Abstract
ALKBH5, a member of AlkB family proteins, has been reported as a mammalian N6-methyladenosine (m6A) RNA demethylase. Here we report the crystal structure of zebrafish ALKBH5 (fALKBH5) with the resolution of 1.65 Å. Structural superimposition shows that fALKBH5 is comprised of a conserved jelly-roll motif. However, it possesses a loop that interferes potential binding of a duplex nucleic acid substrate, suggesting an important role in substrate selection. In addition, several active site residues are different between the two known m6A RNA demethylases, ALKBH5 and FTO, which may result in their slightly different pathways of m6A demethylation.
Keywords: ALKBH5, crystal structure, demethylation, N6-hydroxymethyladenosine
1. Introduction
Among the various RNA modifications, N6-methyladenosine (m6A) is of great interest because it is the most prevalent internal modifications in eukaryotic mRNA [1]. This modification also exists in the virus RNA that is transcribed in host nuclei [2], and plays a important role in yeast meiosis and plant development [3, 4]. A RNA methyltransferase complex with METTL3 as one of the S-adenosylmethionine-binding subunit installs the N6-position methyl group of m6A [5]. Our recent discoveries of two m6A demethylases (FTO and ALKBH5) specifically highlight the importance of the reversal of this modification [6, 7]. Furthermore, the recently mapped transcriptome-wide distributions of m6A in human and mouse cells indicate that this modification could affect a series of biological processes including mRNA splicing, nuclear export, as well as host cellular immune response [8–10].
FTO and ALKBH5 belong to the AlkB family of iron(II)/α-ketoglutarate(α-KG)-dependent dioxygenases [11, 12]. Nine AlkB homologous have been identified in mammals: ALKBH1–8 and FTO, which show different preferences for substrates [13]. AlkB, ALKBH2, and ALKBH3 exhibit demethylation activity of 1-methyladenine (m1A) and 3-methylcytosine (m3C) in DNA [14–16]. AlkB and ALKBH3 show higher activity to single-stranded (ss)DNA than double-stranded (ds)DNA, while ALKBH2 prefers dsDNA over ssDNA [16]. ALKBH8 contains a RNA recognition motif, a tRNA methyltransferase domain, and an AlkB-like domain, which could convert 5-carboxy-methyluridine (cm5U) to (S)-5-methoxycarbonyl-hydroxymethyluridine ((S)-mchm5U) [17, 18]. FTO, a protein associated with human obesity [19–21], was originally shown to demethylate 3-methylthymine (m3T) in ssDNA and 3-methyluracil (m3U) in ssRNA [11, 22]. Recently, our group discovered FTO as the first RNA demethylase that mediates demethylation of m6A to adenosine (A) [6]. Further research in our group indicates that FTO generated two intermediates (N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (fm6A)) during the demethylation process [23], which adds potential complexity to this demethylation regulation [24].
ALKBH5 is the second known m6A RNA demethylase in vitro and in vivo [7]. The over-expression of ALKBH5 leads to reduction of the cellular m6A level, while knockdown of ALKBH5 increases the ratio of m6A to A in mRNA in human cells and mouse testis [7]. Furthermore, aberrant spermatogenesis and apoptosis were observed in mouse testis when Alkbh5 gene was knocked-out [7]. Although ALKBH5 and FTO exhibit similar substrate preference, their reaction pathways seem to be different: as we show in this study, ALKBH5 directly converts m6A to adenosine without any intermediate observed. Whereas, two intermediates of hm6A and fm6A were observed in the FTO-mediated m6A demethylation. Recently, the crystallization conditions for human ALKBH5 (hALKBH5) were published [25]; however, the structure has not been reported. Here we report the crystal structure of a truncated zebrafish ALKBH5 (Protein ID: NP_001070855, residues 38–287, named ΔfALKBH5 here and after), which shares high sequence identity to hALKBH5, as well as the same biological activity. This structure should facilitate our understanding of the substrate-selectivity of the AlkB family proteins, and provide further insights for future investigation into the mechanism of m6A demethylation.
2. Materials and methods
2.1. Cloning, expression, and purification
The truncated zebrafish ALKBH5 (ΔfALKBH5) gene was PCR-amplified from zebrafish cDNA (Thermo Scientific), and subcloned into PMCSG19 vector by ligation independent cloning (LIC) [26], resulting in the ΔfALKBH5-PMCSG19 plasmid with a His6-tag at N-terminal. The constructed plasmid was transformed in BL21(DE3) strain containing PRK1037 plasmid [26]. Cells grew in LB medium containing 50 ug/mL kanamycin and 100 ug/mL Ampicillin at 37 °C. When OD600 reached 0.6, the protein expression was achieved by adding 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) under 16 °C for overnight. Cells were harvested and stored at −80 °C for subsequent steps.
The cell pellet was resuspended with 35 mL buffer A (10 mM Tris-HCl, pH 7.4, 500 mM NaCl and 1 mM DTT), and lysed by sonication. After centrifugation, the supernatant was loaded to pre-equilibrated Ni-NTA column and washed with eight column volumes of buffer A. Target protein was eluted with gradient linear buffer B (10 mM Tris-HCl, pH 7.4, 500 mM NaCl, 500 mM imidazole, and 1 mM DTT). After removing the His-tag by overnight TEV enzyme digestion at 4 °C, the protein solution was applied to MonoS column and eluted with linear concentration of NaCl in 10 mM Tris-HCl, pH 7.4. The eluted fractions were pooled, concentrated, and further purified by gel filtration Superdex200 column in 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1 mM DTT. Over 95% purity of protein was obtained for further use (Supplementary Fig. 1). FTO and hALKBH5 proteins were expressed and purified as reported [7, 22].
2.2. Crystallization and data collection
The sitting-drop vapor diffusion method was employed for the crystallization of ΔfALKBH5/α-KG protein. 1 uL 10 mg/mL protein was mixed with an equal volume of reservoir solution (0.2 M Sodium iodide, pH 7.0, 20% w/v Polyethylene glycol 3,350) in the presence of 1 mM MnCl2 and 2 mM α-KG, and equilibrated against 100 μL of the reservoir solution at 289 K. The crystals of ΔfALKBH5/succinate acid (SIN) were achieved under the same conditions except for the substitution of α-KG for succinate acid. The crystals appeared within 24 hours and were flash-frozen in liquid nitrogen with 25% glycerol (v/v) as the cryoprotectant solution. The crystal diffraction data was collected at the macromolecular crystallography for life science beamline NE-CAT (24-ID-D) at the Advanced Photon Source, Argonne National Laboratory. The data was then integrated and scaled with HKL2000 (Table 1).
Table 1.
Data collection and refinement statistics of ΔfALKBH5 in complex with α-KG or succinate acid (SIN).
| ΔfALKBH5/α-KG | ΔfALKBH5/SIN | |
|---|---|---|
| Data collection | ||
| Space group | P212121 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 65.948, 68.595, 114.909 | 66.067, 69.792, 115.050 |
| α, β, γ (°) | 90.00, 90.00, 90.00 | 90.00, 90.00, 90.00 |
| Resolution (Å) | 30–1.65 | 50–1.80 |
| Rsym or Rmerge | 8.9 (49.0)* | 6.9 (59.4) |
| I / σI | 27.8 (3.0) | 22.5 (3.5) |
| Completeness (%) | 96.1 (93.2) | 99.9 (99.9) |
| Redundancy | 5.7 (4.8) | 6.4 (6.5) |
| Refinement | ||
| Resolution (Å) | 30–1.65 (1.71–1.65) | 50–1.80 (1.86–1.80) |
| No. unique reflections | 60,317 | 49,614 |
| Rwork / Rfree | 20.9/23.8 | 17.2/18.2 |
| No. atoms | ||
| Protein | 3,406 | 3,370 |
| Ligand/ion | 22 | 18 |
| Water | 445 | 471 |
| B-factors | ||
| Protein | 19.8 | 25.3 |
| Ligand/ion | 29.9 | 29.0 |
| Water | 30.1 | 37.2 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.007 | 0.009 |
| Bond angles (°) | 1.140 | 1.161 |
Highest-resolution shell is shown in parentheses.
2.3. Phasing and Refinement
Zebrafish ALKBH5 structures were resolved by molecular replacement with the CCP4i program PHASER using the structure of E.coli AlkB (PDB ID: 2FD8) as the searching model. The structure model building was performed using the computer graphics program Coot, and then refined by using Phenix. The final R/Rfree factor value of ΔfALKBH5/α-KG and ΔfALKBH5/SIN is 20.9/23.8% and 17.2/18.2%, respectively (Table 1).
2.4. MADLI-TOF/TOF MS analysis
As reported previously [7], 1 nmol 9-mer ssRNA substrate (5'-UAAGm6ACUCA-3') was mixed with 1 nmol purified ΔfALKBH5/hALKBH5 protein in 100 uL reaction buffer (25 mM HEPES, pH 7.4, 100 mM KCl, 2 mM MgCl2, 0.2 U/μL RNasin, 2 mM L-ascorbic acid, 300 μM α-KG and 150 μM (NH4)2Fe(SO4)2·6H2O). After incubation at room temperature for 30 min, 10 uL reaction solution was mixed with 50 μl ion exchange resin (Bio-Rad). 1 uL solution was mixed with 1 uL matrix (THAP/Diammonium Citrate) and spotted onto MALDI plate. Then MALDI-TOF/TOF (Bruker) was employed to analyze the results.
2.5. HPLC analysis of ALKBH5 activity
100 uL reaction solution was quenched by 5 mM EDTA followed by heating at 95 °C for 10 min. The reacted ssRNA was digested with 1 uL nuclease P1 at 42 °C for overnight. Then 10 uL 1 M NH4HCO3 and 1 uL alkaline phosphatase were added to the solution and digested for 3 h at 37 °C. HPLC system equipped with an Acclaim 120, C18, 5μm analytical column (Dionex, 059148) was employed to analyze the result. 30 uL digested solution was injected in HPLC and eluted with buffer A (50 mM ammonia acetate) and buffer B (60% acetonitrile, 0.01% TFA, 50 mM ammonia acetate) with a flow rate of 1 ml/min. The analysis was executed at room temperature. The detection wavelength was set at 260 nm.
3. Results and discussion
3.1. Sequence identity and biological activity of hALKBH5 and fALKBH5
ALKBH5 shares high sequence identity among different species. Sequence alignment by ClustalW2 indicates that their differences are mainly distributed at the N- and C-termini with the active site highly conserved, including the five invariant residues (HxD..H..R..R) (Supplementary Fig. 2). After testing ALKBH5 from different species, we succeeded in crystallizing a truncated form of zebrafish ALKBH5 (ΔfALKBH5, residues 38–287) in complex with manganese(II) and α-KG. Full-length fALKBH5 shares 73.9% identity (260/352 residues) with hALKBH5, and the identity for ΔfALKBH5 reaches as high as 80.8% (202/250 residues).
To confirm that the truncation of fALKBH5 did not affect catalytic activity, a 9-mer ssRNA substrate (UAAGm6ACUCA) was treated with equal amounts of hALKBH5 and ΔfALKBH5 for 30 min at room temperature, respectively. MALDI-TOF/TOF was employed to analyze the results. A loss of 14 Da in substrate mass in experiments with both hALKBH5 and ΔfALKBH5 was observed, showing the m6A demethylation activity of ΔfALKBH5 (Fig. 1A). To further confirm this observation, the reacted ssRNA was completely digested using nuclease P1 and alkaline phosphatase to single nucleosides, and then analyzed by HPLC. As shown in Fig. 1B, ΔfALKBH5 could completely demethylate m6A in the ssRNA substrate as hALKBH5.
Fig. 1.
(A) MALDI-TOF/TOF mass spectrometry analysis. hALKBH5 and ΔfALKBH5 showed the same activity of m6A demethylation on ssRNA (ssRNA 5'-UAAGm6ACUCA-3') with loss of 14-Dalton of a methyl group after the reaction in both cases. By contrast, after treating FTO with ssRNA, two intermediates hm6A (m6A-2Da, lost a H2O moiety during MALDI-TOF ionization) and fm6A (m6A+14Da) were observed. The two intermediates were not observed in the reaction with hALKBH5 and ΔfALKBH5 under the same condition. (B) HPLC chromatograms of digested nucleosides from m6A-containing ssRNA. HPLC confirmed the observation that after treatment with hALKBH5 and ΔfALKBH5, m6A underwent complete conversion to adenosine.
3.2. The structure of ΔfALKBH5 and its active site
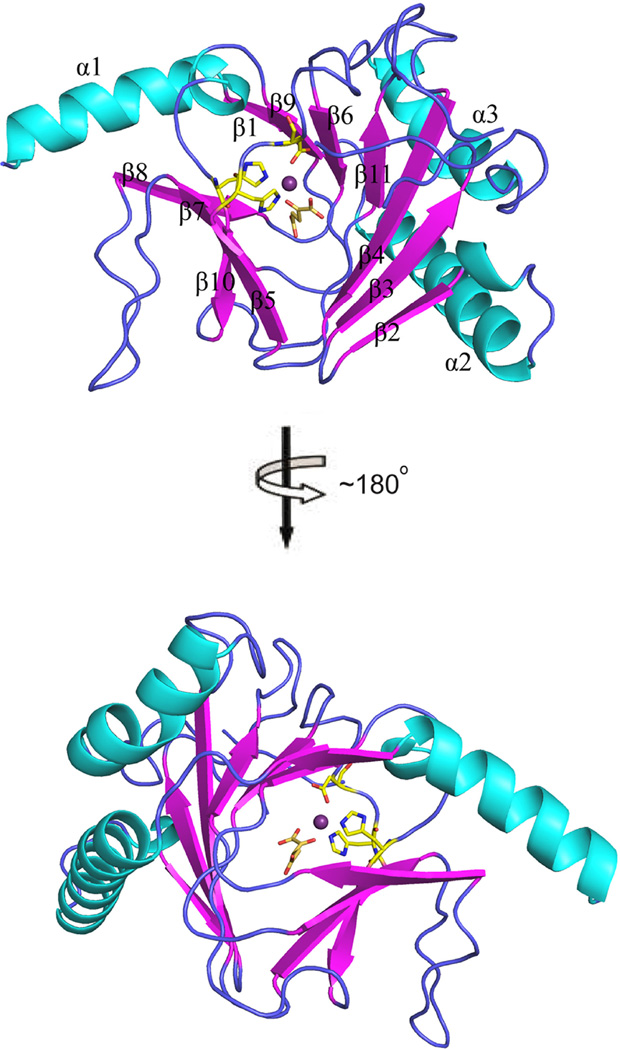
ΔfALKBH5 was crystallized in complex with manganese(II) and α-KG by mixing with an equal volume of reservoir solution (0.2 M sodium iodide, pH 7.0, 20% w/v polyethylene glycol 3,350) at 289 K, and a high-resolution (1.65 Å) x-ray diffraction data set was collected. E.coli AlkB, the closest homologue of ALKBH5, shares 14.8% identity (37/250 residues) and 39.2% similarity (98/250 residues) with ΔfALKBH5. Using the structure of AlkB (PDB ID: 2FD8) as a searching model, the final model of ΔfALKBH5 structure was refined to 1.65 Å (Table 1). fALKBH5 is a monomer in solution, and there are two monomers per asymmetric unit in the P212121 unit cell and they interact with each other mainly through a loop (named L2 here and after) (Supplementary Fig.3). ΔfALKBH5 is composed of 11 β-strands and 3 long α-helixes. As illustrated in Fig. 2, the active site of fALKBH5 is mainly composed of a jelly-roll motif [27], which is formed of eight β-strands (β4-β11). The β sheets are connected through loops and 3 α-helixes buttressing the jelly-roll motif from the outside.
Fig. 2.
The crystal structure of ΔfALKBH5. Two orthogonal views with catalytic core shown. The secondary structural elements are labeled α1-α3 for helices (colored cyan) and β1-β11 for strands (colored magenta).
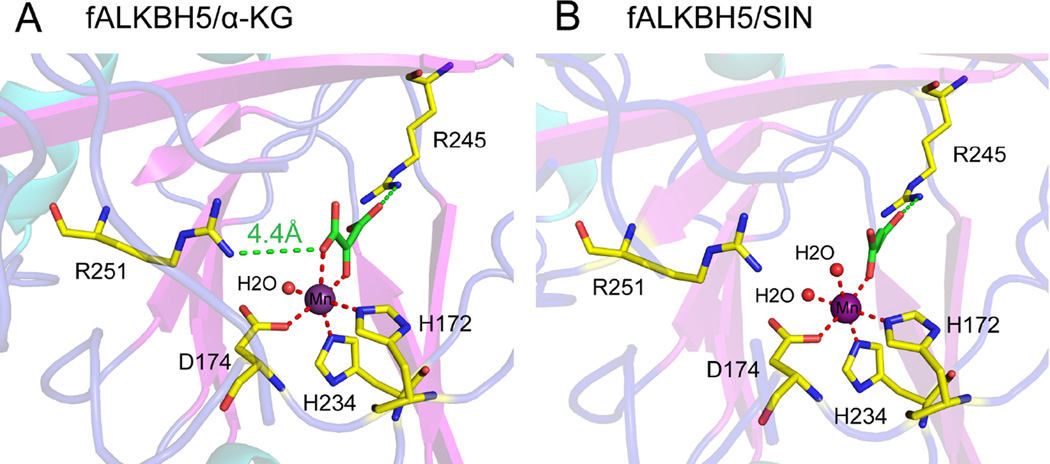
Sequence alignment shows that the consensus HxD..H..R..R residues in the active site of fALKBH5 are highly conserved (Fig. 3A and Supplementary Fig. 2), and structural alignment reveals five invariant residues reside in positions similar to those of other AlkB family proteins (namely, human ALKBH3, hALKBH3; human ALKBH8, hALKBH8; human FTO, FTO; and E. coli AlkB, AlkB) (Supplementary Fig. 4). The root-mean-square deviation (rmsd) between fALKBH5 and its homologues is within 3.5 Å (AlkB: 2.46 Å, hALKBH2: 2.62 Å, hALKBH3: 2.61 Å, hALKBH8:1.75 Å, FTO: 3.16 Å). As showed in Fig. 4A and Supplementary Fig. 5, His172, Asp174, and His234 coordinate to manganese(II), and Arg245 interacts with α-KG through a salt bridge. It is noteworthy that the distance between Arg251 (the second arginine in the motif) and α-KG is 4.4 Å, whereas in other AlkB family proteins the corresponding residues are much closer to α-KG with the distance around 3 Å (Fig. 4A and Supplementary Fig. 6) [28–31]. This observation therefore suggests that Arg251 in ΔfALKBH5 may not bind α-KG, which differs from its equivalents in other AlkB homologous. Previous studies have confirmed that the (HxD..H..R..R) motif is highly conserved in all AlkB family members, and the five invariant residues are essential for enzymatic activity [32]. Usually, the second arginine (Arg251 in fALKBH5) is considered as an α-KG-binding residue, but our structure of ΔfALKBH5 shows a potential weaker interaction between Arg251 and α-KG.
Fig. 3.
The sequence and structure alignment of AlkB family members (FTO (PDB ID: 3LFM), hALKBH2 (PDB ID: 3BTX), hALKBH3 (PDB ID: 2IUW), AlkB (PDB ID: 2FD8), hALKBH8 and fALKBH5). (A) Structure-based sequence alignment of AlkB family proteins. The five invariant residues are highlighted in red. The loop L1 in FTO, L2 in human and fish ALKBH5 as well as the lid in FTO, hALKBH2, hALKBH3 and AlkB are boxed off in red. The secondary structure of fALKBH5 is shown on top of the alignment. (B) Structural comparison of fALKBH5 and hALKBH2-DNA. The L2 loop (colored in blue) in fALKBH5 protrudes into the dsDNA strand (colored in orange) from the superimposed hALKBH2-dsDNA structure. (C) Structure alignment of fALKBH5 and FTO with the extra loop highlighted. (L1 in FTO is colored red, and L2 in fALKBH5 is colored blue.) (D) A hairpin creates a lid over the active site in FTO, AlkB, hALKBH2 and hALKBH3. The structure of fALKBH5 lacks such a lid. The lid is highlighted in a red box.
Fig. 4.
The interaction network around Mn(II) and α-KG (A) or succinate (B). The coordinate bonds between Mn(II) and its ligands are shown with red dash lines, whereas the interactions between Arg245/Arg251 and α-KG/SIN are indicated in green. The distance between Arg251 and α-KG is 4.4 Å, indicating a weak interaction between them.
α-KG is the cofactor for the oxidation reaction catalyzed by ALKBH5, in which it is converted to succinic acid [13]. The addition of α-KG or succinic acid could help stabilize fALKBH5 protein and thus facilitates its crystallization. We also obtained the structure of ΔfALKBH5 in complex with manganese(II) and succinic acid at 1.80 Å resolution (Table 1). In both structures, the manganese(II) adopts a hexa-coordination geometry. Besides the three ligand residues (His172, Asp174, and His234) from the protein, two water molecules and succinate occupy the three remaining coordinate sites of the central manganese(II) in this succinate-bound form (Fig. 4B), while one water and two oxygen from α-KG bind the metal in the α-KG-bound form (Fig. 4A).
3.3. Structure and sequence comparison with other AlkB family proteins
An overlap of the current structure with the structure of human ALKBH2(hALKBH2)-dsDNA [33] yielded some interesting observations. We found that the loop L2 in ΔfALKBH5 protruded into the dsDNA strand in the overlapped hALKBH2-dsDNA structure (Fig. 3B). ALKBH5 has been shown to exhibit much higher activity to ssRNA than dsRNA [7]; this L2 loop most likely plays the role of discriminating against duplex nucleic acids. In addition, the basic residues in the loop are prone to form hydrogen bonds with acidic residues, which may act to enhance the binding of fALKBH5 to ssRNA. It is interesting that FTO also has a loop (referred as L1) that clashes with potential duplex nucleic acid substrates [30]. The sequence alignment shows that the L1 loop in FTO is unique among the AlkB family proteins (Fig. 3A). The L2 loop is highly conserved among ALKBH5 in different species, and has no similarity to other AlkB family proteins as well (Fig. 3A and Supplementary Fig. 2). An overlay of the structure of FTO and fALKBH5 revealed further insights. We found that L1 and L2 loops are both close to the active site. However, the two loops extend from the opposite directions (Fig. 3C). The two loops most likely play similar roles in blocking potential dsDNA/RNA from gaining access to the substrate-binding site. In addition, ΔfALKBH5 lacks a lid composed of two β-strands over the active site, which exists in FTO as well as in AlkB, hALKBH2, and hALKBH3 (Fig. 3A and Fig. 3D). Referred as “nucleotide recognition lid” in AlkB, this lid is conformationally flexible and is involved in binding to nucleotide substrates. It has been proposed to play a role in the substrate recognition [31]. hALKBH2, hALKBH3 and FTO also possess similar lids that were assumed to perform the same function [28, 30, 33]. However, this lid is not conserved among other these proteins in the AlkB family (Fig. 3A). Comparing the structures of fALKBH5 and FTO, the lid in FTO covers the active site while its absence in fALKBH5 makes the active site more exposed (Supplementary Fig. 7).
3.4. Residues comparison near active site between FTO and fALKBH5
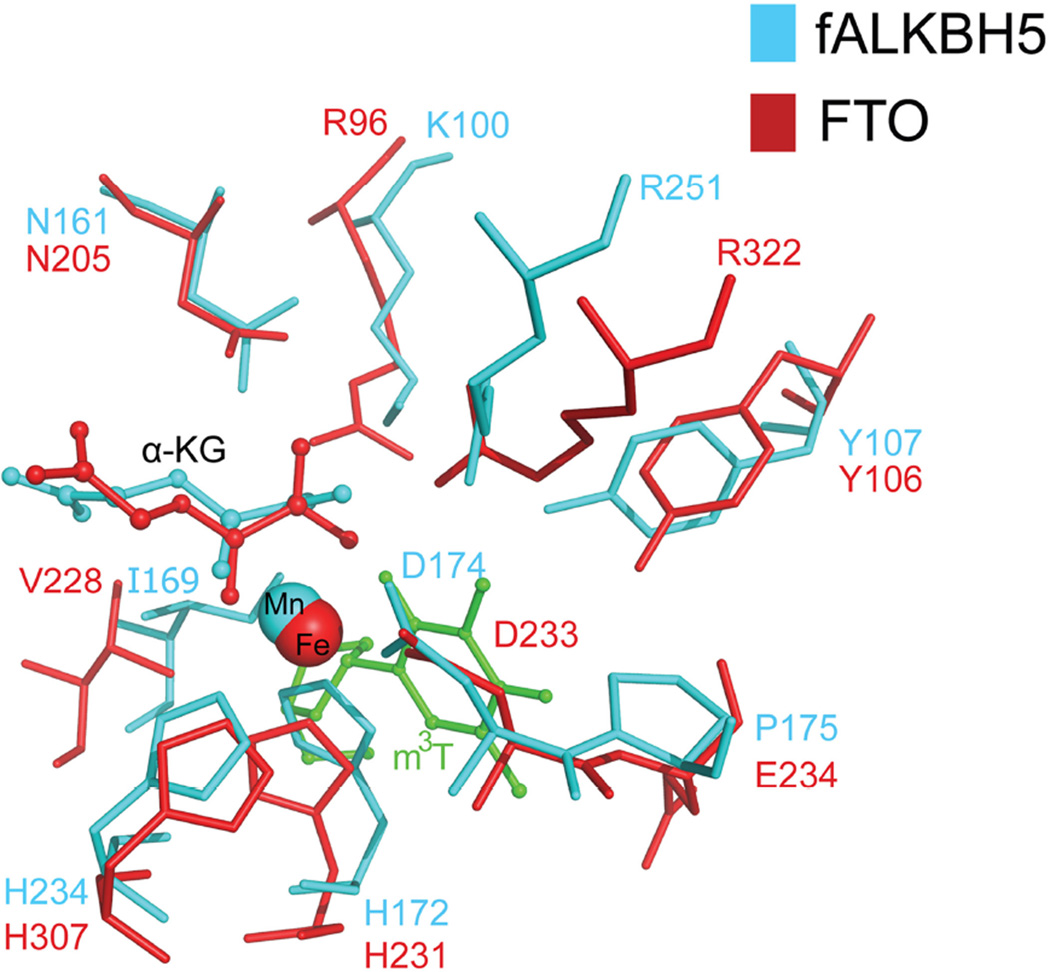
Among all AlkB family proteins, we are most interested in comparisons between ALKBH5 and another m6A demethylase, FTO. We first assayed the demethylation of hALKBH5 with m6A-containing RNA. Under the same conditions that we were able to observe hm6A and fm6A intermediates in the FTO-mediated m6A oxidation, we failed to observe these intermediates in the reaction with hALKBH5 and fALKBH5 (Fig.1A). This observation suggests that ALKBH5 might go through a mechanism of m6A demethylation slightly different from FTO. Supplementary Fig. 8 illustrates the proposed m6A demethylation process. FTO could generate intermediates of hm6A and fm6A in a step-wise manner when converting m6A to A, and both hm6A and fm6A would decompose to adenosine in aqueous solution with both half-lives of around 3 hrs [23]. By contrast, ALKBH5 demethylates m6A to A without observing these intermediates. It is possible that ALKBH5 facilitates/catalyzes decomposition of the generated hm6A when bound to the active site. The exact mechanistic difference related to the production of hm6A will need further biochemical and computational investigations in the future. As viewed through structural alignment, most of residues are visibly conserved except Lys100, Ile169, and Pro175 between fALKBH5 and FTO, which correspond with Arg96, Val228 and Glu234 in FTO (Fig. 5). These three residues in ALKBH5 are also highly conserved among different species (Supplementary Fig. 2). As neutral and hydrophobic residues, Ile169 and Val228 may not directly participate in the demethylation process. Glu234 was reported to form a hydrogen bond with m3T in the FTO-m3T structure, and the mutation of Glu234 to proline resulted in the loss of FTO activity of demethylating m3T [30]. As illustrated in Fig. 5, Pro175 lies in the corresponding position in fALKBH5, which could not form the similar hydrogen bond with nucleic substrates. In addition, mutation of Arg96 in FTO to methionine, glutamine or histidine led to loss of FTO demethylation activity [30, 34], while its equivalent residue is Lys100 in fALKBH5. We have mutated Lys100 to arginine and Pro175 to glutamic acid in fALKBH5, respectively, and tested the demethylation activity of both mutant proteins by MALDI-TOF. As showed in Supplementary Fig. 9, a reduced m6A demethylation activity was observed for ΔfALKBH5 P175E, whereas ΔfALKBH5 K100R almost lost its activity. This result indicated that Lys100 and Pro175 in fALKBH5 are involved in m6A demethylation. Future mechanistic studies are required to study differences between ALKBH5 and FTO.
Fig. 5.
Superimposition of residues near the catalytic core in fALKBH5 and FTO. The mononucleotide m3T in FTO is colored green and residues in fALKBH5 and FTO are labeled in cyan and red, respectively.
4. Conclusions
The non-heme α-KG-dependent AlkB family demethylases mainly catalyze the oxidative demethylation of N-alkylated nucleic acid bases. These members posses a similar catalytic core as well as a highly conserved (HxD..H..R..R) motif. Their substrates differ, however. The molecular basis of substrate-selection and reactions mechanisms have attracted much research attention. Here, we present the high resolution structure of fALKBH5 at 1.65 Å. FTO possesses a loop (L1) that is important for ssRNA-binding, while fALKBH5 possesses a different loop (L2) extended from the opposite direction to accomplish the same function. The presence of this loop explains the preference of ALKBH5 for ssRNA. We also show that unlike FTO, which generates hm6A and fm6A as intermediates in the process of m6A demethylation, such intermediates are not observed in demethylation reactions catalyzed by ALKBH5. Differences in active residues between these two proteins, as well as the weaker interaction between Arg251 and α-KG may explain the light difference in the demethylation pathways of these two proteins. In summary, we report the structure of fALKBH5, which provides a molecular basis for the study of substrate-selection specificity in the AlkB family. With this structure now available, future work will focus on elucidating the potential mechanistic differences between FTO and ALKBH5 m6A demethylation and the potential impact to biology functions.
PDB references
ΔfALKBH5/α-KG 4NPL and ΔfALKBH5/SIN 4NPM.
Supplementary Material
Highlights.
The crystal structure of ALKBH5 from zebrafish.
fALKBH5 is comprised of a conserved jelly-roll motif.
fALKBH5 possesses a loop discriminating against duplex nucleic acids.
Several active site residues are different between fALKBH5 and FTO.
The structure may help understand the RNA demethylation mechanism.
Acknowledgements
We thank Dr. Kay Perry from NE-CAT for structure data procession and structure determination. S.F. Reichard, MA contributed editing. This work is supported by National Institutes of Health GM071440 (to C.H.). W.C. was partially supported by the China Scholar Program, the National Nature Science Foundation of China (91013009 and 21071077) and the Ministry of Science and Technology of China Key Project (2012CB933802).
Abbreviations
- m6A
N6-methyladenosine
- hm6A
N6-hydroxymethyladenosine
- fm6A
N6-formyladenosine
- A
adenosine
- fALKBH5
zebrafish ALKBH5
- α-KG
α-ketoglutarate
- m1A
1-methyladenine
- m3C
3-methylcytosine
- ss
single-stranded
- ds
double-stranded
- cm5U
5-carboxy-methyluridine
- (S)-mchm5U
(S)-5-methoxycarbonyl-hydroxymethyluridine
- m3T
3-methylthymine
- m3U
3-methyluracil
- ΔfALKBH5
truncated zebrafish ALKBH5
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- SIN
succinate acid
- hALKBH3
human ALKBH3
- hALKBH8
human ALKBH8
- hALKBH2
human ALKBH2
- rmsd
root-mean-squaredeviation deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version.
References
- 1.Bokar JA. The biosynthesis and functional roles of methylated nucleosides in eukaryotic mRNA. Fine-tuning of RNA functions by modification and editing, topics in current genetics. 2005 Springer;12:141–177. [Google Scholar]
- 2.Beemon K, Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J. Mol. Biol. 1977;113:165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- 3.Shah J, Clancy M. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:1078–1086. doi: 10.1128/mcb.12.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodi Z, Zhong S, Mehra S, Song J, Graham N, Li H, May S, Fray RG. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front. Plant Sci. 2012;48:1–9. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokar J, Shambaugh M, Polayes D, Matera A, Rottman F. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RPG, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2012;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 9.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Gerken T, Girard CA, Tung YCL, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O’Rahilly S, Schofield CJ. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics. 2003;4:48. doi: 10.1186/1471-2164-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi C, Yang CG, He C. A non-heme iron-mediated chemical demethylation in DNA and RNA. Acc. Chem. Res. 2009;42:519–529. doi: 10.1021/ar800178j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 15.Falnes PØ, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 16.Aas PA, Otterlei M, Falnes PØ, Vågbø CB, Skorpen F, Akbari M, Sundheim O, Bjørås M, Slupphaug G, Seeberg E, E Krokan H. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y, Dai Q, Zhang W, Ren J, Pan T, He C. The AlkB Domain of Mammalian ABH8 Catalyzes Hydroxylation of 5-Methoxycarbonylmethyluridine at the Wobble Position of tRNA. Angew. Chem. Int. Ed. 2010;49:8885–8888. doi: 10.1002/anie.201001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Born E, Vågbø CB, Songe-Møller L, Leihne V, Lien GF, Leszczynska G, Malkiewicz A, Krokan HE, Kirpekar F, Klungland A, Falnes PØ. ALKBH8-mediated formation of a novel diastereomeric pair of wobble nucleosides in mammalian tRNA. Nat. Commun. 2011;2:172. doi: 10.1038/ncomms1173. [DOI] [PubMed] [Google Scholar]
- 19.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Stunff CL, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi Sp, Chèvre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 20.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, shield B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJF, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CNA, Doney ASF, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou Z, He C. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–3319. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Jia G, Pang X, Wang RN, Wang X, Li CJ, Smemo S, Dai Q, Bailey KA, Nobrega MA, Han KL, Cui Q, He C. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C. Grand challenge commentary: RNA epigenetics? Nat. Chem. Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 25.Zhou B, Han Z. Crystallization and preliminary X-ray diffraction of the RNA demethylase ALKBH5. Acta Crystallogr. F. 2013;69:1231–1234. doi: 10.1107/S1744309113024858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly MI, Zhou M, Millard CS, Clancy S, Stols L, Eschenfeldt WH, Collart FR, Joachimiak A. An expression vector tailored for large-scale, high-throughput purification of recombinant proteins. Protein Expres. Purif. 2006;47:446–454. doi: 10.1016/j.pep.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costas M, Mehn MP, Jensen MP, Que L. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 28.Sundheim O, Vågbø CB, Bjørås M, Sousa MM, Talstad V, Aas PA, Drabløs F, Krokan HE, Tainer JA, Slupphaug G. Human ABH3 structure and key residues for oxidative demethylation to reverse DNA/RNA damage. EMBO J. 2006;25:3389–3397. doi: 10.1038/sj.emboj.7601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastore C, Topalidou I, Forouhar F, Yan AC, Levy M, Hunt JF. Crystal structure and RNA binding properties of the RNA recognition motif (RRM) and AlkB domains in human AlkB homolog 8 (ABH8), an enzyme catalyzing tRNA hypermodification. J. Biol. Chem. 2012;287:2130–2143. doi: 10.1074/jbc.M111.286187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Z, Niu T, Chang J, Lei X, Zhao M, Wang Q, Cheng W, Wang J, Feng Y, Chai J. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464:1205–1209. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 31.Yu B, Edstrom WC, Benach J, Hamuro Y, Weber PC, Gibney BR, Hunt JF. Crystal structures of catalytic complexes of the oxidative DNA/RNA repair enzyme AlkB. Nature. 2006;439:879–884. doi: 10.1038/nature04561. [DOI] [PubMed] [Google Scholar]
- 32.Mishina Y, Duguid EM, He C. Direct reversal of DNA alkylation damage. Chem. Rev. 2006;106:215–232. doi: 10.1021/cr0404702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CG, Yi C, Duguid EM, Sullivan CT, Jian X, Rice PA, He C. Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA. Nature. 2008;452:961–965. doi: 10.1038/nature06889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyre D, Proulx K, Kawagoe-Takaki H, Vatin V, Gutiérrez-Aguilar R, Lyon D, Ma M, Choquet H, Horber F, Van Hul W, Van Gaal L, Balkau B, Visvikis-Siest S, Pattou Fo, Farooqi IS, Saudek V, O’Rahilly S, Froguel P, Sedgwick B, Yeo GS. Prevalence of loss-of-function FTO mutations in lean and obese individuals. Diabetes. 2010;59:311–318. doi: 10.2337/db09-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.