Abstract
Introduction and hypothesis
Bony pelvis dimensions have been shown to differ in women with and without pelvic floor dysfunction. The goal of this study was to determine whether bony pelvis dimensions are different when comparing women with severe bilateral levator ani defects (LAD) with those with normal muscles.
Methods
This is a secondary analysis of a case–control study comparing women with and those without pelvic organ prolapse. Subjects underwent pelvic organ prolapse quantification (POP-Q) examination and were classified as either having prolapse or being normal. All underwent pelvic magnetic resonance imaging (MRI). Levator defects were assessed based on the muscles’ appearance on imaging and subjects were stratified into two groups—women with normal muscles (n=99) and women with severe bilateral LAD (n=50). Bony pelvis dimensions were measured via MRI pelvimetry. The subpubic angle, interspinous and intertuberous diameters, and the sacrococcygeal joint-to-infrapubic point (SCIPP) lengths were compared.
Results
Both groups had similar demographics. The SCIPP length was 2.5 % (3 mm) shorter in women with severe LAD than in those without defects (P=0.02). The SCIPP measured 4 % (5 mm) less in women with prolapse and severe LAD than in subjects with prolapse but normal muscles (P=0.01). Logistic regression identified SCIPP length and history of forceps delivery as being independent predictors of severe bilateral LAD.
Conclusions
Severe bilateral LAD are associated with shorter SCIPP length and forceps-assisted vaginal delivery.
Keywords: Bony pelvis, Levator ani defects, Magnetic resonance imaging, Pelvic floor disorders, Pelvic organ prolapse
Introduction
Levator ani defects (LAD) have been associated with pelvic floor dysfunction, including pelvic organ prolapse and fecal incontinence [1, 2]. Vaginal delivery is a well-characterized risk factor for levator ani avulsion injuries; however, relatively few demographic and obstetric characteristics have been identified as being associated with LAD, including older maternal age, use of epidural anesthesia, and forceps assistance [3, 4].
One possible set of maternal characteristics that may be associated with the development of LAD is the size of the bony pelvis. The bony pelvis has historically been assessed by obstetric providers because of its effects on birth mechanics [5]. It has previously been demonstrated that there are differences in bony pelvis dimensions when comparing women with and without pelvic floor dysfunction [6–8]. It is therefore plausible that variations in bony pelvic dimensions may pose as risk factors for delivery-induced levator ani trauma, and hence, pelvic floor dysfunction. Our understanding of these possible effects is limited by the fact that previous studies often involved comparisons of non-demographically matched groups. The differences in pelvic dimensions may therefore reflect differences in race/ethnicity, rather than true associations with pelvic floor dysfunction [9]. It is not known whether bony pelvis dimensions are related to the presence or absence of LAD that might partially mediate the increased occurrence of pelvic floor disorders. The goal of this study was to investigate whether levator ani injuries are associated with bony pelvis dimensions at the level of pelvic floor attachments.
Materials and methods
This is a secondary analysis of an institutional review board-approved case–control study examining levator ani defects in women with clinically diagnosed pelvic organ prolapse and in women with normal pelvic support (University of Michigan Institutional Review Board IRBMED #HUM00043445). Results from the parent study have been published previously [10]. Subjects self-completed questionnaires, including questions about demographics and obstetric and gynecological history. All subjects underwent clinical examination, including pelvic organ prolapse quantification (POP-Q) examination in a semi-recumbent lithotomy position [11]. In the parent study, subjects were classified as cases (with pelvic organ prolapse) if any POP-Q point was measured at least 1 cm beyond the hymen. Controls were identified as subjects with all POP-Q points at least 1 cm above the hymen and who had no demonstrable stress urinary incontinence. The original groups were selected to be of similar age, race, and parity by matching the controls to women already enrolled as cases. The controls were selected if they were within 5 years of the age of a case, if they were of the same race, and if they had similar parity (within one).
All subjects underwent pelvic magnetic resonance imaging (MRI). Complete details of MRI acquisition in these groups have been published previously [10], and include acquisition of 5-mm spaced fast-spin proton density images in the axial, coronal, and sagittal planes in the supine position using a 1.5-T superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI, USA). Levator ani defects were graded on MR scans by two independent investigators blinded to the prolapse status [12]. The right and left muscles were each graded separately, with possible scores 0 (no defect), 1 (less than half of the muscle was missing), 2 (more than half of the muscle was missing), or 3 (complete loss of muscle bulk). The total levator ani defect score was the sum of the scores from each side, with totals ranging from 0 to 6. For this analysis, women were included if they had no levator ani defects (total LAD score 0) or severe bilateral muscle defects (defined as a total LAD score of 5 or 6). Women with LAD scores of 1 to 4 were excluded. All subjects included in this analysis had previously undergone vaginal delivery.
For each subject with digital MRIs available demonstrating all the relevant anatomical landmarks, the interspinous diameter (Fig. 1a), intertuberous diameter (Fig. 1b), subpubic angle (Fig. 1b), and sacrococcygeal joint-to-the-inferior pubic point (SCIPP) distance (Fig. 1c) [13] were measured. These bony dimensions were chosen to reflect boundaries and attachment sites of the pelvic floor musculature. All measurements were made independently by two authors (M.B.B. and S.K.D.) using ImageJ 1.42q software (National Institutes of Health, Bethesda, MD, USA), and the average value of each pelvic dimension was used for analyses. The SCIPP lengths were measured on bony midsagittal slices by drawing a line from the arcuate pubic ligament that forms the caudal end of the pubic symphysis to the inferior–ventral aspect of the periosteum of the sacrococcygeal joint. The length of this line was calculated using ImageJ 1.42q software.
Fig. 1.
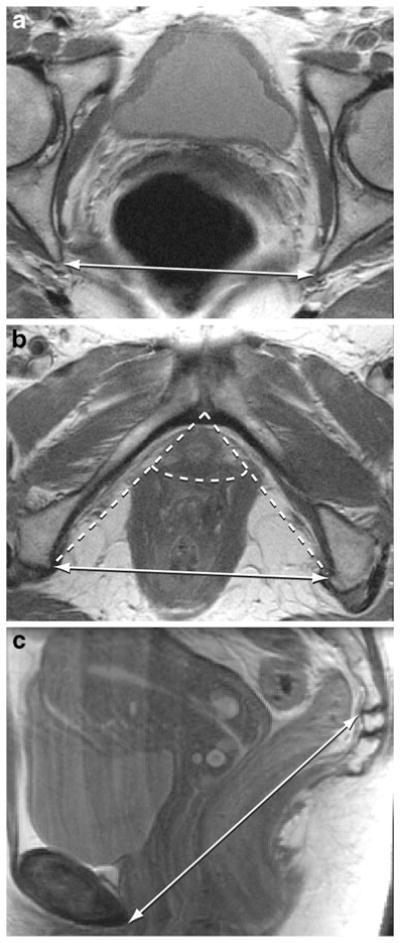
a Axial pelvic magnetic resonance imaging (MRI) slice demonstrating the interspinous diameter (double-headed arrow). b Axial pelvic MRI slice illustrating the intertuberous diameter (solid double-headed arrow) and subpubic angle (dashed lines). c Sagittal pelvic MRI slice demonstrating the sacrococcygeal joint-to-infrapubic point (SCIPP) line (double-headed arrow)
Since an individual subject may have asymmetric anatomy, and the long axis of the subject is not always perpendicular to the axial plane of the MR images, the relevant landmarks for determination of the interspinous and intertuberous diameter lengths and the subpubic angle may not be visualized on the same axial image. We therefore identified the (x, y, z) coordinates for the infrapubic point, the ischial spines, and the ischial tuberosities on axial images, and then calculated the length or angle between them using geometric functions. The infrapubic point was identified as the midline point at the inferior edge of the arcuate pubic ligament on the first image with right-to-left continuity of the ligament while scrolling through the images in a caudad-to-cephalad direction [14]. The coordinates of the ischial spines were identified at the medial aspect of the periosteum at the level of maximal insertion of the sacrospinous ligaments into the spines. The ischial tuberosities were similarly marked at the medial aspect of the periosteum at the medial tip of the insertion site of the sacrotuberous ligaments into the tuberosities, selecting the first image in which the insertion site appears when scrolling through the images from a cephalad-to-caudad direction. Distances between points were calculated using the Pythagorean theorem, and the sub-pubic angle was calculated as the angle between the two lines generated by connecting the infrapubic point to each ischial tuberosity; the angle was calculated using the geometric law of cosines.
We used published differences in pelvic dimensions in women with and without pelvic floor disorders (PFD) as a proxy to calculate an estimate of our study’s power, because data concerning the measures of interest in women with and without severe bilateral levator ani defects were not available. Handa et al., identified statistically significant differences in both the interspinous and intertuberous diameter lengths (amongst other pelvic measurements) in women with pelvic floor disorders compared with women without PFD [6]. Using the results from the Handa study and the sample sizes available in our sample set, we estimate that we have 95.0–99.3 % power with a 95 % two-sided confidence interval to detect similar magnitude differences in the interspinous and intertuberous diameters in our subjects, as measured by Handa and colleagues.
Comparisons were made using the independent Student’s t test or Mann–Whitney U test for continuous variables, and the Chi-squared test for categorical variables. Inter-rater reliability was assessed using intraclass correlation coefficients. PASW version 18.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis, and OpenEpi version 2.3.1. [15] was used for power calculations. P values less than 0.05 were considered statistically-significant.
Results
Of the vaginally parous women in our parent study, there were 99 subjects with no levator ani defects, and 50 subjects with severe bilateral LAD for whom MRIs were available with the relevant bony landmarks visible. Demographics of these two groups were similar (Table 1). However, as levator ani defects are so highly associated with pelvic organ prolapse [10, 16], the proportion of subjects with prolapse in the severe bilateral LAD group was more than 3-fold higher than that in the group with normal muscles (Table 1). This is further reflected by the significantly higher values of POP-Q measurements, i.e., more descent, in the anterior, apical, and posterior vaginal wall compartments in the subjects with severe bilateral LAD than in those with no defects (Table 1).
Table 1.
Demographics and vaginal topography—women with normal muscles and women with severe bilateral levator ani defects
| Characteristic | Normal muscles (n=99) | Severe bilateral LAD (n=50) | P value |
|---|---|---|---|
| Age (years) | 54.8±12.1 | 55.4±12.5 | 0.79 |
| BMI (kg/m2) | 26.8±5.0 | 25.8±5.0 | 0.26 |
| Height (inches) | 64.2±2.6 | 64.4±3.3 | 0.66 |
| Vaginal parity | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.54 |
| Caucasian (%) | 92.8 (90/97) | 94 (47) | 1.00 |
| Forceps delivery (%) | 25.9 (22/85) | 74.4 (32/43) | <0.001 |
| Cesarean delivery (%) | 7.1 (7/98) | 4.0 (2) | 0.72 |
| Weight of heaviest baby (gm) | 3,585±588 | 3,760±396 | 0.06 |
| Hysterectomy (%) | 13.3 (13/98) | 16.0 (8) | 0.63 |
| Postmenopausal (%) | 63.2 (60/95) | 66.7 (32/48) | 0.72 |
| Taking ERT (%) | 18.8 (18/96) | 24.5 (12/49) | 0.52 |
| Prolapse (%) | 31.3 (31) | 98 (49) | <0.001 |
| POP-Q point Aa (cm) | −1.0 (−2.0, −1.0) | 0 (0, 1.0) | <0.001 |
| POP-Q point Ba (cm) | −1.0 (−2.0, −1.0) | 2.0 (0, 3.0) | <0.001 |
| POP-Q point C (cm) | −6.0 (−7.0, −5.0) | −1.0 (−4.0, 3.0) | <0.001 |
| POP-Q point Ap (cm) | −2.0 (−2.0, −1.0) | 0 (−2.0, 1.0) | 0.005 |
| POP-Q point Bp (cm) | −2.0 (−2.0, −1.0) | 0 (−2.0, 1.0) | 0.001 |
All data are presented as mean ± standard deviation, median (25th percentile, 75th percentile), or percentage (number of subjects). Denominators are presented in the number of subjects if there are missing data
Forceps delivery was defined as the percentage of women having had at least one forceps-assisted vaginal delivery
Cesarean delivery was defined as the percentage of women having had at least one Cesarean delivery
LAD levator ani defects, BMI body mass index, ERT estrogen replacement therapy, POP-Q Pelvic Organ Prolapse Quantification
Inter-rater reliability for all bony pelvic dimension measurements was high, with intraclass correlation coefficients (ICCs) for the various pelvic measurements ranging from 0.975 to 0.995. P s (using average measures) for all of these ICCs were less than 0.001.
Bony pelvis measurements from the group with normal muscles and the group with severe bilateral LAD are presented in Table 2. The only pelvic dimension for which there is a statistically significant difference is the length of the SCIPP line, which is 2.5 % (3 mm) shorter in women with severe levator ani defects than in women with normal muscles.
Table 2.
Bony pelvis dimensions from all subjects, comparing women with normal muscles and women with severe bilateral levator ani defects
| Bony pelvis measurement | Normal muscles (n=99) | Severe bilateral LAD (n=50) | P |
|---|---|---|---|
| Interspinous diameter (cm) | 10.7±0.8 | 10.6±0.7 | 0.77 |
| Intertuberous diameter (cm) | 11.2±1.0 | 11.1±0.8 | 0.40 |
| Subpubic angle (°) | 89.5±7.4 | 88.2±7.2 | 0.31 |
| SCIPP length (cm) | 11.9±1.0 | 11.6±0.7 | 0.02 |
All data are presented as mean ± standard deviation
LAD levator ani defects, SCIPP sacrococcygeal joint-to-the-inferior pubic point
Since LAD are so highly associated with pelvic organ prolapse, we separately analyzed bony pelvis dimensions only in the subset of subjects (both with normal muscles and with severe bilateral LAD) with clinically diagnosed prolapse (Table 3). There were 31 subjects with normal muscle morphology who had prolapse, and 49 subjects in the group with severe bilateral levator ani defects. Again, the only pelvic dimension for which there was a statistically significant difference between the two groups is the length of the SCIPP line, which is approximately 4 % (5 mm) shorter in the women with severe muscle defects, compared with those with normal levator ani appearance. A similar analysis looking at the subset of women with normal pelvic support (controls) could not be meaningfully performed as there was only one subject with normal support and severe bilateral LAD.
Table 3.
Bony pelvis dimensions from subjects with prolapse, comparing women with normal muscles and women severe bilateral levator ani defects
| Bony pelvis measurement | Normal muscles (n=31) | Severe bilateral LAD (n=49) | P |
|---|---|---|---|
| Interspinous diameter (cm) | 10.7±0.9 | 10.6±0.7 | 0.44 |
| Intertuberous diameter (cm) | 11.5±1.1 | 11.1±0.8 | 0.09 |
| Subpubic angle (degrees) | 90.6±8.2 | 88.2±7.3 | 0.19 |
| SCIPP length (cm) | 12.1±0.9 | 11.6±0.7 | 0.01 |
All data are presented as mean ± standard deviation
LAD levator ani defects, SCIPP sacrococcygeal joint-to-the-inferior pubic point
Logistic regression was used to identify characteristics that could plausibly play a role in the development of severe bilateral levator ani defects. We first analyzed demographics, obstetric and gynecological factors, and bony pelvic measurements in univariate analyses (Table 4). The only variables with statistically significant associations with the presence of severe bilateral LAD are a history of forceps-assisted vaginal delivery and the length of the SCIPP line. Multivariate logistic regression was then performed, with the final model shown in Table 4. This model further confirmed that the length of the SCIPP line is associated with severe muscle injuries, even after controlling for forceps delivery.
Table 4.
Logistic regression models predicting severe bilateral levator ani defects
| Independent variable | Odds ratio | 95 % CI | Regression coefficient | Standard error | P |
|---|---|---|---|---|---|
| Univariate analyses | |||||
| Age (years) | 1.00 | 0.98–1.03 | 0.004 | 0.01 | 0.78 |
| Vaginal parity | 0.92 | 0.73–1.16 | −0.08 | 0.12 | 0.49 |
| Height (inches) | 1.03 | 0.91–1.16 | 0.03 | 0.06 | 0.66 |
| BMI (kg/m2) | 0.96 | 0.89–1.03 | −0.04 | 0.04 | 0.26 |
| Forceps delivery | 8.33 | 3.60–19.29 | 2.12 | 0.43 | <0.001 |
| Cesarean delivery | 0.54 | 0.11–2.71 | −0.61 | 0.82 | 0.46 |
| Weight of heaviest baby (gm) | 1.00 | 1.00–1.00 | 0.001 | <0.001 | 0.06 |
| Hysterectomy | 1.25 | 0.48–3.24 | 0.22 | 0.49 | 0.65 |
| Postmenopausal | 1.17 | 0.56–2.42 | 0.15 | 0.37 | 0.68 |
| Taking ERT | 1.41 | 0.61–3.22 | 0.34 | 0.42 | 0.42 |
| Interspinous diameter (cm) | 0.99 | 0.95–1.04 | −0.007 | 0.02 | 0.78 |
| Intertuberous diameter (cm) | 0.99 | 0.95–1.02 | −0.01 | 0.02 | 0.43 |
| Subpubic angle (degrees) | 0.98 | 0.93–1.02 | −0.02 | 0.02 | 0.31 |
| SCIPP length (cm) | 0.96 | 0.92–1.00 | −0.05 | 0.02 | 0.03 |
| Multivariate analysis | |||||
| Intercept | – | – | 5.98 | 2.99 | 0.05 |
| Forceps delivery | 10.70 | 4.27–26.81 | 2.37 | 0.47 | <0.001 |
| SCIPP length (cm) | 0.94 | 0.89–0.99 | −0.07 | 0.03 | 0.01 |
Forceps delivery is coded as 1 if the subject had at least one forceps-assisted vaginal delivery, 0 if she never had a forceps delivery
Cesarean delivery is coded as 1 if the subject had at least one Cesarean delivery, 0 if she never had a Cesarean section
C.I. confidence interval, BMI body mass index, POP-Q Pelvic Organ Prolapse Quantification, ERT estrogen replacement therapy, SCIPP sacrococcygeal joint-to-the-inferior pubic point
Discussion
The results of this study show that the anterior-to-posterior bony pelvis dimension, as measured by the SCIPP length, is shorter in women with severe bilateral levator ani defects than in women with normal pelvic floor muscles. This observation holds true whether analyzing all subjects or only women with pelvic organ prolapse. We are unable to detect differences in the remainder of the bony pelvis dimensions comparing women with severe bilateral LAD and those with no levator defects.
There are two conceptual models with which the association between bilateral LAD and a shorter SCIPP length might be explained: first, levator ani avulsions result in changes to the bony pelvis, and second, shorter anterior-to-posterior (A-P) dimension of the bony pelvis predisposes women to birth trauma resulting in severe bilateral LAD. As our case–control study design does not allow for causal determination, we cannot definitively prove or disprove either hypothesis. However, we favor the latter postulate, i.e., a shorter SCIPP distance increases a woman’s risk of birth-related levator ani trauma. On average, a shorter pelvis would be associated with shorter muscles. For a given fetal size, the shorter the muscle length before delivery, the more it will have to stretch to allow for birth to occur. It is certainly possible that a short A-P pelvic dimension predisposes women to difficult delivery, and thereby increases the frequency with which they are delivered with forceps assistance, a known factor causing LAD. However, the results from our multivariate logistic regression suggest that a shorter SCIPP length might not lead to severe bilateral LAD solely by increasing women’s risk of operative vaginal delivery. Furthermore, our prior work reveals that levator injury occurs in the portion of the muscle that undergoes the greatest degree of stretch [17]. It is therefore teleologically plausible that a shorter anterior-to-posterior pelvic dimension would result in greater stretch of the levator ani during vaginal delivery, thereby predisposing to muscle injuries. By contrast, the normal role of the levator ani musculature is to maintain constant tension to compress the pelvic organs in a caudad-to-cephalad direction [18]. If major avulsion injuries of these muscles were to result in bony pelvis changes, they would therefore likely cause lengthening of the SCIPP line, compared with the shorter distance seen in our analysis.
Despite the strong association between levator ani defects and pelvic organ prolapse [10, 16], and the findings in this study that a shorter SCIPP distance is associated with severe bilateral levator ani defects, several published studies suggest that anterior-to-posterior bony pelvis dimensions might be similar in women with pelvic organ prolapse and those with normal support [19, 20]. At this time, it is not clear why a shorter SCIPP length would predispose to severe bilateral levator ani defects, but not pelvic organ prolapse. One possibility is that we are only looking at the extremes of muscle morphology, whereas the aggregate of women with prolapse fall within a spectrum of levator ani defects [10, 21]. Furthermore, the development of pelvic organ prolapse involves a complex interplay of genetics, tissue biochemistry, and pelvic floor muscle function [22–24]. It is therefore likely that risk factors for levator ani injury may not always prove to play similar roles in the development of prolapse. However, it is becoming increasingly clear that levator ani defects may play a role in pelvic floor structure and function, irrespective of pelvic organ prolapse [25].
There are several strengths to this study, including large sample sizes, availability of high resolution images for measurement from women with known symptom status and assessment of pelvic organ support on physical examination, minimization of confounders through demographic matching, the use of objective measures of levator ani defects with high inter-rater reliability, and the use of control subjects who were all healthy, asymptomatic volunteers rather than women seeking care for other reasons. Limitations of the study include the case–control design, which prevents the ability to determine causality or temporal changes, the relative racial homogeneity of our subject pool, which may limit the generalizability of the findings, the risk of recall bias for obstetric and gynecological factors, inherent limitations of image analysis, and the small magnitude of the differences in the bony pelvic dimensions identified. Our study was not powered to detect smaller differences between measurements, but we would wonder whether such small differences would be clinically significant. Furthermore, the magnitude of the differences in pelvic dimensions we identified is similar to what has been shown in other studies, suggesting that this might be a widely reproducible finding [7, 26, 27]. We acknowledge that this study may be limited by spectrum bias as we only evaluated women at the extremes of LAD scores. Future studies evaluating associations between pelvic dimensions and the entire range of LAD scores would be helpful in better characterizing these relationships.
Conclusion
The results of this study suggest the hypothesis that a shorter anterior-to-posterior bony pelvis dimension might be associated with an elevated risk of traumatic birth injury. These findings supplement the growing body of evidence that pelvic shape and size are associated with the development of pelvic floor disorders [6–8, 19, 26–28]. Although these studies utilized a variety of imaging techniques to assess pelvic dimensions, and a wide range of pelvic floor disorders were investigated, they collectively highlight the influence that the bony pelvis may have not only on childbirth, but with lasting effects throughout a woman’s lifetime.
Acknowledgments
We gratefully acknowledge support from United States National Institutes of Health grants NICHD R01 HD038665 and ORWH P50 HD044406.
Footnotes
Data in this manuscript were presented as oral posters at the 36th Annual Meeting of the International Urogynecological Association, Lisbon, Portugal, on 30 June 2011 and at the 32nd Annual Scientific Meeting of the American Urogynecologic Society, Providence, RI, USA, on 15 September 2011.
Conflicts of interest Dr Doumouchtsis has received travel grants from Pfizer and Astellas. The Pelvic Floor Research Group, of which Dr DeLancey is the director, receives research support from American Medical Systems, Johnson & Johnson, Kimberly Clark, and Proctor & Gamble through the University of Michigan. No conflicts of interest were declared for Dr Berger.
Contributor Information
Mitchell B. Berger, Email: mitcberg@umich.edu, Pelvic Floor Research Group, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA. L4100 Women’s Hospital, 1500 E. Medical Center Drive, SPC 5276, Ann Arbor, MI 48109-5276, USA
Stergios K. Doumouchtsis, Department of Obstetrics and Gynecology, St. George’s University of London, London, UK
John O. DeLancey, Pelvic Floor Research Group, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA
References
- 1.Heilbrun ME, Nygaard IE, Lockhart ME, Richter HE, Brown MB, Kenton KS, Rahn DD, Thomas JV, Weidner AC, Nager CW, Delancey JO. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. Am J Obstet Gynecol. 2010;202(5):488. e481–486. doi: 10.1016/j.ajog.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwertner-Tiepelmann N, Thakar R, Sultan AH, Tunn R. Obstetric levator ani muscle injuries—current status. Ultrasound Obstet Gynecol. 2011;39(4):372–383. doi: 10.1002/uog.11080. [DOI] [PubMed] [Google Scholar]
- 3.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG. 2010;117(12):1485–1492. doi: 10.1111/j.1471-0528.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick S, Garrison E. Normal labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and problem pregnancies. 5. Churchill Livingstone Elsevier; Philadelphia: 2007. pp. 303–321. [Google Scholar]
- 6.Handa VL, Pannu HK, Siddique S, Gutman R, VanRooyen J, Cundiff G. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102(6):1283–1290. doi: 10.1016/j.obstetgynecol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Berger MB, Doumouchtsis SK, DeLancey JO. Bony pelvis dimensions in women with and without stress urinary incontinence. Neurourol Urodyn. 2012 doi: 10.1002/nau.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KM. Three-dimensional shape differences in the bony pelvis of women with pelvic floor disorders. Int Urogynecol J. 2012 doi: 10.1007/s00192-012-1876-y. [DOI] [PubMed] [Google Scholar]
- 9.Hoyte L, Thomas J, Foster RT, Shott S, Jakab M, Weidner AC. Racial differences in pelvic morphology among asymptomatic nulliparous women as seen on three-dimensional magnetic resonance images. Am J Obstet Gynecol. 2005;193(6):2035–2040. doi: 10.1016/j.ajog.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 10.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 11.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 12.Morgan DM, Umek W, Stein T, Hsu Y, Guire K, DeLancey JO. Interrater reliability of assessing levator ani muscle defects with magnetic resonance images. Int Urogynecol J. 2007;18(7):773–778. doi: 10.1007/s00192-006-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noll LE, Hutch JA. The SCIPP line—an aid in interpreting the voiding lateral cystourethrogram. Obstet Gynecol. 1969;33(5):680–689. [PubMed] [Google Scholar]
- 14.Chou Q, DeLancey JO. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol. 2001;185(1):44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- 15.Dean AG, Sullivan KM, Soe MM. [accessed 1 February 2012];OpenEpi: Open source epidemiologic statistics for public health. Version 2.3.1. 2006 www.OpenEpi.com, updated 23 June 2011.
- 16.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115(8):979–984. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 17.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103(1):31–40. doi: 10.1097/01.AOG.0000109207.22354.65/103/1/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashton-Miller JA, DeLancey JOL. Functional anatomy of the female pelvic floor. Ann NY Acad Sci. 2007;1101(1):266–296. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 19.Stein TA, Kaur G, Summers A, Larson KA, DeLancey JO. Comparison of bony dimensions at the level of the pelvic floor in women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2009;200(3):241. e241–e245. doi: 10.1016/j.ajog.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues AA, Jr, Bassaly R, McCullough M, Terwilliger HL, Hart S, Downes K, Hoyte L. Levator ani subtended volume: a novel parameter to evaluate levator ani muscle laxity in pelvic organ prolapse. Am J Obstet Gynecol. 2012;206(3):244, e241–e249. doi: 10.1016/j.ajog.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Dietz HP. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol. 2007;29(3):329–334. doi: 10.1002/uog.3951. [DOI] [PubMed] [Google Scholar]
- 22.Kerkhof MH, Hendriks L, Brolmann HA. Changes in connective tissue in patients with pelvic organ prolapse—a review of the current literature. Int Urogynecol J. 2009;20(4):461–474. doi: 10.1007/s00192-008-0737-1. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol. 2011;186(5):1768–1772. doi: 10.1016/j.juro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Campeau L, Gorbachinsky I, Badlani GH, Andersson KE. Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int. 2011;108(8):1240–1247. doi: 10.1111/j.1464-410X.2011.10385.x. [DOI] [PubMed] [Google Scholar]
- 25.Clark NA, Brincat CA, Yousuf AA, Delancey JO. Levator defects affect perineal position independently of prolapse status. Am J Obstet Gynecol. 2010;203(6):595 e517–522. doi: 10.1016/j.ajog.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stav K, Alcalay M, Peleg S, Lindner A, Gayer G, Hershkovitz I. Pelvis architecture and urinary incontinence in women. Eur Urol. 2007;52(1):239–244. doi: 10.1016/j.eururo.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Handa VL, Nygaard I, Kenton K, Cundiff GW, Ghetti C, Ye W, Richter HE. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J. 2009;20(12):1407–1411. doi: 10.1007/s00192-009-0937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. Computed tomography comparison of bony pelvis dimensions between women with and without genital prolapse. Obstet Gynecol. 1999;93(2):229–232. doi: 10.1016/s0029-7844(98)00376-7. [DOI] [PubMed] [Google Scholar]


