Abstract
Thiolation of carbon-2 of uridine located in the first position of the anticodons of tRNAGlnUUG, tRNAGluUUC, and tRNALysUUU is a conserved RNA modification event requiring the 2-thiouridine synthetase Ncs6/Ctu1 in archaea and eukaryotes. Ncs6/Ctu1 activates uridine by adenylation, but its role in sulfur transfer is unclear. Here we show that Mmp1356, the Ncs6/Ctu1 homolog in the archaeon Methanococcus maripaludis, forms a persulfide enzyme adduct with an active site cysteine; this suggests that Mmp1356 directly participates in sulfur transfer as a persulfide carrier. Transposon mutagenesis shows that Mmp1356 is likely to be an essential protein.
Keywords: 2-thiouridine, tRNA modification, sulfur, methanogen, archaea
1. Introduction
2-Thiouridine (s2U) derivatives are present as the first anticodon base (position 34) in tRNAGlnUUG, tRNAGluUUC, and tRNALysUUU in all three domains of life. This RNA modification enhances aminoacylation kinetics [1-3] and improves translation efficiency and accuracy [4-6]. The lack of s2U in yeast causes a pleotropic phenotype leading to defects in invasive growth [7], hypersensitivity to rapamycin, caffeine, or oxidative stress [8], and inability to maintain normal metabolic cycles [9]. These observations suggest that 2-thiolation of U34 plays essential roles in challenging growth environments.
The s2U biosynthetic pathway recruits a series of proteins for sulfur transfer. In bacteria, the sulfur relay starts with the generation of a protein-bound Cys persulfide by the cysteine desulfurase IscS employing free Cys as the sulfur donor [10,11]. Then the sulfur is transferred sequentially to TusA, TusBCD, and TusE as persulfide [12]. Finally, the persulfide is transferred to the bacterial s2U synthetase (MnmA), a PP-loop ATPase domain-containing protein. MnmA activates U34 through adenylation and functions as the proximal sulfur donor for U34 thiolation [13]. In eukaryotic cytosol, the sulfur also derives from free Cys activated by a cysteine desulfurase (Nfs1) [14]. Then the persulfide is transferred to a rhodanese domain-containing protein (Tum1/Yor251c), which subsequently transfers the persulfide to the rhodanese domain of Uba4/MOCS3, an E1-like protein [15]. Uba4/MOCS3 activates and then thiolates an ubiquitin-like (Ubl) protein Urm1 to form a thiocarboxylate, which probably donates sulfur to generate s2U [8,15-19]. The eukaryotic s2U synthetase (Ncs6/Ctu1), which also contains a PP-loop like the bacterial MnmA, binds tRNA and activates U34 by adenylation [18,20]; however, its role in sulfur transfer is unclear.
The archaeal s2U biosynthetic pathway probably resembles that present in the eukaryotic cytosol. This assumption is based upon the observation that mutagenesis of SAMP2 (an archaeal Ubl protein) or UbaA (an E1-like protein that actives SAMPs) in Haloferax volcanii prevents formation of s2U in tRNALysUUU [21]. This demonstrates that an Ubl protein similar to Urm1 is required for archaeal s2U biosynthesis. However, the direct evidence that SAMP2 serves as sulfur donor remains to be determined. Furthermore, the enzymes Nfs1 and Tum1 that are involved in the early steps of the eukaryotic sulfur transfer are missing in Methanococcus maripaludis and many other archaea [22]. In addition, the methanococcal Uba4 homolog lack the rhodanese domain required for S transfer. Therefore, the nature of the sulfur source for archaeal tRNA thiolation is unclear. Here we show that the putative 2-thiouridine synthetase (Mmp1356, an Ncs6/Ctu1 homolog) in M. maripaludis can function as a sulfur carrier by forming a persulfide group. This suggests that Mmp1356 directly participates in sulfur transfer and may be the proximal sulfur donor for tRNA thiolation.
2. Materials and methods
2.1. Media and culture conditions for M. maripaludis growth
M. maripaludis was grown in 28-ml aluminum seal tubes with 275 kPa of H2:CO2 (4:1, v/v) at 37 °C in 5 ml McC medium as described previously [23]. The 1.5 L cultures were grown in 2-L bottles in formate medium [24] buffered with 0.2 M glycylglycine (pH 7.0). Neomycin (500 μg/ml in plates and 1 mg/ml in broth) was added when needed. Before inoculation, 3 mM of sodium sulfide was added as the sulfur source.
2.2. Cloning, expression, and purification of recombinant Mmp1356
The M. maripaludis mmp1356 with a C-terminal His6-tag was cloned into the vector pCDFDuet-1 (Novagen) for expression in the E. coli Rosetta 2(DE3) strain. The same construct was cloned into the vector pQE2 (Qiagen) for expression in the E. coli strains from the Keio collection BW25113 (parent strain), JW2514 (ΔiscS), JW2513 (ΔiscU), and JW1670 (ΔsufS) [25]. The mutations of Mmp1356 (C142S, C145S, and C233S) were constructed using the QuikChange mutagenesis kit (Agilent).
For expression of recombinant Mmp1356, the transformed E. coli cells were grown in 1 L of Luria-Bertani (LB) medium at 37 °C with shaking until they reached an absorbance at 600 nm of 0.6~0.8. Then 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce overnight production at 25 °C. For anaerobic protein purification, the harvested E. coli cells were transferred into the anaerobic chamber (atmosphere of 95 % N2 and 5 % H2) and resupended in 10 ml buffer A (50 mM sodium HEPES, 0.3 M NaCl, 5 mM MgCl2, 20 mM imidazole, pH 7.5). The cells were disrupted by addition of 1 ml BugBuster (Novagen). The cell lysate was centrifuged at 100,000 × g for 30 min at 4 °C, and the supernatant was applied to 1 ml of TALON Metal Affinity Resin (Clontech) equilibrated with buffer A. Proteins bound to the column were eluted with buffer B (50 mM sodium HEPES, 0.3 M NaCl, 5 mM MgCl2, 0.2 M imidazole, pH 7.5) and dialyzed against buffer C (50 mM sodium HEPES, 0.3 M NaCl, 5 mM MgCl2, 20% [v/v] glycerol, pH 7.5). The purified proteins were stored at −80 °C until use.
2.3. Identification of modifications of Mmp1356 by mass spectrometry
The anaerobically purified recombinant Mmp1356 was digested with trypsin as described [26]. Tryptic peptides were separated on a Waters nanoACQUITY column (75μm × 250mm eluted at 300nl/min, 80min run time) followed by tandem MS analysis on an Orbitrap Elite mass spectrometer at the Keck MS & Proteomics Resource Laboratory at Yale University. The peptides were identified by MASCOT search with the following parameters: trypsin digestion with two possible missed cleavages, peptide mass tolerance of 15 parts-per-million, fragment mass tolerance of 0.2 Da, and variable Cys modifications of disulfide (−1.0078 Da; needs two Cys with this modification to form a disulfide), persulfide (+31.9721 Da), and trisulfide (+29.9564 Da; needs one Cys with this modification and an unmodified Cys to form a trisulfide). Confidence level was set to 95% within the MASCOT search engine for protein hits.
2.4. Sulfur transfer assay with radioactive sulfur
The sulfur transfer assay was carried out anaerobically as described [26]. The maltose-binding protein (MBP) tagged-IscS (80 μM) was incubated with Mmp1356 (40 μM) in the presence of L-[35S]cysteine (0.3 mM) at 37 °C in the reaction buffer containing 50 mM sodium HEPES (pH 7.3), 150 mM KCl, 10 mM MgCl2, and 10 μM PLP for 30 min. The reaction was stopped by addition of non-reducing SDS loading dye. The proteins mixture was then separated by SDS-PAGE, and the radioactivity retained on the gel was followed by autoradiography.
2.5. Affinity purification and protein identification by mass spectrometry
The N-terminal His6-tagged mmp1356 was cloned into the vector pMEV2 for expression in M. maripaludis strain S2 [27]. For affinity purification, the M. maripaludis cells anaerobically harvested from 1.5 L cultures were resupended in 10 ml buffer D (20 mM sodium HEPES, 0.1 M NaCl, 5 mM MgCl2, 20 mM imidazole, pH 7.5). The cells were disrupted by two times freezing (−80 °C) and thawing. DNA and RNA were digested with 10 U of Benzonase Nuclease (Sigma). Then the cell lysate was centrifuged at 100,000 × g for 30 min at 4 °C, and the supernatant was applied to 1 ml of TALON Metal Affinity Resin (Clontech) equilibrated with buffer D. Proteins bound to the column were eluted with buffer E (20 mM sodium HEPES, 0.1 M NaCl, 5 mM MgCl2, 0.2 M imidazole, pH 7.5). The purified proteins were applied to SDS-PAGE and silver stained. The protein bands were excised, in-gel digested with trypsin, and analyzed by LCMS/MS at Keck MS & Proteomics Resource Laboratory at Yale University.
3. Results and discussion
3.1. Mmp1356 contains conserved residues of the eukaryotic s2U synthetase
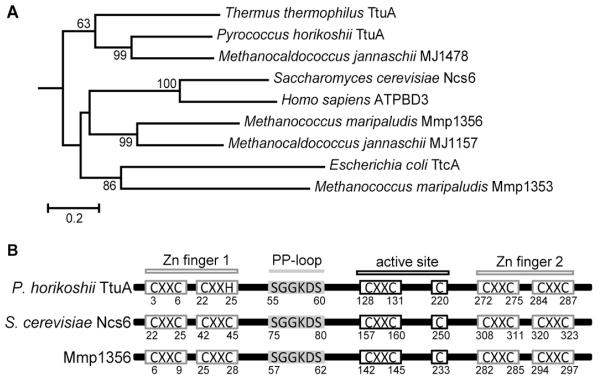
Mmp1356 is the most closely related homolog of the eukaryotic s2U synthetase (Ncs6/Ctu1) in M. maripaludis (Fig. 1A). It shares 31% identity with the Saccharomyces cerevisiae Ncs6 and 27% identify with the Thermus thermophilus TtuA. Both Ncs6/Ctu1 and TtuA belong to the group II of the TtcA tRNA 2-thiolation enzyme family, which has five conserved CXXC/H motifs and a signature PP-loop motif [28]. Structural and mutational studies of Pyrococcus horikoshii TtuA reveal that three conserved cysteines of the putative active site are necessary for the 2-thiolation activity [29]. Multiple sequence alignments recognized that the CXXC and PP-loop motifs as well as the active site cysteines are all conserved in Mmp1356 (Fig. 1B); thus Mmp1356 is a putative 2-thiouridine synthetase.
Fig. 1.
(A) Phylogeny of tRNA 2-thiolation enzymes. The sequence-based phylogenetic tree was constructed with the Minimum-Evolution algorithm of MEGA5. Bootstrap analysis was performed with 1,000 replicates, and values greater than 60% are labeled on the nodes. The E. coli 2-thiouridine synthetase MnmA was used as an outgroup. TtuA, Ncs6/ATPBD3, and TtcA are involved in the 2-thiolation reactions of tRNA nucleotides T54, U34, and C32, respectively. (B) Conserved motifs and residues of the group II of the TtcA family. Four CXXC/H motifs are involved in Zn-binding, and three cysteines are located at the putative active site. The numbers under the schemes are the amino acid positions of the P. furiosus TtuA (locus tag: PH0300), S. cerevisiae Ncs6 (locus tag: YGL211W), and Mmp1356.
3.2. Mmp1356 is a sulfur carrier
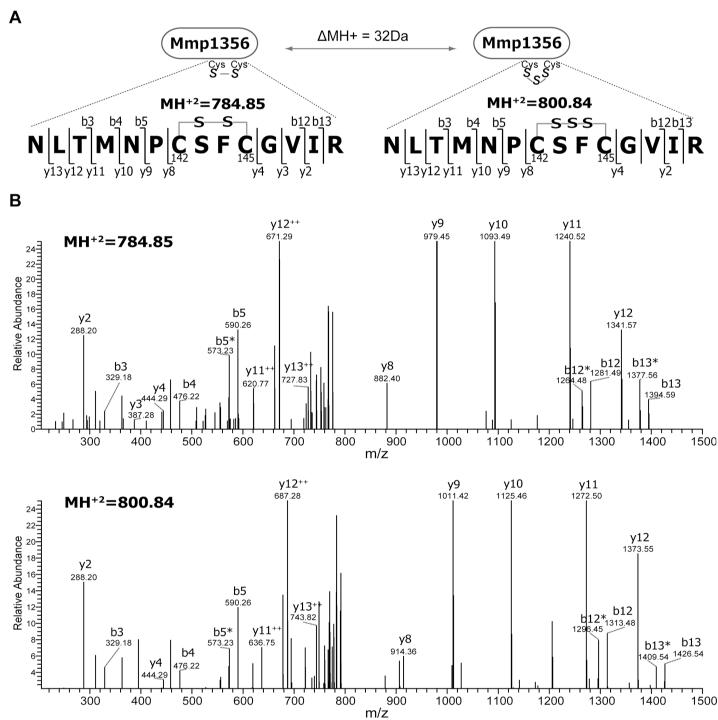
The requirement of three cysteines at the active site of the group II TtcA family resembles that of the tRNA 4-thiouridine synthetase (ThiI) [30] and of Sep-tRNA:Cys-tRNA synthase (SepCysS) [26] in methanogenic archaea. Since ThiI and SepCysS form a persulfide enzyme adduct for sulfur transfer [26], the group II TtcA probably also participates in sulfur transfer with a similar reaction mechanism to that of ThiI and SepCysS. To examine whether Mmp1356 is a sulfur carrier, the modifications of the anaerobically purified recombinant Mmp1356 expressed in E. coli was analyzed by LC-MS/MS. For the peptide 136NLTMNPCSFCGVIR149, the precursor ions were observed with −2 Da (mass of −2H) and +30 Da (mass of −2H + 1S) shifts (Fig. 2A). Analysis of the MS/MS spectra matched them with intra-peptide disulfide and trisulfide modifications at C142XXC145, respectively (Fig. 2B). Due to the instability of a Cys persulfide, the detected trisulfide presumably resulted from oxidation (loss of dihydrogen) of a Cys persulfide and a nearby Cys thiol. This modification pattern is similar to that observed for SepCysS [26] and ThiI [30], suggesting that a fraction of the purified Mmp1356 carries an oxygen-sensitive persulfide group at an active site cysteine.
Fig. 2.
Identification of sulfur modifications of Mmp1356 by LC-MS/MS. (A) Two precursor ions with a mass difference of 32 Da were observed for the tryptic peptide 136NLTMNPCSFCGVIR149 (theoretical MH+2 =785.86 with an oxidized Met). (B) MS/MS fragmentation spectra of the −2 Da (upper panel) and the +30 Da (lower panel) precursor ions. The detected b- and y- ions are labeled in the spectra. * indicates immonium ions.
The E. coli cysteine desulfurase IscS is a versatile sulfur donor [31-34]. It generates a persulfide group from free Cys and donates sulfur for the biosynthesis of multiple cofactors (e.g. Fe-S clusters, thiamine, and molybdopterin) and thionucleotides (e.g. 2-thiouridine and 4-thiouridine). Because IscS can deliver persulfide to diverse downstream sulfur carrier proteins, it is possible that the persulfide of the recombinant Mmp1356 came from IscS. To test this possibility, Mmp1356 was expressed in an E. coli ΔiscS strain. As control experiments, Mmp1356 was also expressed in the deletion strains of iscU (a scaffold protein that receives sulfur from IscS for Fe-S cluster assembly) and sufS (a cysteine desulfurase that functions as the sulfur donor for Fe-S cluster biosynthesis under stress conditions). Although the disulfide modification of C142XXC145 was present in all cases, the trisulfide modification was not detected in Mmp1356 purified from the ΔiscS strain. This suggests that IscS generates the persulfide on Mmp1356 when expressed in E. coli. In contrast, the trisulfide modification was present in both the ΔiscU and ΔsufS strains, suggesting that this process is independent of Fe-S cluster assembly.
To further confirm that E. coli IscS delivers sulfur to Mmp1356, Mmp1356 was incubated with [35S]Cys in the absence or presence of IscS (Fig. 3). The protein(s) was then subjected to SDS-PAGE analysis under non-reducing conditions. Mmp1356 was only radiolabeled in the presence of IscS (lane3 of Fig. 3), indicating that Mmp1356 can accept 35S from IscS. The radiolabeling was sensitive to the reductant 2-mercaptoethanol (lane 4 of Fig. 3), suggesting that 35S was attached to IscS and Mmp1356 as persulfide. Mutations of Cys142, Cys145, or Cys233 in Mmp1356 to Ser markedly reduced its 35S-labelling (lanes 5-7 of Fig. 3), indicating that these three cysteines are essential for the generation or stabilization of the persulfide under in vitro conditions.
Fig. 3.
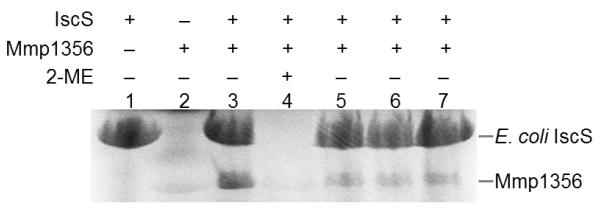
Persulfide formation on Mmp1356. The E. coli IscS (lane 1), the wild-type Mmp1356 (lane 2), and the two proteins together (lane 3) were incubated with 300 μM [35S]Cys at 37 °C for 30 min, and then the incubation mixtures were analyzed by SDS-PAGE under non-reducing condition. Lane 4, the IscS and Mmp1356 mixture (as in lane 3) was analyzed by SDS-PAGE under reducing condition with 1% (v/v) 2-mercaptoethanol (2-ME). Lane 5-7, the E. coli IscS was incubated with the C142S, C145S, and C233S variants of Mmp1356, respectively. The positions of the MBP-tagged IscS (~ 75 kDa) and the His-tagged Mmp1356 (~ 37 kDa) are labeled.
Overall, these results imply that the C142XXC145 motif of Mmp1356 can form a persulfide enzyme adduct. Therefore, Mmp1356 probably directly participates in sulfur transfer and functions as the proximal sulfur donor for tRNA thiolation. Although an IscS homolog is not encoded in M. maripaludis, the capability of IscS to donate sulfur to Mmp1356 suggests that Mmp1356 can receive sulfur from a protein sulfur donor or sulfide directly, resembling the case of ThiI in methanogens [30].
3.3. Mmp1356 interacts with Mmp1357 in vivo
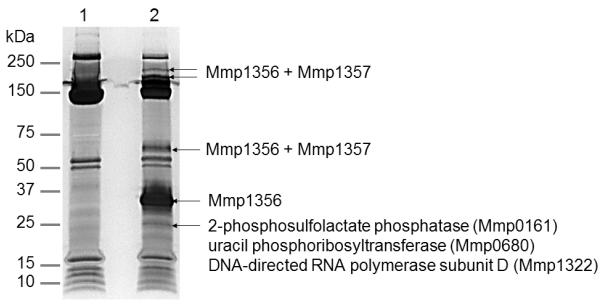
To identify the physiological partner proteins of Mmp1356, the His6-tagged Mmp1356 was overexpressed in M. maripaludis for a pull-down experiment. Affinity purification followed by MS analysis demonstrated that Mmp1357 (~ 10 kDa) co-purified with Mmp1356 (~ 37 kDa). They appeared as major components of two >200 kDa and one ~ 65 kDa protein bands separated by SDS-PAGE (Fig. 4). This result suggests that Mmp1356 interacts with Mmp1357 in vivo and they are likely to be covalently linked into large molecular mass conjugates. Other proteins co-purified specifically with Mmp1356 (identified with > 40% coverage in MS analysis) included 2-phosphosulfolactate phosphatase (Mmp0161), uracil phosphoribosyltransferase (Mmp0680), and DNA-directed RNA polymerase subunit D (Mmp1322). These proteins may directly or indirectly associate with Mmp1356.
Fig. 4.
Pull-down of Mmp1356 expressed in M. maripaludis. The proteins purified by metal affinity chromatography from M. maripaludis cells transformed with the empty vector (lane 1) or the shuttle vector expressing His6-tagged Mmp1356 (lane 2) were analyzed with SDS-PAGE and stained with silver. The protein bands marked with arrows in lane 2 were excised, digested with trypsin, and analyzed by LC-MS/MS. The corresponding regions in lane 1 were analyzed as controls. The proteins specifically identified in lane 2 with > 40% coverage are listed.
Mmp1357 is homologous to the archaeal Ubl protein SAMP, and it is encoded upstream of the mmp1356 gene. According to the transcriptomic tiling array data [35], mmp1357 and mmp1356 are most likely to be co-transcribed, suggesting that they represent an operon. This genetic association is present in several archaeal lineages [36], including some species of the methanogens (Methanoregula, Methanocorpusculum, Methanococcus, and Methanosphaerula), the crenarchaeotes (Aeropyrum, Hyperthermus, Pyrolobus, Staphylothermus, and Stygioglobus), and the korarchaeotes (Korarchaeum). These associations suggest that SAMPs are often functionally linked to tRNA thiolation [36,37].
The functional link of Mmp1356 and Mmp1357 is supported by their physiological interaction, which has also been observed for their homologs in haloarchaea [38], Thermus thermophilus [39], and mammalian cells [40]. In all these cases, the C-terminal Gly of the Ubl proteins are appended to Lys residues of the 2-thiolation enzymes via isopeptide bonds, similar to ubiquitylation. In H. volcanii, 29 proteins are conjugated (sampylated) with SAMP2, including homologs of Uba4, Tum1, and Ncs6 associated with the s2U biosynthetic pathway [38]. Furthermore, SAMP2 also sampylates its own Lys to form polymeric chains [38]. Deletion of SAMP2 causes retarded growth at high temperatures and lack of s2U in tRNA [21]; this provides direct evidence that SAMP is required for s2U biosynthesis. However, whether SAMP can be thiolated to form a C-terminal thiocarboxylate and donate sulfur to tRNA are unclear. The Δsamp2 phenotype permits two interpretations: (i) SAMP acts as a sulfur carrier; or (ii) sampylation of s2U synthetase controls the thiolation activity.
3.4. Mmp1356 is likely to be essential in M. maripaludis
A whole-genome analysis of gene function by the Tn-seq methodology has recently been completed in M. maripaludis [41]. Two libraries of mutants were generated for M. maripaludis using a derivative of the Tn5 transposon comprising puromycin resistance [41]. Because M. maripaludis is polyploid and contains 30-55 copies of the chromosome per cell [42], the transposon was inserted into both essential and nonessential genes during growth with puromycin selection [41]. When puromycin was removed, transposon insertions in essential genes were quickly replaced by the wild-type alleles [41]. This is because gene conversion rapidly homogenizes the genome to prevent accumulation of heterologous genes [42]. Using this method, 526 genes were classified as possibly essential or strongly advantageous for growth in rich medium, corresponding to 30% of the genome [41]. The genomic region of mmp1357-mmp1356 (1335578-1336834) contained 37 unique insertions at T0 (growth with puromycin for 20 generations) and only one insertion at T2 (growth without puromycin for 14 generations). This suggests that (i) mmp1356 is likely to be essential and (ii) the upstream mmp1357 is either essential or falsely appeared essential because of polarity.
3.5. Concluding remarks and future prospects
Our biochemical characterizations of Mmp1356, a putative s2U synthetase, indicate that it functions as a sulfur carrier. This provides a new perspective of the function of the Ncs6/Ctu1 enzymes, suggesting that they serve as a sulfur donor for s2U biosynthesis. Three cysteines at the active site of Mmp1356 are required to generate a persulfide enzyme adduct, resembling the archaeal ThiI and SepCysS. This suggests that these enzymes may use a common mechanism for sulfur transfer. The functional link of Mmp1356 with an archaeal ubiquitin-like protein (Mmp1357) is supported by their conserved genetic association and physiological interaction. However, whether Mmp1357 forms a thiocarboxylate group and directly participates in sulfur transfer remains to be determined. Finally, knowledge of the nature of the physiological sulfur donor used to form the persulfide on Mmp1356 and the ultimate sulfur source for archaeal s2U biosynthesis awaits future investigations.
Highlights.
Mmp1356 is a putative 2-thiouridine synthetase and acts as a sulfur carrier.
Three conserved cysteines of Mmp1356 are required for sulfur transfer.
Mmp1356 interacts with the ubiquitin-like protein Mmp1357 in vivo.
Mmp1356 is likely to be an essential protein in Methanococcus maripaludis.
Acknowledgements
We thank Dr. John Leigh (University of Washington) and Dr. Nitin Baliga (Institute for Systems Biology) for providing the transcriptomic data of M. maripaludis prior to its publication. This work was supported by grants from the Department of Energy Office of Basic Energy Sciences (DE-FG02-98ER20311) and the National Institute for General Medical Sciences (GM22854) to DS and from the Office of the Vice President for Research of the University of Georgia to WW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rodriguez-Hernandez A, et al. Structural and mechanistic basis for enhanced translational efficiency by 2-thiouridine at the tRNA anticodon wobble position. J Mol Biol. 2013;425:3888–906. doi: 10.1016/j.jmb.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sylvers LA, Rogers KC, Shimizu M, Ohtsuka E, Söll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry. 1993;32:3836–41. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- [3].Madore E, Florentz C, Giegé R, Sekine S, Yokoyama S, Lapointe J. Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl-tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. Eur J Biochem. 1999;266:1128–35. doi: 10.1046/j.1432-1327.1999.00965.x. [DOI] [PubMed] [Google Scholar]
- [4].Yarian C, Townsend H, Czestkowski W, Sochacka E, Malkiewicz AJ, Guenther R, Miskiewicz A, Agris PF. Accurate translation of the genetic code depends on tRNA modified nucleosides. J Biol Chem. 2002;277:16391–5. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- [5].Ashraf SS, Sochacka E, Cain R, Guenther R, Malkiewicz A, Agris PF. Single atom modification (OS) of tRNA confers ribosome binding. RNA. 1999;5:188–94. doi: 10.1017/s1355838299981529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johansson MJ, Esberg A, Huang B, Björk GR, Byström AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28:3301–12. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Goehring AS, Rivers DM, Sprague GF., Jr. Urmylation: a ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol Biol Cell. 2003;14:4329–41. doi: 10.1091/mbc.E03-02-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leidel S, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–32. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- [9].Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154:416–29. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nilsson K, Lundgren HK, Hagervall TG, Björk GR. The cysteine desulfurase IscS is required for synthesis of all five thiolated nucleosides present in tRNA from Salmonella enterica serovar typhimurium. J Bacteriol. 2002;184:6830–5. doi: 10.1128/JB.184.24.6830-6835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kambampati R, Lauhon CT. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry. 2003;42:1109–17. doi: 10.1021/bi026536+. [DOI] [PubMed] [Google Scholar]
- [12].Ikeuchi Y, Shigi N, Kato J, Nishimura A, Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [13].Numata T, Ikeuchi Y, Fukai S, Suzuki T, Nureki O. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature. 2006;442:419–24. doi: 10.1038/nature04896. [DOI] [PubMed] [Google Scholar]
- [14].Nakai Y, Umeda N, Suzuki T, Nakai M, Hayashi H, Watanabe K, Kagamiyama H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J Biol Chem. 2004;279:12363–8. doi: 10.1074/jbc.M312448200. [DOI] [PubMed] [Google Scholar]
- [15].Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–52. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmitz J, Chowdhury MM, Hanzelmann P, Nimtz M, Lee EY, Schindelin H, Leimkuhler S. The sulfurtransferase activity of Uba4 presents a link between ubiquitin-like protein conjugation and activation of sulfur carrier proteins. Biochemistry. 2008;47:6479–89. doi: 10.1021/bi800477u. [DOI] [PubMed] [Google Scholar]
- [17].Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci U S A. 2008;105:18255–60. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283:27469–76. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- [19].Huang B, Lu J, Bystrom AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14:2183–94. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dewez M, Bauer F, Dieu M, Raes M, Vandenhaute J, Hermand D. The conserved wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci U S A. 2008;105:5459–64. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miranda HV, et al. E1- and ubiquitin-like proteins provide a direct link between protein conjugation and sulfur transfer in archaea. Proc Natl Acad Sci U S A. 2011;108:4417–22. doi: 10.1073/pnas.1018151108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu Y, Sieprawska-Lupa M, Whitman WB, White RH. Cysteine is not the sulfur source for iron-sulfur cluster and methionine biosynthesis in the methanogenic archaeon Methanococcus maripaludis. J Biol Chem. 2010;285:31923–9. doi: 10.1074/jbc.M110.152447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Whitman WB, Shieh J, Sohn S, Caras DS, Premachandran U. Isolation and characterization of 22 mesophilic methanococci. Syst Appl Microbiol. 1986;7:235–240. [Google Scholar]
- [24].Lupa B, Hendrickson EL, Leigh JA, Whitman WB. Formate-dependent H2Production by the mesophilic methanogen Methanococcus maripaludis. Appl Environ Microbiol. 2008;74:6584–90. doi: 10.1128/AEM.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Y, Dos Santos PC, Zhu X, Orlando R, Dean DR, Söll D, Yuan J. Catalytic mechanism of Sep-tRNA:Cys-tRNA synthase: sulfur transfer is mediated by disulfide and persulfide. J Biol Chem. 2012;287:5426–33. doi: 10.1074/jbc.M111.313700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin W, Whitman WB. The importance of porE and porF in the anabolic pyruvate oxidoreductase of Methanococcus maripaludis. Arch Microbiol. 2004;181:68–73. doi: 10.1007/s00203-003-0629-1. [DOI] [PubMed] [Google Scholar]
- [28].Jäger G, Leipuviene R, Pollard MG, Qian Q, Björk GR. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J Bacteriol. 2004;186:750–7. doi: 10.1128/JB.186.3.750-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakagawa H, et al. Crystallographic and mutational studies on the tRNA thiouridine synthetase TtuA. Proteins. 2013;81:1232–44. doi: 10.1002/prot.24273. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Zhu X, Nakamura A, Orlando R, Söll D, Whitman WB. Biosynthesis of 4-thiouridine in tRNA in the methanogenic archaeon Methanococcus maripaludis. J Biol Chem. 2012;287:36683–92. doi: 10.1074/jbc.M112.405688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mueller EG. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2:185–94. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- [32].Kessler D. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol Rev. 2006;30:825–40. doi: 10.1111/j.1574-6976.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- [33].Mihara H, Esaki N. Bacterial cysteine desulfurases: their function and mechanisms. Appl Microbiol Biotechnol. 2002;60:12–23. doi: 10.1007/s00253-002-1107-4. [DOI] [PubMed] [Google Scholar]
- [34].Hidese R, Mihara H, Esaki N. Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl Microbiol Biotechnol. 2011;91:47–61. doi: 10.1007/s00253-011-3336-x. [DOI] [PubMed] [Google Scholar]
- [35].Yoon SH, et al. A systems level predictive model for global gene regulation of methanogenesis in a hydrogenotrophic methanogen. Genome Res. 2013;23:1839–51. doi: 10.1101/gr.153916.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Makarova KS, Koonin EV. Archaeal ubiquitin-like proteins: functional versatility and putative ancestral involvement in tRNA modification revealed by comparative genomic analysis. Archaea. 2010 doi: 10.1155/2010/710303. 2010, doi:10.1155/2010/710303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maupin-Furlow JA. Ubiquitin-like proteins and their roles in archaea. Trends Microbiol. 2013;21:31–8. doi: 10.1016/j.tim.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Humbard MA, et al. Ubiquitin-like small archaeal modifier proteins (SAMPs) in Haloferax volcanii. Nature. 2010;463:54–60. doi: 10.1038/nature08659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shigi N. Posttranslational modification of cellular proteins by a ubiquitin-like protein in bacteria. J Biol Chem. 2012;287:17568–77. doi: 10.1074/jbc.M112.359844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van der Veen AG, Schorpp K, Schlieker C, Buti L, Damon JR, Spooner E, Ploegh HL, Jentsch S. Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc Natl Acad Sci U S A. 2011;108:1763–70. doi: 10.1073/pnas.1014402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sarmiento F, Mrazek J, Whitman WB. Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcus maripaludis. Proc Natl Acad Sci U S A. 2013;110:4726–31. doi: 10.1073/pnas.1220225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hildenbrand C, Stock T, Lange C, Rother M, Soppa J. Genome copy numbers and gene conversion in methanogenic archaea. J Bacteriol. 2011;193:734–43. doi: 10.1128/JB.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]