar 30;144(3):345–347. doi: 10.1007/BF00341734. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Davis N., Ghosh S., Simmons D. L., Tempst P., Liou H. C., Baltimore D., Bose H. R., Jr Rel-associated pp40: an inhibitor of the rel family of transcription factors. Science. 1991 Sep 13;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. Transcriptional control of Drosophila fushi tarazu zebra stripe expression. Genes Dev. 1989 Mar;3(3):384–398. doi: 10.1101/gad.3.3.384. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Tripoulas N., Biggs J., Shearn A. Molecular consequences of awdb3, a cell-autonomous lethal mutation of Drosophila induced by hybrid dysgenesis. Dev Biol. 1988 Sep;129(1):169–178. doi: 10.1016/0012-1606(88)90171-6. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Muller O., Ziemiecki A., Mayeux P., Drucker B., Djiane J., Wilks A., Harpur A. G., Fischer S., Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994 Jun 1;13(11):2583–2591. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Drosophila extracellular matrix. Annu Rev Cell Biol. 1989;5:309–339. doi: 10.1146/annurev.cb.05.110189.001521. [DOI] [PubMed] [Google Scholar]
- Firmbach-Kraft I., Byers M., Shows T., Dalla-Favera R., Krolewski J. J. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990 Sep;5(9):1329–1336. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993 Sep 24;74(6):1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
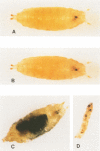
- Gateff E., Schneiderman H. A. Neoplasms in mutant and cultured wild-tupe tissues of Drosophila. Natl Cancer Inst Monogr. 1969 Jul;31:365–397. [PubMed] [Google Scholar]
- Gerttula S., Jin Y. S., Anderson K. V. Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal-ventral pattern formation. Genetics. 1988 May;119(1):123–133. doi: 10.1093/genetics/119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
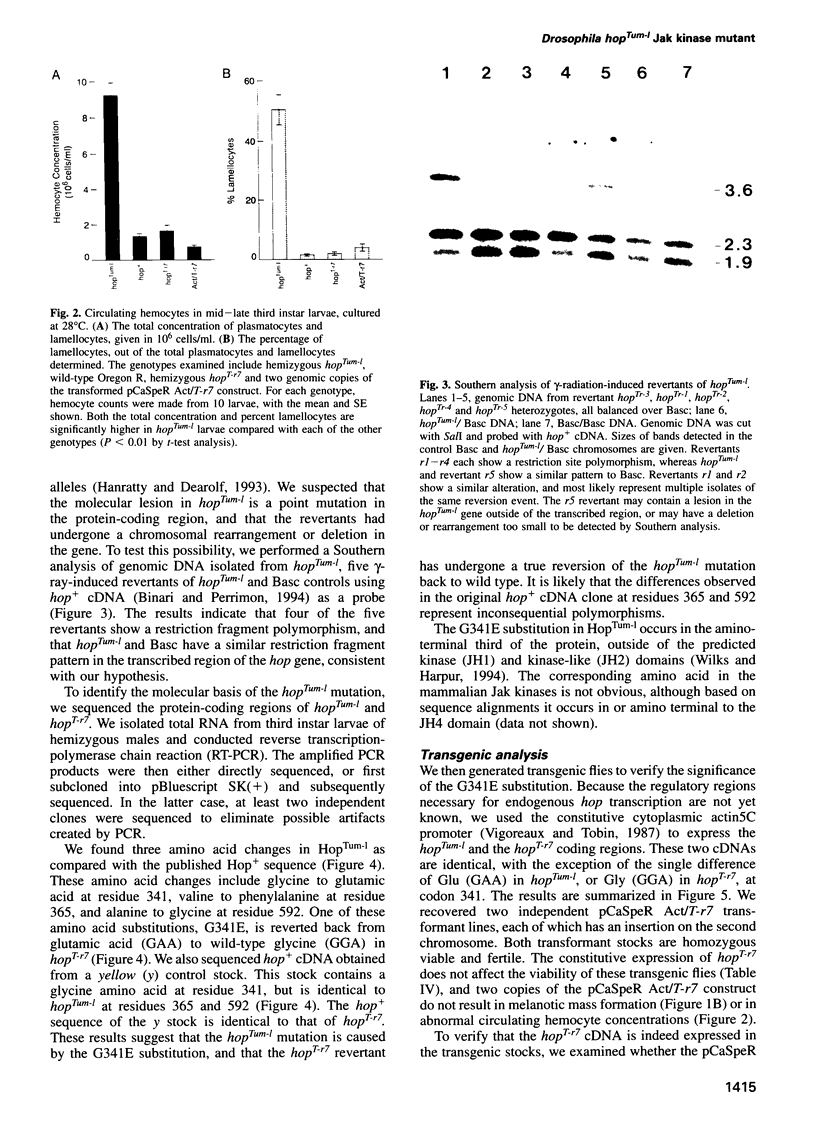
- Hanratty W. P., Dearolf C. R. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993 Apr;238(1-2):33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Hanratty W. P., Ryerse J. S. A genetic melanotic neoplasm of Drosophila melanogaster. Dev Biol. 1981 Apr 30;83(2):238–249. doi: 10.1016/0012-1606(81)90470-x. [DOI] [PubMed] [Google Scholar]
- Harpur A. G., Andres A. C., Ziemiecki A., Aston R. R., Wilks A. F. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992 Jul;7(7):1347–1353. [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Konrad L., Becker G., Schmidt A., Klöckner T., Kaufer-Stillger G., Dreschers S., Edström J. E., Gateff E. Cloning, structure, cellular localization, and possible function of the tumor suppressor gene lethal(3)malignant blood neoplasm-1 of Drosophila melanogaster. Dev Biol. 1994 May;163(1):98–111. doi: 10.1006/dbio.1994.1126. [DOI] [PubMed] [Google Scholar]
- Lefevre G., Jr Salivary chromosome bands and the frequency of crossing over in Drosophila melanogaster. Genetics. 1971 Apr;67(4):497–513. doi: 10.1093/genetics/67.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Nappi A. J., Silvers M. Cell surface changes associated with cellular immune reactions in Drosophila. Science. 1984 Sep 14;225(4667):1166–1168. doi: 10.1126/science.6433482. [DOI] [PubMed] [Google Scholar]
- Narazaki M., Witthuhn B. A., Yoshida K., Silvennoinen O., Yasukawa K., Ihle J. N., Kishimoto T., Taga T. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Huang Z. M. Interleukin-1-mediated enhancement of mouse factor B gene expression via NF kappa B-like hepatoma nuclear factor. Mol Cell Biol. 1990 Dec;10(12):6283–6289. doi: 10.1128/mcb.10.12.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Howell B. W., Broome M. A., Cooper J. A. Deletion of the SH3 domain of Src interferes with regulation by the phosphorylated carboxyl-terminal tyrosine. J Biol Chem. 1993 Aug 25;268(24):18070–18075. [PubMed] [Google Scholar]
- Perrimon N., Mahowald A. P. l(1)hopscotch, A larval-pupal zygotic lethal with a specific maternal effect on segmentation in Drosophila. Dev Biol. 1986 Nov;118(1):28–41. doi: 10.1016/0012-1606(86)90070-9. [DOI] [PubMed] [Google Scholar]
- Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994 Jul;14(7):4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki R. M., Rizki T. M. Basement membrane abnormalities in melanotic tumor formation of Drosophila. Experientia. 1974 May 15;30(5):543–546. doi: 10.1007/BF01926343. [DOI] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. Topology of the caudal fat body of the tumor-w mutant of Drosophila melanogaster. J Invertebr Pathol. 1974 Jul;24(1):37–40. doi: 10.1016/0022-2011(74)90161-x. [DOI] [PubMed] [Google Scholar]
- Schneider D. S., Hudson K. L., Lin T. Y., Anderson K. V. Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev. 1991 May;5(5):797–807. doi: 10.1101/gad.5.5.797. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers M., Hanratty W. P. Alterations in the production of hemocytes due to a neoplastic mutation of Drosophila melanogaster. J Invertebr Pathol. 1984 Nov;44(3):324–328. doi: 10.1016/0022-2011(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Sparrow J. C. The genetics of some second chromosome melanotic tumour mutants of Drosophila melanogaster. Genet Res. 1974 Feb;23(1):13–21. doi: 10.1017/s0016672300014622. [DOI] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Stewart M. J., Denell R. Mutations in the Drosophila gene encoding ribosomal protein S6 cause tissue overgrowth. Mol Cell Biol. 1993 Apr;13(4):2524–2535. doi: 10.1128/mcb.13.4.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga G., Fumagalli S., Koegl M., Courtneidge S. A., Draetta G. Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J. 1993 Jul;12(7):2625–2634. doi: 10.1002/j.1460-2075.1993.tb05923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Fessler L. I., Aziz A., Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994 Jul;120(7):1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Vigoreaux J. O., Tobin S. L. Stage-specific selection of alternative transcriptional initiation sites from the 5C actin gene of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1161–1171. doi: 10.1101/gad.1.10.1161. [DOI] [PubMed] [Google Scholar]
- WILSON L. P., KING R. C., LOWRY J. L. Studies on the tuW strain of Drosophila melanogaster. I. Phenotypic and genotypic characterization. Growth. 1955 Sep;19(3):215–244. [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Watson K. L., Johnson T. K., Denell R. E. Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev Genet. 1991;12(3):173–187. doi: 10.1002/dvg.1020120302. [DOI] [PubMed] [Google Scholar]
- Watson K. L., Konrad K. D., Woods D. F., Bryant P. J. Drosophila homolog of the human S6 ribosomal protein is required for tumor suppression in the hematopoietic system. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11302–11306. doi: 10.1073/pnas.89.23.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G. Cytokine signal transduction and the JAK family of protein tyrosine kinases. Bioessays. 1994 May;16(5):313–320. doi: 10.1002/bies.950160505. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I T. Two New Hereditary Tumors in Drosophila. Genetics. 1924 Jul;9(4):343–362. doi: 10.1093/genetics/9.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yin T., Yasukawa K., Taga T., Kishimoto T., Yang Y. C. Identification of a 130-kilodalton tyrosine-phosphorylated protein induced by interleukin-11 as JAK2 tyrosine kinase, which associates with gp130 signal transducer. Exp Hematol. 1994 May;22(5):467–472. [PubMed] [Google Scholar]
- Zinyk D. L., McGonnigal B. G., Dearolf C. R. Drosophila awdK-pn, a homologue of the metastasis suppressor gene nm23, suppresses the Tum-1 haematopoietic oncogene. Nat Genet. 1993 Jun;4(2):195–201. doi: 10.1038/ng0693-195. [DOI] [PubMed] [Google Scholar]