Abstract
Smooth muscle cells (SMCs) have been shown to migrate in response to insulin-like growth factor I (IGF-I). However, the mechanism mediating this response has not been determined. The migration rates of porcine and human vascular SMCs were assessed in a monolayer wounding assay. IGF-I and IGF-II induced increases of 141% and 97%, respectively, in the number of cells that migrated in 4 days. The presence of 0.2% fetal bovine serum in the culture medium was necessary for the IGFs to stimulate migration over uncoated plastic surfaces. However, if vitronectin was used as the substratum, IGF-I stimulated migration by 162% even in the absence of serum. To determine the role of integrins in mediating this migration, SMC surface proteins were labeled with 125I and immunoprecipitated with specific anti-integrin antibodies. Integrins containing alpha-V (vitronectin receptor), alpha5 (fibronectin receptor), and alpha3 (collagen/laminin receptor) subunits were the most abundant. IGF-I treatment caused a 73% reduction in alpha5-integrin subunit protein and a 25% increase in alpha-V subunit. More importantly, ligand binding of alpha-V-beta3 was increased by 2.4-fold. We therefore examined whether the function of the alpha-V-beta3 integrin was important for IGF-I-mediated migration. The disintegrin kistrin was shown by affinity crosslinking to specifically bind with high affinity to alpha-V-beta3 and not to alpha5-beta1 or other abundant integrins. The related disintegrin echistatin specifically inhibited 125I-labeled kistrin binding to alpha-V-beta3, while a structurally distinct disintegrin, decorsin, had 1000-fold lower affinity. The addition of increasing concentrations of either kistrin or echistatin inhibited IGF-I-induced migration, whereas decorsin had a minimal effect. The potency of these disintegrins in inhibiting IGF-I-induced migration paralleled their apparent affinity for the alpha-V integrin. Furthermore, an alpha-V-beta3 blocking antibody inhibited SMC migration by 80%. In summary, vitronectin receptor activation is a necessary component of IGF-I-mediated stimulation of smooth muscle migration, and alpha-V-beta3 integrin antagonists appear to be important reagents for modulating this process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
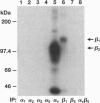
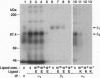
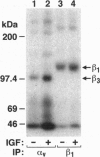
- Bartfeld N. S., Pasquale E. B., Geltosky J. E., Languino L. R. The alpha v beta 3 integrin associates with a 190-kDa protein that is phosphorylated on tyrosine in response to platelet-derived growth factor. J Biol Chem. 1993 Aug 15;268(23):17270–17276. [PubMed] [Google Scholar]
- Basson C. T., Kocher O., Basson M. D., Asis A., Madri J. A. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J Cell Physiol. 1992 Oct;153(1):118–128. doi: 10.1002/jcp.1041530116. [DOI] [PubMed] [Google Scholar]
- Bornfeldt K. E., Raines E. W., Nakano T., Graves L. M., Krebs E. G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994 Mar;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992 Mar 25;267(9):5790–5796. [PubMed] [Google Scholar]
- Bürk R. R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973 Feb;70(2):369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercek B., Fishbein M. C., Forrester J. S., Helfant R. H., Fagin J. A. Induction of insulin-like growth factor I messenger RNA in rat aorta after balloon denudation. Circ Res. 1990 Jun;66(6):1755–1760. doi: 10.1161/01.res.66.6.1755. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993 Jul;306(1):42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R. Exposure to platelet-derived growth factor modulates the porcine aortic smooth muscle cell response to somatomedin-C. Endocrinology. 1985 Jul;117(1):77–83. doi: 10.1210/endo-117-1-77. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Kramer R. H. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992 Jun;200(2):272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- Dennis M. S., Henzel W. J., Pitti R. M., Lipari M. T., Napier M. A., Deisher T. A., Bunting S., Lazarus R. A. Platelet glycoprotein IIb-IIIa protein antagonists from snake venoms: evidence for a family of platelet-aggregation inhibitors. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin R. G., Busby W. H., Jr, Clemmons D. R. An insulin-like growth factor (IGF) binding protein enhances the biologic response to IGF-I. Proc Natl Acad Sci U S A. 1987 May;84(10):3254–3258. doi: 10.1073/pnas.84.10.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. E., Siegall C. B., Biro S., Fu Y. M., FitzGerald D., Pastan I. Cytotoxic effects of a recombinant chimeric toxin on rapidly proliferating vascular smooth muscle cells. Circulation. 1991 Aug;84(2):778–787. doi: 10.1161/01.cir.84.2.778. [DOI] [PubMed] [Google Scholar]
- Gockerman A., Prevette T., Jones J. I., Clemmons D. R. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995 Oct;136(10):4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- Gould R. J., Polokoff M. A., Friedman P. A., Huang T. F., Holt J. C., Cook J. J., Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990 Nov;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- Grant M. B., Wargovich T. J., Ellis E. A., Caballero S., Mansour M., Pepine C. J. Localization of insulin-like growth factor I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. A potential treatment for restenosis? Circulation. 1994 Apr;89(4):1511–1517. doi: 10.1161/01.cir.89.4.1511. [DOI] [PubMed] [Google Scholar]
- Grant M., Jerdan J., Merimee T. J. Insulin-like growth factor-I modulates endothelial cell chemotaxis. J Clin Endocrinol Metab. 1987 Aug;65(2):370–371. doi: 10.1210/jcem-65-2-370. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Reidy M. A. The role of plasminogen activation in smooth muscle cell migration after arterial injury. Ann N Y Acad Sci. 1992 Dec 4;667:141–150. doi: 10.1111/j.1749-6632.1992.tb51606.x. [DOI] [PubMed] [Google Scholar]
- Janat M. F., Argraves W. S., Liau G. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor beta1 and by platelet-derived growth factor-BB. J Cell Physiol. 1992 Jun;151(3):588–595. doi: 10.1002/jcp.1041510319. [DOI] [PubMed] [Google Scholar]
- Jones J. I., Gockerman A., Busby W. H., Jr, Camacho-Hubner C., Clemmons D. R. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993 May;121(3):679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. I., Gockerman A., Busby W. H., Jr, Wright G., Clemmons D. R. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. P., Zhang K., Chen J. D., Kramer R. H., Woodley D. T. Vitronectin-driven human keratinocyte locomotion is mediated by the alpha v beta 5 integrin receptor. J Biol Chem. 1994 Oct 28;269(43):26926–26932. [PubMed] [Google Scholar]
- Krezel A. M., Wagner G., Seymour-Ulmer J., Lazarus R. A. Structure of the RGD protein decorsin: conserved motif and distinct function in leech proteins that affect blood clotting. Science. 1994 Jun 24;264(5167):1944–1947. doi: 10.1126/science.8009227. [DOI] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L., Skinner M. P., Raines E. W., Ross R., Cheresh D. A., Schwartz S. M., Giachelli C. M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995 Feb;95(2):713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle M., Napier M. A., McLean J. W. Cloning and expression of a divergent integrin subunit beta 8. J Biol Chem. 1991 Oct 15;266(29):19650–19658. [PubMed] [Google Scholar]
- Nakao-Hayashi J., Ito H., Kanayasu T., Morita I., Murota S. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis. 1992 Feb;92(2-3):141–149. doi: 10.1016/0021-9150(92)90273-j. [DOI] [PubMed] [Google Scholar]
- Pfeifle B., Boeder H., Ditschuneit H. Interaction of receptors for insulin-like growth factor I, platelet-derived growth factor, and fibroblast growth factor in rat aortic cells. Endocrinology. 1987 Jun;120(6):2251–2258. doi: 10.1210/endo-120-6-2251. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough R. M., Rose J. W., Naughton M. A., Phillips D. R., Nannizzi L., Arfsten A., Campbell A. M., Charo I. F. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J Biol Chem. 1993 Jan 15;268(2):1058–1065. [PubMed] [Google Scholar]
- Shoji S., Ertl R. F., Linder J., Koizumi S., Duckworth W. C., Rennard S. I. Bronchial epithelial cells respond to insulin and insulin-like growth factor-I as a chemoattractant. Am J Respir Cell Mol Biol. 1990 Jun;2(6):553–557. doi: 10.1165/ajrcmb/2.6.553. [DOI] [PubMed] [Google Scholar]
- Stracke M. L., Engel J. D., Wilson L. W., Rechler M. M., Liotta L. A., Schiffmann E. The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem. 1989 Dec 25;264(36):21544–21549. [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994 Dec 2;266(5190):1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]