Abstract
In Drosophila, the GAL4/UAS/GAL80 repressible binary expression system is widely used to manipulate or mark tissues of interest. However, complex biological systems often require distinct transgenic manipulations of different cell populations. For this purpose, we recently developed the Q system, a second repressible binary expression system. We describe here the basic steps for performing a variety of Q system experiments in vivo. These include how to generate and use Q system reagents to express effector transgenes in tissues of interest, how to use the Q system in conjunction with the GAL4 system for the generation of intersectional expression patterns that precisely limit which tissues will be experimentally manipulated, and how to use the Q system to perform mosaic analysis. The protocol described here can be adapted to a wide range of experimental designs.
Keywords: MARCM, QF, QS, quinic acid, QUAS, transcription factor, coupled MARCM, reporter expression, fly, technique
INTRODUCTION
Binary expression is a powerful strategy for regulating expression of an effector transgene for the purpose of interrogating the development or function of cells and tissues in multicellular organisms. In such a strategy, one transgene contains a specific promoter driving an exogenous transcription factor, while the other transgene uses a promoter specifically activated only by that introduced transcription factor. An additional layer of control is afforded if the transcription factor itself can be specifically inhibited by an exogenous element. The yeast GAL4 system is such a repressible binary expression system, and has revolutionized experimental manipulations in flies1,2. The GAL4 transcription factor binds to an Upstream Activation Sequence (UAS) to induce expression of a reporter transgene (UAS-geneX). Only when GAL4 and UAS-geneX are in the same animal is geneX expressed in the GAL4 expression pattern. Thousands of GAL4 lines have been characterized for tissue and developmental expression patterns, and can be used in combination with thousands of effector lines. Effector lines range from cell markers (e.g., membrane tagged GFP) to signaling molecules (e.g., activated Ras) to inhibitory molecules (e.g., neurotoxins or RNAi constructs). Furthermore, GAL4 activity can be inhibited by GAL80, a natural suppressor of GAL43. Thus, when GAL80 is co-expressed with GAL4, UAS-geneX reporters are silent. This allows for further effector refinement, including the Mosaic Analysis with a Repressible Cell Marker (MARCM) technique3. The combination of the three GAL4 components (GAL4, UAS-geneX, GAL80) allows for a rich diversity of experimental investigations.
Nonetheless, the GAL4 system has its limitations. UAS-geneX effectors can only be expressed in the one population of cells defined by GAL4. In complex cellular organisms, it is often desirable to express an effector in a fraction of a cellular population, and then examine the effects on the other population of cells. Likewise, one might want to differentially label and manipulate two different types of tissues- neurons labeled with GFP and glia labeled with RFP. Such techniques would be invaluable for determining non-cell autonomous effects (such as ligand/receptor interactions).
We have recently characterized the Q system for these and other purposes4. The Q system utilizes genes from the qa gene cluster of the filamentous fungus Neurospora crassa. This gene cluster, consisting of 7 genes, is required for the catabolism of quinic acid (quinate) under conditions of limited glucose levels5–9. This gene cluster contains two regulatory genes: qa-1f (encoding a protein of 816 aa) and qa-1s (encoding a protein of 918 aa). qa-1f (shortened as QF hereafter) is a transcription factor, and qa-1s (shortened as QS hereafter) is a repressor of QF. The other 5 genes in the qa gene cluster encode enzymes or cofactors required for the catabolism of quinic acid. The promoters for the 7 qa genes in Neurospora contain binding sites for QF, and expression of the qa genes can be induced by the QF transcription factor. The binding site for QF is the sequence “GGRTAA RYRY TTATCC” (R is A/G, Y is C/T). Under normal growth conditions where glucose is high, QS binds to and inhibits QF, and prevents expression of the qa gene cluster. However, when glucose is limiting and quinic acid is present, quinic acid binds to and inhibits QS. This releases QF from QS suppression, allowing QF now to induce expression from the qa gene cluster. This results in the expression of the factors required for the catabolism of quinic acid as an energy source. In effect, the catabolite (quinic acid) controls expression of the genes required for its catabolism.
Development of the Q system in Drosophila
The Q system introduced into Drosophila consists of three components: the QF transcription factor, a QUAS-geneX effector, and the QS suppressor (Fig. 1, Fig. 2). The QUAS element contains 5 QF binding sites, and allows for robust QF-dependent expression of the effector. As such, the Q system contains the same 3 basic components (QF, QUAS, QS) as the analogous GAL4 system (GAL4, UAS, GAL80). In addition, the molecule quinic acid can inhibit the QS suppressor in flies fed a diet containing quinic acid (Fig. 1, Fig. 2). This allows temporal control of the Q system upon treatment with this non-toxic molecule (Fig. 1).
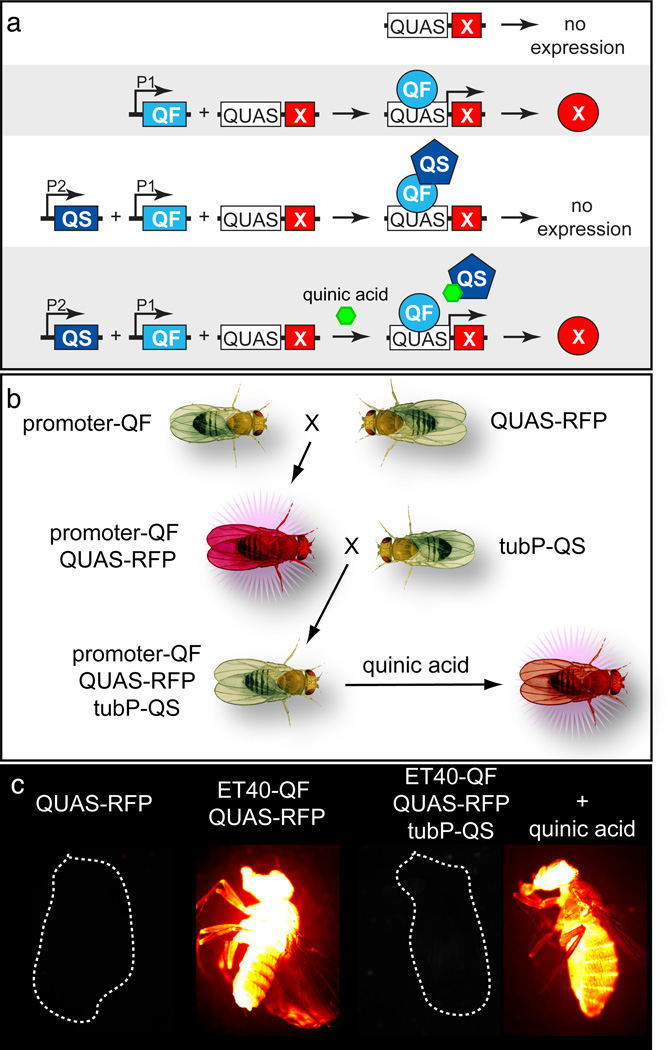
Figure 1. Schematic and example of Q system components in Drosophila.
(a) Schematic representing the function of the Q system components. P1 and P2 indicate Promoter 1 and Promoter 2.
(b) Diagram illustrating a crossing scheme for Q system transgenic flies. tubP indicates the tubulin promoter.
(c) Transgenic Drosophila examples of the genotypes shown in (b). Transgenic flies not expressing the RFP reporter are outlined by a dashed white circle. The quinic acid treated flies developed on quinic acid containing fly food (see Step 6C). Images and schematics reprinted with permission from ref. 4.
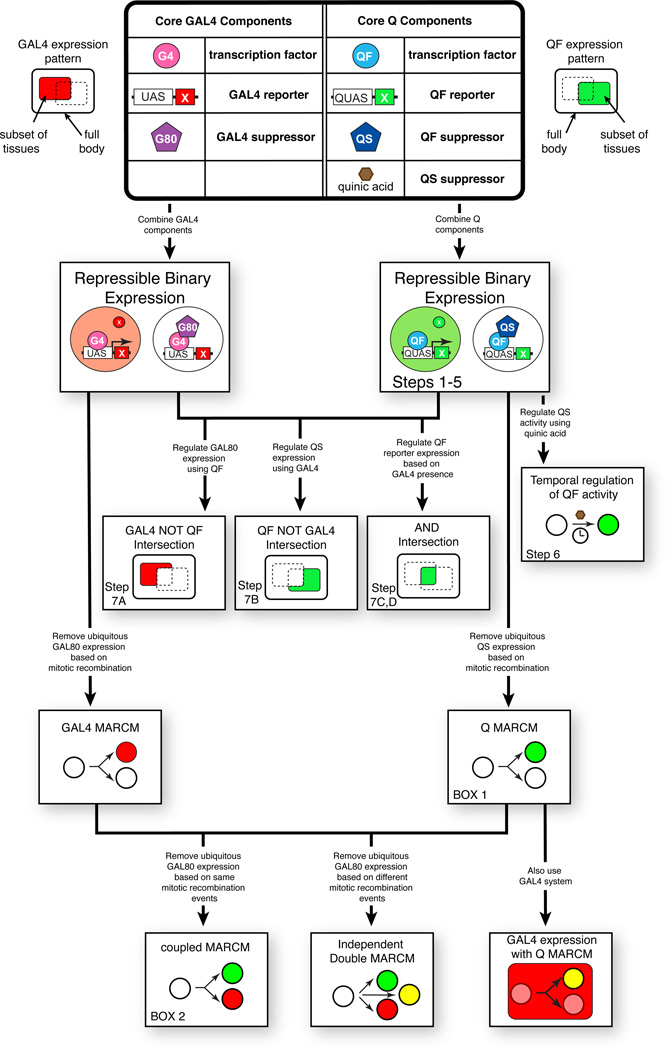
Figure 2. Flowchart of example GAL4 and Q system applications.
The main box illustrates the basic GAL4 and Q system components: the transcription factors GAL4 and QF, the GAL4 and QF reporters UAS-geneX and QUAS-geneX, and the GAL4 and QF suppressors GAL80 and QS. In addition, the Q system includes a small drug inhibitor of QS (quinic acid). The manipulation and combination of these core components (arrows) allows for a number of in vivo applications. The Procedure Step describing the application is listed.
Applications of the method
The Q system contains the same basic components as the GAL4 system, and so can be used for the same applications as the GAL4 system: binary expression in a subset of tissues, refinement of that expression by using the QS inhibitor, and MARCM analysis1,3,10 (Fig. 2). In addition, temporal control of QF activity can be achieved by using quinic acid and QS expression (Fig. 1c, Fig. 4). However, a key experimental advantage is obtained when the Q repressible binary system is used in conjunction with the GAL4 repressible binary system. Figure 2 shows some of the possible applications achievable. Highlighted is the ability to define intersectional expression patterns whereby finer precision of tissue manipulations can be achieved (Figs. 5–8, Fig. 11). In addition, the Q system can be used for MARCM analysis (Fig. 9), which has a variety of in vivo applications3,10–13. Since the Q system and GAL4 system function independently in vivo4, Q-MARCM and GAL4 MARCM can be coupled to the same mitotic event. As such, an unlabeled progenitor cell would give rise by mitosis to one cell that is positively labeled by the Q system (as it lacks the QS repressor), and a sister cell that is positively labeled by the GAL4 system (as it lacks the GAL80 repressor). This is called “coupled MARCM” as the segregation of the QS and GAL80 suppressor are coupled to the same mitotic event (Fig. 10). This allows for the differential marking and manipulation of all progeny from a single mitotic event. If segregation of the QS and GAL80 suppressor were not coupled to the same mitotic event, then the cell progeny could independently be labeled or unlabelled by the GAL4 or Q system. This is called “independent double MARCM” (Fig. 2).
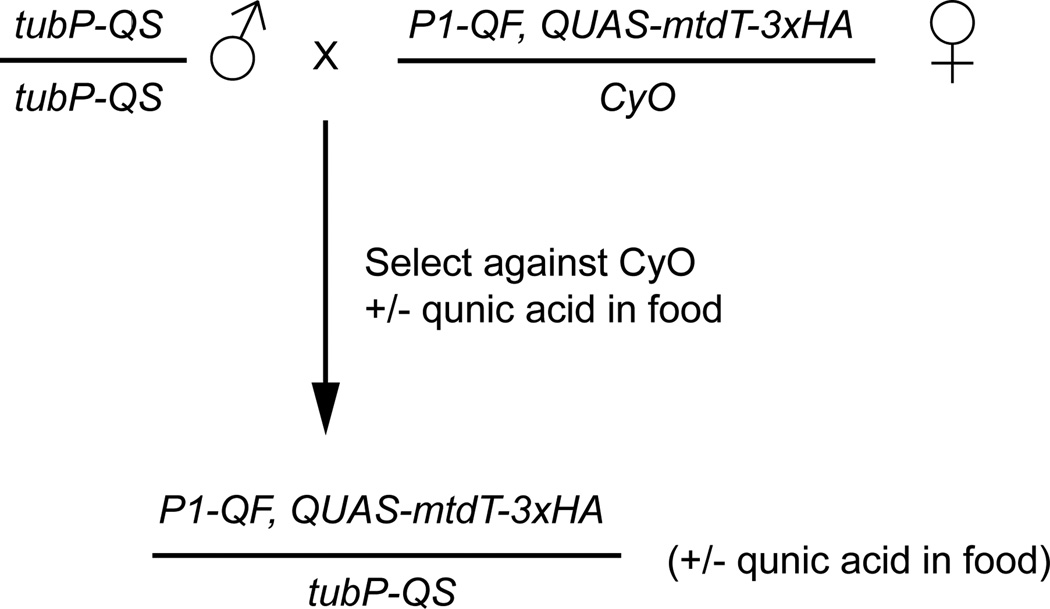
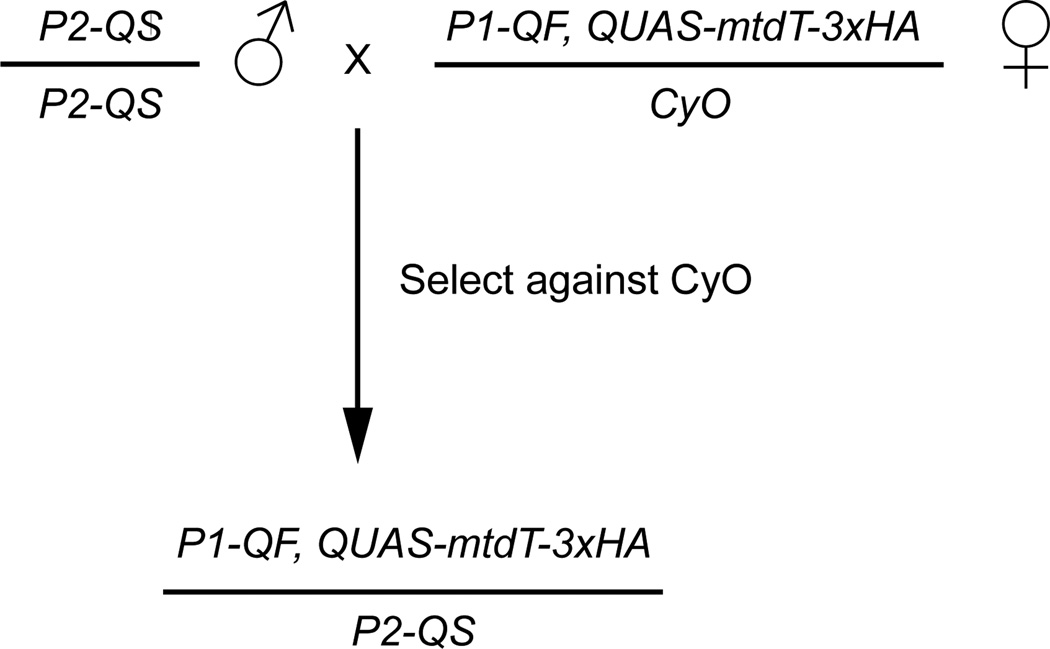
Figure 4. Crossing scheme for ubiquitous QS mediated suppression of QF coupled with quinic acid treatment.
Ubiquitous QS expression is achieved by using a tubulin promoter to drive QS (tubP-QS). Crossing tubP-QS to a P1-QF, QUAS-geneX recombinant and selecting against the CyO balancer will result in progeny that no longer express the QUAS-geneX effector in any tissues. This QS mediated suppression can be inhibited by feeding developing flies quinic acid, or by feeding adult flies quinic acid. If treated with quinic acid, the QUAS-geneX reporter induced by P1-QF will be expressed. Differing levels of QS suppression can be achieved by altering the concentration of quinic acid fed to the flies.
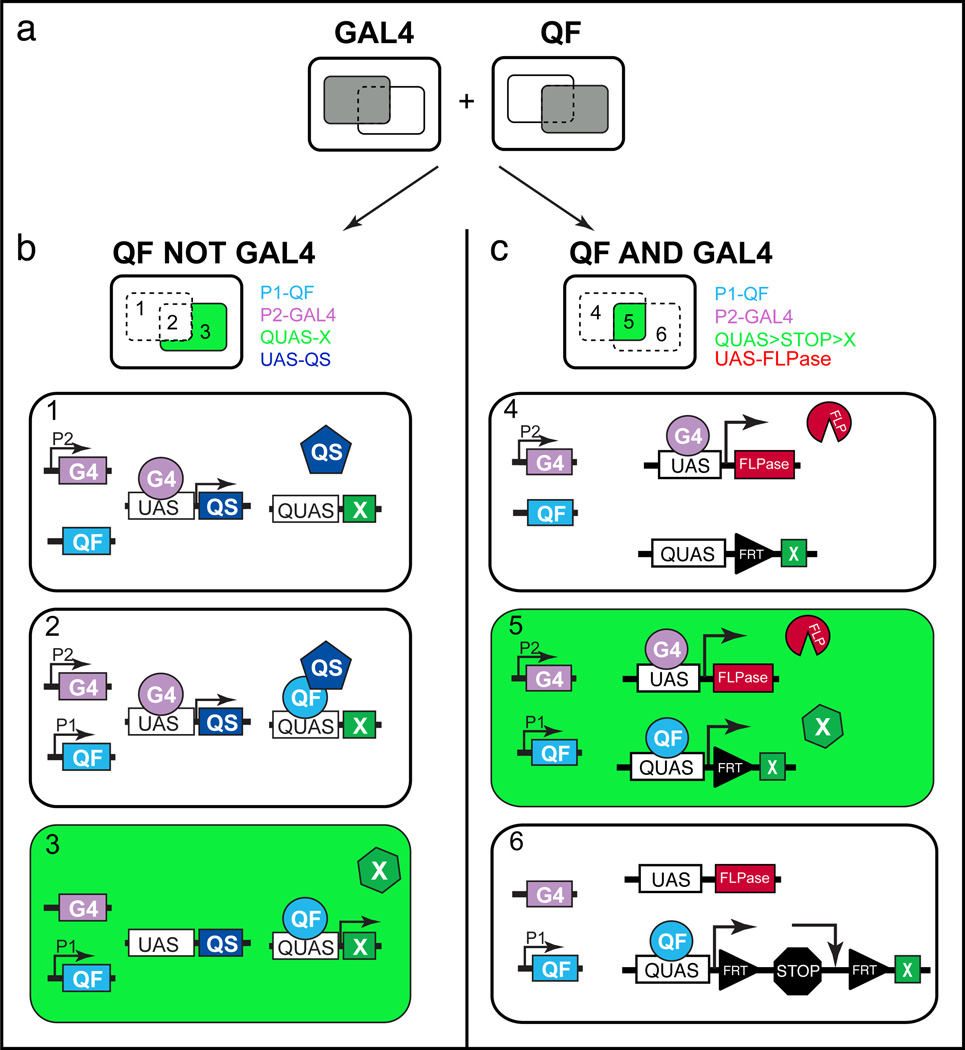
Figure 5. Using the Q system with the GAL4 system for generating intersectional expression patterns.
(a) The gray squares represent the extent of the GAL4 or QF expression pattern.
(b) In the “QF NOT GAL4” example, a GAL4 line (P2-GAL4) is used to drive expression of the QS suppressor (UAS-QS) to restrict QUAS-geneX expression. This results in a final expression pattern reflecting where QF is expressed but not where GAL4 is also expressed. Region 1 does not express the QUAS-geneX since P1-QF is not expressed in this region. Region 2 expresses both P2-GAL4 and P1-QF but does not express the QUAS-geneX due to expression of QS. Only region 3 expresses the QUAS-geneX. See Step 7B.
(c) In the “QF AND GAL4” example, QUAS-geneX expression is limited to regions where both QF and GAL4 are expressed. The QUAS-geneX contains a ‘FRT-transcription stop-FRT’ cassette (>stop>) between the QUAS promoter and the reporter gene. This cassette can be excised by the activity of the FLPase recombinase. Region 4 does not express the QUAS>stop>geneX since QF is not expressed in this region. Region 5 expresses the QUAS>stop>geneX since P2-GAL4 induces UAS-FLPase expression, which removes the transcription stop cassette, allowing for P2-QF induced expression. Region 6 does not express the QUAS>stop>geneX since P2-GAL4 is not expressed in this region. ‘>’ indicates FRT. See Step 7C.
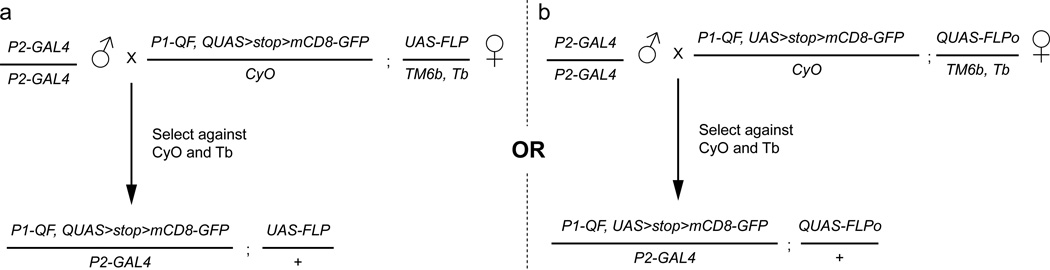
Figure 8. Crossing scheme for QF AND GAL4 intersectional experiments.
There are two strategies to perform an AND intersectional cross. Both strategies require 4 components to be combined: P1-QF and P2-GAL4 along with (a) UAS-FLP, QUAS>stop>mCD8-GFP or (b) QUAS-FLPo, UAS>stop>mCD8-GFP. In these examples, QF AND intersectional ready flies are shown for each strategy. These AND intersectional ready females flies contain all the necessary components except for the P2-GAL4. Crossing these stocks to any GAL4 line and selecting against the balancers will result in progeny that only have expression where both QF and GAL4 are expressed. These crossing schemes simplify the experimental design required to quickly test many different GAL4 lines for their intersection with a characterized QF line. Although both strategies limit expression to only where GAL4 and QF are expressed, they are not equivalent. In (a) the resulting expression pattern is determined by the developmental expression pattern of the GAL4 line, and the final expression pattern of the QF line. Conversely, in (b) the resulting expression pattern is determined by the developmental expression pattern of the QF line, and the final expression pattern oftheGAL41ine.
Figure 11. Example intersectional expression experiments between GAL4 and QF olfactory projection neuron lines.
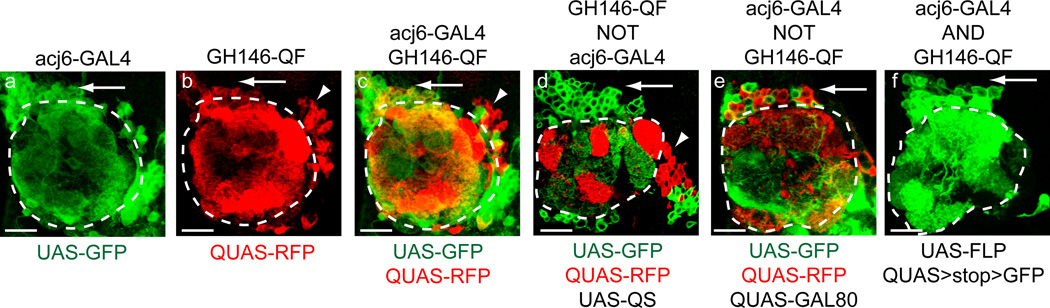
(a) Shown is the antennal lobe innervation of acj6-GAL4 projection neurons labeled by UAS-mCD8-GFP. The antennal lobe is circled. The arrow in all panels points to the dorsal population of projection neuron cell bodies, (b) Shown is the antennal lobe innervation of GH146-QF labeled by QUAS-mtdt-3xHA. The arrowhead in all panels points to a GH146+ lateral population of projection neuron cell bodies. (c) GH146-QF expresses in a subset of acj6-GAL4 expressing dorsal projection neurons (labeled in yellow). GH146-QF and acj6-GAL4 do not express in the same population of lateral projection neurons. (d) Example of the GH146-QF NOT acj6-GAL4 intersectional expression pattern. QUAS-mtdT-3xHA is no longer expressed in any of the dorsal projection neurons (arrow) due to acj6-GAL4 expression (green) driving UAS-QS. The lateral GH146-QF projection neurons remain labeled (arrowhead) since they do not express acj6-GAL4. (e) Example of the acj6-GAL4 NOT GH146-QF intersectional expression pattern. UAS-mCD8-GFP is no longer expressed in a subset of dorsal projection neurons due to GH146-QF expression (red) driving QUAS-GAL80. (f) Example of the acj6-GAL4 AND GH146-QF intersectional expression pattern. The QUAS>GFP reporter is only expressed in a subset of dorsal projection neurons that coexpress both acj6-GAL4 and GH146-QF (arrow). The lateral projection neurons are not labeled. GFP, mCD8-GFP; RFP, mtdT-3xHA; Scale bars: 20 µm. Panels (d) and (f) reprinted with permission from ref. 4.
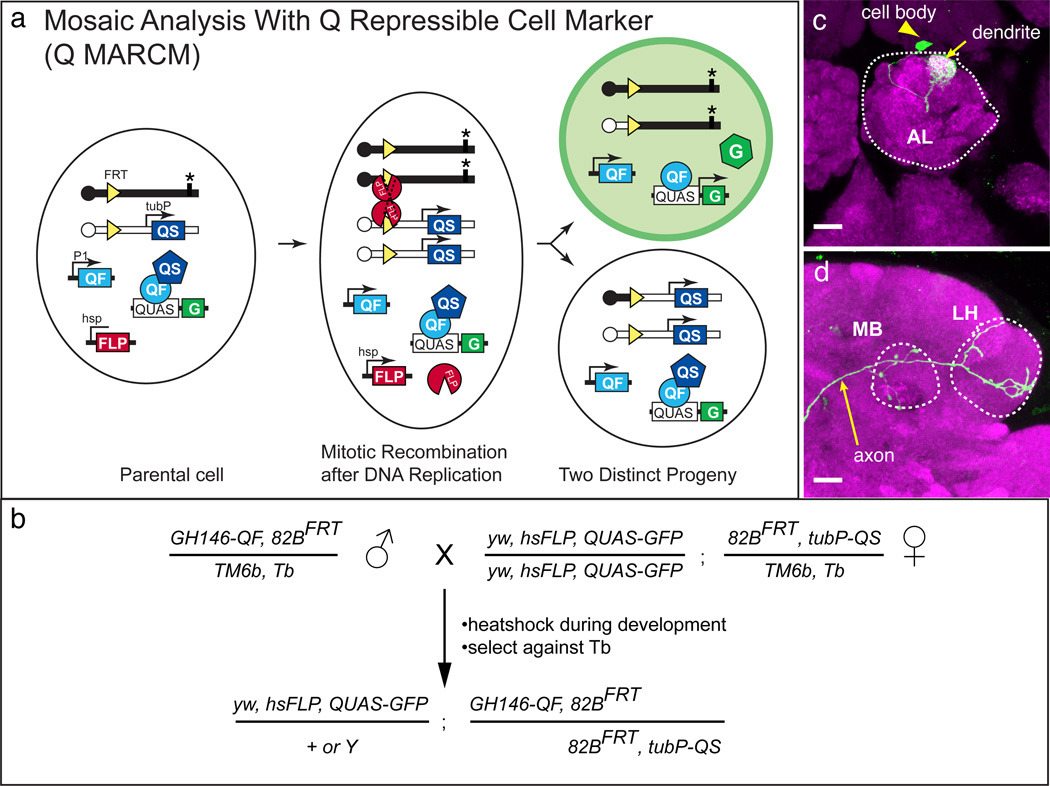
Figure 9. Schematic and example of Q-based Mosaic Analysis with a Repressible Cell Marker (Q MARCM).
(a) In a MARCM experiment, ubiquitous expression of the QS suppressor (driven by the tubulin promoter) is removed by a mitotic recombination event mediated by the FLP/FRT system, which allows for QF to activate QUAS-geneX reporters in a subset of cells. The parental cell contains sister chromosomes (black bar and white bars) containing the same FRT insertion (yellow triangle) distal to the centromere (circles). Distal to one of the FRT sites is the tubP-QS transgene. The other sister chromosome could contain a mutation of interest (*). FLPase expression is under control of a heat shock promoter (hsp). A heat shock pulse induces FLPase expression (red pacman) at or before mitosis. FLP/FRT mediated mitotic recombination at the G2 phase of the cell cycle (dotted black cross) followed by the chromosome segregation shown causes the top cell progeny to lose both copies of tubP-QS, restore QF activity, and become capable of expressing the QUAS-GFP marker (G). It also becomes homozygous for a mutation (*). hsFLP, QF, and QUAS-geneX transgenes can be located on any other chromosome arm. Schematic modified from ref. 4.
(b) Example crossing strategy for the Q MARCM experiment in (c–d).
(c–d) Example of a single DL1 olfactory projection neuron labeled by Q MARCM. The antennal lobe (AL), mushroom body calyx (MB), and lateral horn (LH) are outlined. Reprinted with permission from ref. 4. Scale bars: 20 µm.
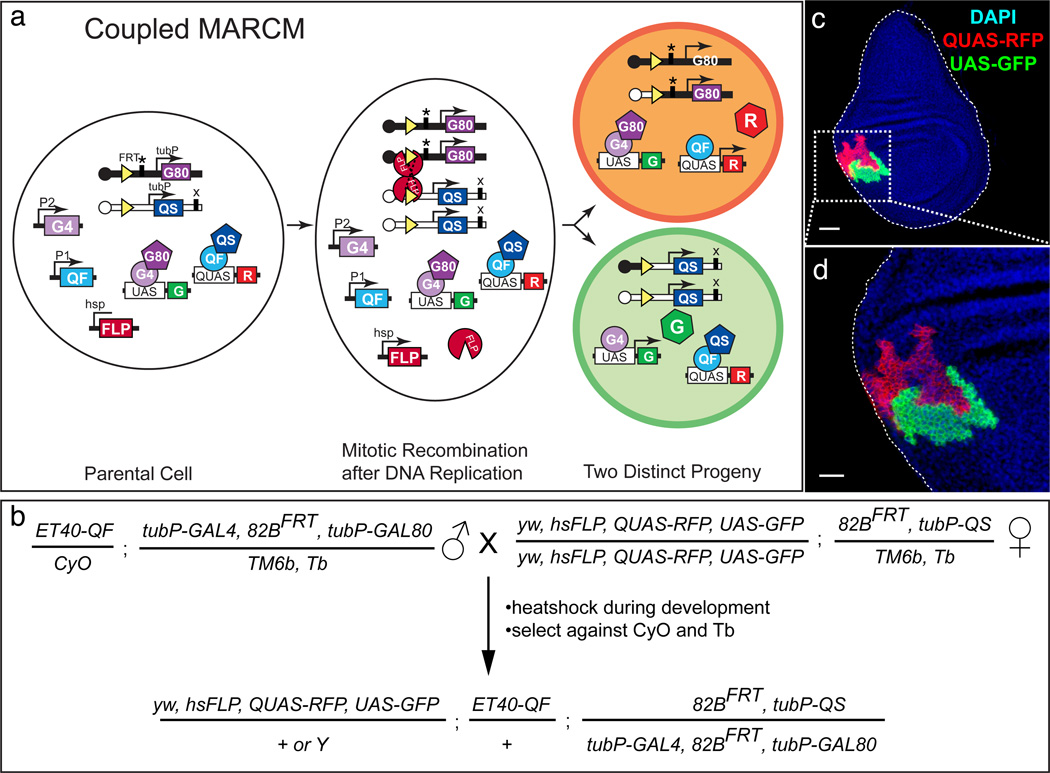
Figure 10. Schematic and example of coupled MARCM.
(a) In a coupled MARCM experiment, ubiquitous expression of the QS and GAL80 suppressors (driven by the tubulin promoter) are simultaneously segregated to different progeny by an experimentally induced mitotic recombination event. This results in two distinct progeny- one that has an active QF (due to loss of the QS suppressor) and the other that has an active GAL4 (due to loss of the GAL80 suppressor). See Figure 9 for additional details. ‘*’ and ‘x’ designate two independent mutations that can be rendered homozygous in sister progeny. hsFLP, QF, GAL4, UAS-geneX, and QUAS-geneX transgenes can be located on any other chromosome arm. Schematic modified from ref. 4.
(b) Example crossing strategy for the coupled MARCM clone shown in (c–d). ET40-QF is a QF enhancer trap on the second chromosome which expresses QF in imaginal discs.
(c–d) Example coupled MARCM clone in a third instar larval wing imaginal disc. Cell nuclei are labeled with DAPI. Larvae were heat shocked for 30 minutes at 48 hrs after egg laying. Scale bars: 20 µm.
Comparisons with other methods
Binary Expression Systems
The bacteria LexA/LexAop binary expression system has also been used to express effectors independently of GAL414. LexA contains a DNA binding domain specific for the LexA operator (LexAop), yet does not contain a transcriptional activation domain. In Drosophila, LexA is either fused to the viral acidic activation domain VP16 or the GAL4 activation domain (GAD). The LexA-VP16 protein is insensitive to GAL80, whereas the LexA-GAD protein can be inhibited by GAL80. The LexA/LexAop system does not contain an endogenous suppressor, and so cannot be used to generate some intersectional expression patterns or for GAL4 independent MARCM analysis. The LexAop-geneX reporter also exhibits a higher basal level of expression compared to UAS-geneX or QUAS-geneX reporters4. Nonetheless, recent progress has been made to optimize the LexA/LexAop binary expression system for use in vivo15,16.
Intersectional Expression Patterns
Limiting GAL4 expression patterns can also be achieved by expressing GAL80 in the tissue of interest3,17. However, GAL80 expression patterns are difficult to determine, and GAL80 levels need to be higher than GAL4 for effective suppression. This can make it difficult to precisely define the resulting GAL4 expression pattern. A better approach, as detailed in Figure 2 and Step 7A, is to use a binary expression system to drive GAL80 expression. Similarly, the LexA/LexAop system could be used to refine GAL4 expression patterns. In this case, LexA-VP16 would be used to drive LexAop-GAL80. However, given the lack of an independent repressor of LexA, the reciprocal experiment (using GAL4 to limit LexAop-geneX reporter expression) is not possible. This approach is possible using the Q system (Step 7B, Fig. 5b).
Limiting expression patterns to overlapping subsets is also achieved by using the “split GAL4” method in which GAL4 is split into two halves- one half containing the DNA binding domain and the other half containing the activation domain18. The two GAL4 halves can be reconstituted in vivo by the addition of leucine zippers to the split GAL4 proteins. This technique can achieve precise intersectional expression patterns18. However, split GAL4 can not utilize existing characterized GAL4 lines for intersectional expression, the reconstituted GAL4 is not as robust as the original GAL4, and split GAL4 transgenes are not useful for many other purposes (in contrast to a new QF reagent that can be used for binary expression or MARCM experiments).
Mosaic labeling methods
Coupled MARCM allows for the labeling of all progeny from a single mitotic event. It can also be used for independent gain- and loss-of-function genetic manipulations of both progeny. A number of other techniques also allow for the marking of both sister progenies.
“Dual expression control MARCM” uses LexA-GAD in conjunction with GAL4 based MARCM to visualize progeny from a cell division14. This technique allows labeling of different populations of cells (one labeled by the LexA driver and the other by the GAL4 driver) that arise from a common progenitor. However, since both LexA-GAD and GAL4 are suppressed by GAL80, this prevents labeling and manipulation of all progeny from a cell division. This technique has been used successfully for lineage analysis of certain neuronal populations14,19.
“Twin-spot MARCM” uses UAS-Inverse Repeat (UAS-IR) transgenes as the source of repressors against two different fluorescent proteins. Similar in design to coupled MARCM which utilizes the differential loss of tubP-GAL80 and tubP-QS, twin-spot MARCM follows the coupled loss of the UAS-IR repressors20. This creates two sibling cells, each losing one of the RNAi repressor genes. Twin-spot MARCM is simpler in design than coupled MARCM (since it utilizes fewer transgenes). However, both progeny are labeled by the same GAL4 driver, which could miss labeling of a cell progeny that lies outside this expression pattern. In addition, since the system is GAL4 only based, cell progeny can not be independently manipulated. Nonetheless, this technique is a powerful method for resolving the lineage pattern of a GAL4 expression pattern20,21.
“Twin-spot generator” (TSG) does not utilize a binary expression system but instead places two split chimeric fluorescent proteins on the same chromosome arm in trans22. Upon FLP/FRT-mediated recombination, the two fluorescent proteins are reconstituted and can be segregated to daughter cells. This is similar in design to the mouse MADM system for mosaic analysis23. The advantage of the TSG method over other methods that use a repressible binary system is the ability to examine clones shortly after clonal induction since there is no perdurance of a repressor molecule. However, a major limitation is low marker expression due to the lack of binary system-based amplification. In addition, both markers are driven by a ubiquitous promoter, which severely limits the utility for tracking complex lineages. And since TSG does not utilize a repressible binary system, cell progeny cannot be easily manipulated by effector transgene expression.
Limitations of the Q system
As the Q system has only been recently introduced, a number of Q reagents, such as QUAS-geneX effectors or promoter-QF lines, remain to be generated. However, as more studies use the Q system, the availability of useful reagents will grow. Alternatively, in cases where the GAL4 system is not sufficient, the LexA/LexAop system could be used if LexA system reagents have already been generated and validated for a tissue of interest, and experimental designs do not require an endogenous LexA suppressor.
Experimental Design
Generation of QF transgenic flies
The first step for many Q system studies is the generation of Q system reagents for the manipulation of target tissues. The most straightforward approach is to clone a previously characterized enhancer/promoter region of interest into a QF DNA construct. A number of suitable QF DNA constructs are shown in Supplementary Table 1. There are two basic choices for cloning QF constructs: pattB-QF-hsp70 and pattB-QF-SV40. These differ in their 3’ transcriptional terminators. SV40 terminators lead to increased mRNA stability and higher protein levels. We have found that in most cases this increased protein level is not necessary or desirable when generating QF constructs due to potential toxicity of high level QF expression in as-yet-unidentified tissues. We therefore recommend that the pattB-QF-hsp70 construct be used for routine enhancer and promoter cloning.
There are three basic strategies for generating QF transgenics utilizing previously characterized expression patterns. The first involves the cloning of gene promoters. In many cases, an enhancer and promoter region will be the genomic region immediately upstream of the ATG start site of a gene up to the preceding gene24. A PCR reaction that introduces flanking BamHI and EcoRI restriction sites can be used to amplify this genomic region for placement into the pattB-QF-hsp70 construct.
The second strategy to generate QF expression patterns of interest is to clone the genomic region associated with enhancer trap insertions. The expression pattern of an enhancer trap could be mimicked by cloning a large genomic region immediately preceding the insertion site of an enhancer trap4,11,25. In this case, a promoter would also need to be included, such as either the P-element promoter or the synthetic DSCP promoter26 with the QF-hsp70 cassette following the cloned genomic region.
When the above two approaches fail to recapitulate the expression pattern of interest, a third strategy is to clone a larger genomic region associated with the gene or enhancer trap insertion. BAC recombineering could be used to insert a promoter-QF-hsp70 cassette into a larger genomic region (20kb or 80kb) to more likely recapitulate a complex regulatory locus27. These BAC resources are compatible with PhiC31 integration for the generation of transgenic animals28,29.
In some cases, generating promoter-QF transgenic lines might be difficult, especially for constructs that would result in widespread expression of QF. This could be due to QF being more toxic than GAL4. To reduce QF expression (and potential QF toxicity), the QF cDNA has been codon non-optimized for Drosophila expression. This allows for the generation of promoter-QF constructs that were previously difficult to generate, such as a pan-neuronal synaptobrevin-QF (CJP, unpublished results).
In addition, success rates for generating QF transgenic lines can be improved when using P-element or piggyBac based vectors instead of attB vectors (CJP, unpublished results). Alternative QF coding variants and QF cloning vectors that use piggyBac or P-elements are available from the authors upon request.
Generation of QF enhancer trap lines
Enhancer trap lines can often give rise to an expression pattern that is difficult to reproduce by cloning. In addition, a small scale enhancer trap screen can quickly generate many new expression patterns in parallel. A number of suitable QF enhancer trap DNA constructs are shown in Supplementary Table 1. These constructs can be injected with P-element transposase to generate new QF enhancer trap lines. Note that the available QF enhancer traps are P-element based and use an SV40 terminator. Alternative QF enhancer traps that use hsp70 terminators or piggyBac vectors are available from the authors upon request.
In addition, existing QF enhancer trap lines (Supplementary Table 2) can be mobilized by crossing to a stable P-element transposase (e.g., Δ2–3, Bloomington Stock #1798) to generate additional QF lines exhibiting new expression patterns. A small screen of ~25 lines has already identified QF enhancer trap lines that label trachea (ET14-QF), glia (ET31-QF), imaginal discs (ET40-QF), and many tissues including neuronal and epithelial (ET49-QF).
QF enhancer traps (and occasionally promoter-QF transgenes) can exhibit tracheal expression, especially if the trapped enhancers are weak. This is likely due to a cryptic weak tracheal enhancer in the QF coding sequence. Constructs that use QF coding variants (and no longer contain the cryptic tracheal enhancer) exhibit decreased or no tracheal expression in enhancer traps (CJP, unpublished results). In addition, tracheal-promoter-QS transgenic lines can be used to inhibit tracheal QF induced reporter expression (CJP, unpublished results). These reagents are available from the authors upon request.
Generation of QUAS-geneX effector lines
Another important Q system reagent is the QF inducible reporter- QUAS-geneX. A number of QUAS-geneX transgenic flies are available (Supplementary Table 2). To simplify the generation of additional QUAS-geneX transgenic flies, the pQUAST vector (Supplementary Table 1) contains the same multicloning site as the pUAST vector (EcoRI-BglII-NotI-SacII-XhoI-KpnI-XbaI), which allows for easy exchange of inserts between pUAST and pQUAST Vectors. If the pUAST-geneX plasmid is not available, genomic DNA from flies containing the UAS-geneX transgene can be used as the source of the geneX insert4.
By using P-element based transgenesis30, many independent insertions of the same QUAS-geneX construct will be generated. It is often useful to keep a single transgenic line on each of the 3 major chromosomes (X, 2nd, 3rd). Each transgenic line should be tested for inducibility and for lack of position effect. Even though most QUAS-geneX insertions are silent without a QF inducer, occasionally a QUAS-geneX line might be expressed due to induction of the minimal hsp70 promoter by local strong enhancer elements. Such lines should be discarded.
Generation of QS effector lines
QS expression can be used to limit QF reporter expression patterns. Similar to the approaches for cloning QF transgenic animals, a promoter region known to express in defined tissues can be cloned into a QS-SV40 transformation vector (Supplementary Table 1). For example, the EcoRI/KpnI flanked tubulin promoter in ptubP-QS-SV40 could be replaced with the promoter of choice. Alternatively, the QS coding region from pBS-KS-QS (that has restriction sites KpnI-ApaI-HindIII-EcoRI-QS-XbaI-NotI-EagI) could be cloned into an existing promoter-containing vector of choice.
For Q MARCM experiments (BOX 1), ubiquitous QS expression is required. Lines expressing ubiquitous QS (driven by the tubulin promoter) have been recombined with FRT sites for every chromosome arm as well as inserted onto the CyO and TM6B balancers (Supplementary Table 2). In addition, by using a UAS-QS transgenic animal (Supplementary Table 2), GAL4 patterns can be used to direct QS expression with the purpose of limiting QF expression patterns (see Step 7B; Fig. 5b).
BOX 1 Performing Q MARCM experiments. • TIMING Variable, depending on generation of fly stocks (1– 5 generations).
MARCM experiments can serve a variety of purposes including generating mosaic tissues that are mutant for a gene of interest, or for identifying the anatomy of a single neuron. Any QF driver line can be used for Q MARCM experiments (Fig. 9). The protocol below is adapted from a Nature Protocol for performing MARCM experiments10.
Generate Q MARCM ready flies
-
(i)
Use standard genetic techniques to introduce the following genetic components into a single fly: 1) FLP recombinase under the control of a heat-shock promoter, 2) a QUAS-geneX reporter to visualize the Q MARCM clone, such as QUAS-mCD8-GFP, 3) an FRT site and tubP-QS recombined onto the chromosome arm of interest (Fig 9b). tubP-QS insertions recombined with FRT sites are available for each of major chromosome arms (Supplementary Table 2).
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
Generate a promoter-QF line that is Q MARCM ready
-
(ii)
Use standard genetic techniques to combine a QF line (e.g., GH146-QF) with an FRT chromosome that uses the same FRT site as the Q MARCM ready flies generated in the previous step. For example, to be compatible with an 82BFRT, tubP-QS stock, an 82BFRT line with GH146-QF could be used. The GH146-QF insertion can be on any chromosome arm.
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
•CRITICAL STEP The promoter-QF insertion can be located distal to the desired FRT (for example, 82BFRT, promoter-QF). However, since this chromosome arm will become homozygous after the mitotic recombination event, it might affect the tissue of interest in cases where the transgene insertion disrupts proper gene functions. It is recommended instead to position the promoter-QF insertion on any other chromosome arm. If possible, recombine the promoter-QF onto the chromosome arm opposite to the utilized FRT (e.g., promoter-QF, 82BFRT) which can simplify future MARCM experiments.
Perform Q MARCM cross and generate MARCM clones
-
(iii)
Cross 5–10 promoter-QF MARCM ready males to 10–20 Q MARCM ready virgins in a freshly yeasted vial. Depending on the birth date of the tissues of interest, heat shock the progeny in a 37°C water bath for 30 minutes to 2 hours (see ref. 10 for additional details). For example, to generate olfactory projection neuron clones, a 1.5 hr heat shock can be performed from embryonic to third instar stages. For imaginal wing disc MARCM clones, a 30 minute heat shock is performed at 48 hrs after egg laying.
•CRITICAL STEP The developmental time point and extent of the heat shock needs to be experimentally determined for each target tissue. The Q MARCM ready flies often contain a hsFLP insertion on the X chromosome (e.g., Fig. 9b). Using females of these flies for the Q MARCM cross will ensure that both males and female progeny will contain Q MARCM clones.
Analyze and examine Q MARCM clones
-
(iv)
Analyse Q MARCM clones using an appropriate technique10; live or fixed tissues can be used.
? TROUBLESHOOTING
Potential applications of the Q system
The Q system can be used for a variety of in vivo applications. In many cases, the experimental question will determine which QUAS-geneX effector is used. Table 1 presents a sampling of possible studies, the geneX effectors for QUAS-geneX constructs that might be used, and the method of detection or analysis.
Table 1.
Example Applications of the Q system
| Application | geneX for QUAS-geneX | Detection/analysis method |
Reference |
|---|---|---|---|
| Labeling tissues |
mCD8-GFP mtDT-3xHA CD2-HRP |
Live imaging Immunohistochemistry Electron microscopy |
3,4,31,32 |
| Marking different cellular compartments |
EYFP-Mito (mitochondria) EYFP-Golgi (golgi) DenMark (dendrites) synaptotagmin-HA (pre-synaptic termini) nuclearLacZ (nucleus) GFP-α-tubulin (microtubules) |
Live imaging Immunohistochemistry |
12,33–35 |
| Ectopically expressing a gene of interest |
Tsc1/Tsc2 (cell growth/proliferation) Akt (cell growth) TβH (enzyme for synthesis of octopamine) |
Live imaging Electron Microscopy Immunohistochemistry Behavior |
36–38 |
| Cell ablation |
reaper hid grim |
Immunohistochemistry | 39–41 |
| Report cell activity |
GCAMP3 (neural activity) tGPH (PIP3 signaling) |
2-photon microscopy Immunohistochemistry |
42 43 |
| Gene knockdown |
Interfering DNA against geneX (RNAi) microRNA against geneX |
Behavior Live imaging Immunohistochemistry |
44 45 |
| Neuronal activation |
Channel Rhodopsin (blue light activation) TRPA1 (high temperature activation) TRPM8 (low temperature activation) |
Behavior Calcium imaging |
46–49 |
| Neuronal inactivation |
shibirets1 (inhibits vesicle recycling) Kir2.1 (hyperpolarizes neuron) tetanus toxin (cleaves synaptobrevin) |
Behavior | 50–53 |
| Mosaic analysis | reporter (to label clones and/or mutant tissue) | Immunohistochemistry | 3,10,54 |
MATERIALS
REAGENTS
Q system cloning vectors: many Q system cloning vectors (Supplementary Table 1) are available from Addgene (http://www.addgene.org/pgvec1?identifier=Luo.p9EJQGBAq0qGJ7t4LCsvD2Yax9w&cmd=findpub)
Drosophila fly stocks: many Q system fly stocks are available from the Bloomington Stock Center (Supplementary Table 2; http://flystocks.bio.indiana.edu/Browse/misc-browse/Qintro.htm)
quinic acid (Sigma-Aldrich, cat. no. 138622)
Active dry yeast (Red Star Active Dry Yeast, Flystuff.com, cat. no. 62-103)
propionic acid (Flysuff.com, cat. no. 20-271)
EQUIPMENT
Standard fly-culturing equipment
Wide Polystyrene Vials (cat. no. 32-110, Flystuff.com)
Fly Vial Plugs (Wide Flugs, cat. no. 49-101, Flystuff.com)
Dissecting microscope (Stemi 2000, Zeiss).
fluorescent dissecting microscope (Stereo Discovery V8 Pentafluar, Zeiss)
RFP filter cube for V8 Pentafluar (KSC 295-834D DS RED, Zeiss)
GFP filter cube for V8 Penatfluar (KSC 295-814D GFP CUBE, Zeiss)
37 °C water bath for heat-shock (if using heat-shock promoter for FLP expression during MARCM experiments)
humidified 25°C incubator to maintain fly crosses (Environmental Chamber 3940, Forma Scientific)
Imaging microscope and software (e.g., Zeiss LSM 510 confocal microscope)
Sharp forceps for brain dissections (Ted Pella, Inc, cat. no. 503, Dumont Biology Grade Tweezers Style 3)
Three-well glass dissection dishes (Fisher Scientific, cat. no. 21-379)
REAGENT SETUP
Quinic acid solution Dissolve quinic acid in water to achieve desired concentration. Saturated concentration is ~ 300 mg/mL (roughly equivalent to 1.56 M). The solution might need to be incubated at 37°C for ~15 min to help dissolve the quinic acid. Solution can be stored as aliquots at −20°C. The aliquot size for making ~10 quinic acid vials is ~3.5 mL.
0.5% propionic acid (weight/vol) In 1 L bottle, mix 5 grams of propionic acid with 999 mL of water.
Yeast Paste In small container, mix approximately equal volumes of active dry yeast with 0.5% propionic acid. Mix with metal spatula until yeast paste has dissolved. Mix in additional dried yeast as needed to achieve creamy peanut butter consistency.
EQUIPMENT SETUP
Quinic acid containing vials Poke ~10 holes into medium of standard fly vials with wooden sticks. Apply ~300 µl of quinic acid solution to medium, making sure all holes are covered. Cover vials with cotton plug, and allow to dry on benchtop overnight. Vials should be used fresh (within 3–4 days if stored at RT), but can be stored at 4°C for ~ 2 weeks.
PROCEDURE
Performing repressible binary expression experiments. •TIMING ~15 days
-
1|
In a yeasted vial, cross 3–5 promoter1-QF transgenic animals to 3–5 transgenic animals containing the appropriate QUAS-geneX reporter (Fig. 1b; Fig. 2; Table 1; Supplementary Table 2).
-
2.
Depending on the goal of the experiment and the identity of geneX, determine the effect of binary expression on F1 progeny at an appropriate developmental stage using an appropriate method (see Table 1). Alternatively, if promoter1-QF and QUAS-geneX are on the same chromosome, you may wish to proceed directly to Step 3 to generate a stable binary expression stock for subsequent analyses.
Generating a stable binary expression stock. •TIMING 2–3 generations
-
3|
It is often convenient to recombine the promoter1-QF and QUAS-geneX reporter onto the same chromosome for future expression experiments. This requires that the promoter1-QF and QUAS-geneX are both located to the same chromosome. Common QUAS-geneX reporters are available with insertions on each of the 3 major chromosomes (Supplementary Table 2). Choose 5–10 virgin F1 females of genotype promoter1-QF/QUAS-geneX from the progeny in Step 1 and cross to a balancer stock.
•CRITICAL STEP: To get a successful recombinant, it is essential to use F1 heterozygote females since meiotic recombination occurs only in females, and not males.
-
4|
Select single male progeny that contain both copies of the selectable marker (usually two copies of the mini-white+ gene) and set up individual crosses to virgin females from an appropriate balancer stock. Carry out appropriate sib-crosses with the progeny to generate a balanced promoter1-QF,QUAS-geneX stock derived from each original male.
•CRITICAL STEP: Single males are used for establishing balanced recombinant stocks since recombination does not occur in males. The use of single male crosses ensures that a generated stock will be genetically homogeneous.
•CRITICAL STEP If the expression pattern of the promoter1-QF + QUAS-geneX reporter can be visualized in live animals, this expression activity can be used to select for recombinant animals (instead of scoring for both copies of the selectable marker).
-
5|
If desired, use the balanced stocks to analyze the effects of binary expression. Alternatively, proceed to Step 6 to repress or temporally control binary expression or to Step 7 to carry out intersectional experiments in conjunction with the GAL4 system.
Repression and temporal control of QF induced binary expression. •TIMING 1 generation
-
6|
QF induced QUAS-geneX expression can be effectively silenced by the presence of QS. To refine a QF expression pattern, for example to remove a subset of QF labeled tissues, follow option (A). To completely abolish QF expression, for example when performing quinic acid treatment experiments, follow option (B). QS suppression of QF induced reporters can be relieved by quinic acid treatment, resulting in temporal suppression of QF (Fig. 1, Fig. 2, Fig. 4). To relieve QS suppression of QF during larval development, follow option (C). To relieve QS suppression of QF only in adult animals, follow option (D). Ubiquitous expression of QS that is linked to a mitotic recombination event can also be used for mosaic analysis with a repressible cell marker (Q MARCM) (BOX 1). Coupling both GAL4 based MARCM and Q based MARCM to the same mitotic event can be used for coupled MARCM (BOX 2).
- Expressing QS in a subset of tissues
-
(i)Generate (or select an existing) promoter2-QS line that results in the desired expression pattern of QS. Cross promoter2-QS flies to promoter1-QF,QUAS-geneX flies (generated in Step 4) (Fig. 3) and maintain in standard fly food vials.
-
(II)Use an appropriate method to analyse the effects of QS in F1 progeny with the genotype promoter1-QF,QUAS-geneX; promoter2-QS. Where QS is expressed, the QUAS-geneX reporter will no longer be expressed even if QF is present. As a control, reporter expression without QS presence should also be examined i.e. in parental flies of genotype promoter1-QF, QUAS-geneX. Alternatively, raise F1 to adulthood and proceed to Step 6D to relieve QS-mediated suppression of QF using quinic acid.•CRITICAL STEP Promoter2-QS transgenic lines should express QS in the same pattern as promoter2-QF transgenic animals that use the same promoter. This should be verified by crossing the promoter2-QS transgenic fly to a promoter2-QF, QUAS-geneX recombinant to confirm that the entire promoter2-QF reported expression pattern is silenced. Different insertions of the promoter2-QS might need to be tested to find a line that effectively suppresses promoter2-QF.
-
(i)
- Expressing QS in all tissues.
-
Ubiquitous expression of QS can be achieved by using the tubulin promoter to drive QS (tubP-QS). Select an appropriate tubP-QS stock (Supplementary Table 2) and cross to stable promoter1-QF, QUAS-geneX lines (from Step 4); maintain on standard fly food.•CRITICAL STEP It is highly recommended to use a promoter1-QF, QUAS-geneX recombinant for ubiquitous QS experiments. Since the outcome of tubP-QS experiments is lack of expression, it is vital to know with 100% certainty that both promoter1-QF and QUAS-geneX components are present. The lack of either of these components will appear identical to tubP-QS suppression.
- Examine the F1 progeny for suppression of QF using an appropriate method (Table 1). The effects of ubiquitous QS expression can be confirmed by the lack of signal from the QUAS-geneX reporter. As a control for effectiveness of tubP-QS, reporter expression of parental flies of genotype promoter1-QF, QUAS-geneX can be examined. Alternatively, raise F1 to adulthood and proceed to Step 6D to relieve QS-mediated suppression of QF using quinic acid.
-
- Quinic acid treatment of developing animals.
-
Prepare fresh quinic acid containing food vials (see REAGENT SETUP).•PAUSE POINT Quinic acid fly food can be stored for up to a week if kept at 4°C.
-
Cross ~10 tubP-QS animals to ~10 promoter1-QF, QUAS-geneX animals (from Step 4) and let them lay eggs in quinic acid containing food for 6–12 hours. Transfer adults to fresh quinic acid food vials approximately every 12 hours to prevent overcrowding of progeny. The developing larval progeny will ingest sufficient quinic acid for suppression of QS and re-expression of the QUAS-geneX effector (Fig. 1c).•CRITICAL STEP: Alternatively, to target a specific developmental period, crosses could be set up on standard fly food and larvae at the required developmental stages transferred to grape plates or food containing quinic acid.•CRITICAL STEP Quinic acid suppression of QS occurs within ~2 hours of animals being placed on quinic acid containing plates4. However, different tissues might respond differently to quinic acid feeding due to variations in proliferation rates or the extent of exposure to quinic acid. To reduce the level of quinic acid suppression, lower concentrations of quinic acid solution can be used when generating quinic acid food vials.
- Analyse expression at the appropriate developmental stage using an appropriate technique (Table 1).
-
- Quinic acid treatment of adult animals.
-
Place adults of genotype tubP-QS+promoter1-QF, QUAS-geneX (Step 6Bii) in a fresh food vial containing quinic acid solution (Fig. 4).•CRITICAL STEP Although quinic acid mediated relief of ubiquitous QS expression is detailed here, tissue specific promoter2-QS expression can also be relieved by quinic acid treatments as described above by using flies generated as described in Step 6A.? TROUBLESHOOTING
- For continued suppression, transfer flies to fresh quinic acid containing food vials every 24–48 hours. Quinic acid is non-toxic to flies and can be supplemented in their diet with no adverse effects.
-
BOX 2 Performing coupled MARCM experiments. •TIMING Variable, depending on generation of fly stocks (1– 6 generations).
To label or manipulate all progeny of a mitotic division, coupled MARCM experiments can be used (Fig. 10). This involves combining both Q MARCM and GAL4 MARCM techniques.
Generate coupled MARCM ready flies containing tubP-QS
-
(i)
Use standard genetic techniques to introduce the following genetic components into a single fly: 1) FLP recombinase under the control of a heat-shock promoter, 2) a QUAS-geneX reporter to visualize the Q MARCM clone, such as QUAS-mtdT-3xHA, 3) a UAS-geneX reporter to visualize GAL4 MARCM clones, such as UAS-mCD8-GFP, 4) an FRT site and tubP-QS recombined onto the chromosome arm of interest (Fig. 10b). tubP-QS insertions recombined with FRT sites are available for each major chromosome arm (Supplementary Table 2).
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments. This fly line could also be used for Q MARCM experiments.
Generate coupled MARCM ready flies containing tubP-GAL80
-
(ii)
Use standard genetic techniques to introduce the following genetic components into a single fly: 1) tubP-GAL80 recombined distally to an FRT chromosome that is the same FRT site as the coupled MARCM ready flies generated in the previous step, 2) promoter2-GAL4, 3) promoter1-QF (Fig. 10b).
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
•CRITICAL STEP. The promoter2-GAL4 and promoter1-QF insertions can technically be located on any chromosome arm to generate coupled MARCM clones. However, as mentioned for Q MARCM in Box 1, it is best to avoid recombining these reagents distal to the FRT site being used in case these lines, when homozygous, disrupt endogenous gene functions. The crossing scheme diagramed in Fig. 10b allows for different promoter-GAL4 or promoter-QF lines to be used with the same coupled MARCM ready flies. However, promoter2-GAL4 and/or promoter1-QF could also be combined to other components in the previous step. The positioning of such components depends on the simplicity in generating a compatible coupled MARCM stock.
Perform coupled MARCM cross and generate coupled MARCM clones
-
(iii)
In a freshly yeasted vial, cross 5–10 coupled MARCM ready males containing tubP-GAL80 to 10–20 Q MARCM ready virgins containing tubP-QS. Depending on the birth date of the tissues of interest, heat shock the progeny in a 37°C water bath for 30 minutes to 2 hours.
•CRITICAL STEP The developmental time point and extent of the heat shock needs to be experimentally determined for each target tissue. The coupled MARCM ready flies with the tubP-QS often contain a hsFLP insertion on the X chromosome (e.g., Fig. 10b). Using females of these flies for the Q MARCM cross will ensure that both males and female progeny will contain Q MARCM clones.
Analyze and examine coupled MARCM clones
Figure 3. Crossing scheme for tissue specific QS suppression of QF.
To simplify analysis of QS suppression on a QF induced expression pattern, the QUAS-geneX reporter (QUAS-mtdT-3xHA) is recombined with P1-QF. Crossing this stable expression line to a P2-QS fly and selecting against the CyO balancer will result in progeny that have a subset of tissues no longer expressing the QUAS-geneX reporter. This can be directly compared to the original expression pattern.
Performing intersectional expression experiments. • TIMING Variable, depending on generation of fly stocks. ~5 fly generations to generate fly stocks and perform intersectional experiments
-
7|
There are 12 intersectional expression patterns possible by using GAL4 and QF systems together (examples are shown in Fig. 2, Fig. 5, Fig. 11). Each of these 12 intersectional expression patterns represent an effector expression profile that is a subset of the GAL4 and QF expression patterns used in the experiment. See Figure S7 in ref. 4 for a full list of expression patterns possible, including required genotypes. Below are details for 3 of the intersectionals which illustrate the basic principles for performing these genetic experiments. Choose option (A) to use QF expression patterns to limit the extent of GAL4 expression patterns. Choose option (B) to use GAL4 expression patterns to limit the extent of QF expression patterns. Choose options (C) or (D) to limit expression of an effector to only tissues that express both GAL4 and QF transgenes.
• CRITICAL STEP Even though the strategies in (C) and (D) reflect the overlapping intersection between QF and GAL4, they are not equivalent. Whichever line is driving FLPase expression will capture the entire developmental profile of that expression pattern, which could be much broader than the expression pattern at the target stage (e.g., the adult stage). The final effector expression level is reflected by whichever transcription factor is driving the final effector transgene (e.g., QF driving QUAS>geneX).
? TROUBLESHOOTING
- GAL4 NOT QF intersectional experiments
-
Recombine promoter2-GAL4 and the UAS-geneX onto the same chromosome and generate a balanced stock (as described in Steps 3 and 4 for promoter1-QF and QUAS-geneX).•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
To this promoter2-GAL4, UAS-geneX stock, cross in a QUAS-GAL80 transgene and generate a balanced stock (Fig. 6). QUAS-GAL80 transgenes are available on each chromosome (Supplementary Table 2).• CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
Cross a promoter1-QF to the promoter2-GAL4, UAS-geneX; QUAS-GAL80 stock (Fig. 6). Select progeny that contain all four genetic components required (promoter1-QF, promoter2-GAL4, UAS-geneX, and QUAS-GAL80) (Fig. 6; Supplementary Table 2). As a control, also choose animals for analysis that do not contain the QUAS-GAL80 transgene (e.g. select for Tubby animals in Fig. 6).•CRITICAL STEP These genetic components may be located on any chromosome just as long as progeny contain all four components. The scheme above is designed to simplify the testing of many different promoter-QF lines on altering GAL4 expression patterns.
-
Analyse UAS-geneX expression using an appropriate technique (Table 1)• CRITICAL STEP UAS-geneX effector expression will be refined based on the expression pattern of the promoter1-QF. For example, if promoter1-QF overlaps a portion of the promoter2-GAL4 expression pattern, than the overlapping tissues would no longer express the UAS-geneX effector.
-
- QF NOT GAL4 intersectional experiments
- Recombine promoter1-QF and the QUAS-geneX onto the same chromosome and generate a balanced stock (see Steps 3 and 4).
-
To the promoter1-QF, QUAS-geneX stock, cross in a UAS-QS transgene (Supplementary Table 2) and generate a balanced stock (Fig. 7).•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
Cross a promoter2-GAL4 to the promoter1-QS, QUAS-geneX; UAS-QS stock (Fig. 7). Select progeny that contain all four genetic components required (promoter1-QF, promoter2-GAL4, QUAS-geneX, and UAS-QS) (Fig. 5b; Fig. 7; Supplementary Table 2). As a control, also choose animals for imaging that do not contain the UAS-QS transgene (e.g. select for Tubby animals in Fig. 7).•CRITICAL STEP These genetic components may be located on any chromosome just as long as progeny contain all four components. The scheme shown in Fig. 7 is designed to simplify the testing of many different promoter-GAL4 lines for their effects on QF expression patterns.
-
Analyse QUAS-geneX expression using an appropriate technique (Table 1).•CRITICAL STEP QUAS-geneX effector expression will be refined based on the expression pattern of the promoter2-GAL4. For example, if promoter2-GAL4 is tubulin-GAL4, then there would be no expression of the QUAS-geneX effector. If promoter2-GAL4 overlaps a portion of the promoter1-QF expression pattern, than only the overlapping tissues would no longer express the QUAS-geneX effector (Fig. 5b).? TROUBLESHOOTING
- QF AND GAL4 intersectional experiment that captures the developmental profile of promoter-QF expression
-
(i)Recombine promoter1-QF with a QUAS “FLP-out” reporter, such as QUAS>stop>mCD8-GFP (Supplementary Table 2) and generate a balanced stock.•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
(ii)To the promoter1-QF, QUAS>stop>mCD8-GFP stock, cross in a UAS-FLP transgene and generate a balanced stock (Fig. 8a).•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
(iii)Cross a promoter2-GAL4 animals to the promoter1-QF, QUAS>stop>mCD8-GFP; UAS-FLP stock. Select progeny that contain all four genetic components required for QUAS reporter expression (Fig. 8a). In this case, GAL4 will drive FLPase expression, which will excise the transcription stop from the QUAS>stop>mCD8-GFP effector. QF is then able to induce expression from the resulting QUAS>mCD8-GFP transgene (Fig. 5c). As a control, also select animals for imaging that do not contain the UAS-FLP transgene (e.g. select for Tubby animals in Fig. 8a).•CRITICAL STEP These four genetic components may be located on any chromosome just as long as progeny contain all four components. The scheme shown in Fig. 8a is designed to simplify the testing of many different promoter-GAL4 lines to determine their overlapping expression pattern with a promoter1-QF line. Unbalanced lines could be used for these experiments since only when all four components are together will there be any reporter expression. However, using unbalanced lines will reduce the efficiency of the cross and increase the number of animals that need to be processed to ensure a positive result.
-
(iv)Analyse QUAS>mCD8-GFP expression by immunohistochemistry or on live animals by fluorescent microscopy (Table 1).
-
(i)
-
QF AND GAL4 intersectional experiment that captures the developmental profile of promoter-GAL4 expression.
-
(xiii)
Recombine promoter1-QF with a UAS “FLP-out” reporter, such as UAS>stop>mCD8-GFP (Supplementary Table 2) and generate a balanced stock.
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
(xiv)
To the promoter1-QF, QUAS>stop>mCD8-GFP stock, cross in a QUAS-FLPo transgene (Supplementary Table 2) and generate a balanced stock (Fig. 8b).
•CRITICAL STEP This balanced stock is a valuable reagent and should be maintained for future experiments.
-
(xv)
Cross promoter2-GAL4 animals to the promoter1-QF, UAS>stop>mCD8-GFP; QUAS-FLPo stock. Select progeny that contain all four genetic components required for UAS reporter expression (Fig. 8b). In this case, QF will drive FLPase expression, which will excise the transcription stop from the UAS>stop>mCD8-GFP effector. GAL4 is then able to induce expression from the resulting UAS>mCD8-GFP transgene. As a control, also choose animals for imaging that do not contain the QUAS-FLPo transgene (e.g., select for Tubby animals in Fig. 8b).
•CRITICAL STEP These four genetic components may be located on any chromosome just as long as progeny contain all four components. The scheme shown in Fig. 8b is designed to simplify the testing of many different promoter-GAL4 lines to determine their overlapping expression pattern with a promoter1-QF line. Unbalanced lines could be used for these experiments as only when all four components are together will there be any reporter expression. However, using unbalanced lines will reduce the efficiency of the cross and increase the number of animals that need to be processed to insure a positive result.
-
(xvi)
Analyse UAS>mCD8-GFP expression by immunohistochemistry or by using a fluorescent dissecting scope (Table 1).
-
(xiii)
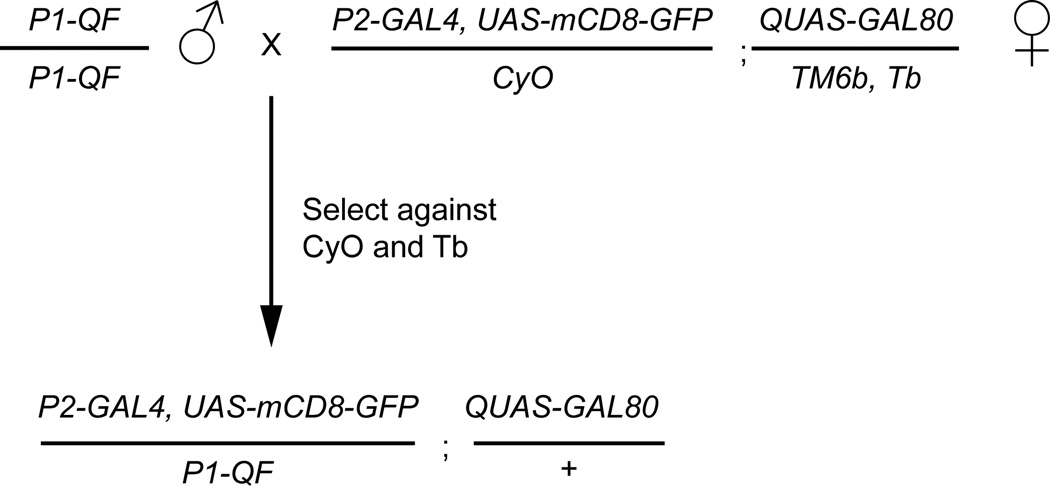
Figure 6. Crossing scheme for GAL4 NOT QF intersectional experiments.
For this NOT intersectional strategy to work, four components (P1-QF, P2-GAL4, UAS-geneX, and QUAS-GAL80) need to be combined into a fly. In this example, a GAL4 NOT intersectional ready female fly is diagramed. This fly contains a P2-GAL4 line recombined with a UAS-mCD8-GFP marker, as well as the QUAS-GAL80 transgene on the third chromosome. Crossing this stock to any QF line and selecting against the balancers will result in progeny that have reduced GAL4 expression based on the QF expression pattern. This simplifies the experimental setup for testing the intersectional results for many different QF lines.
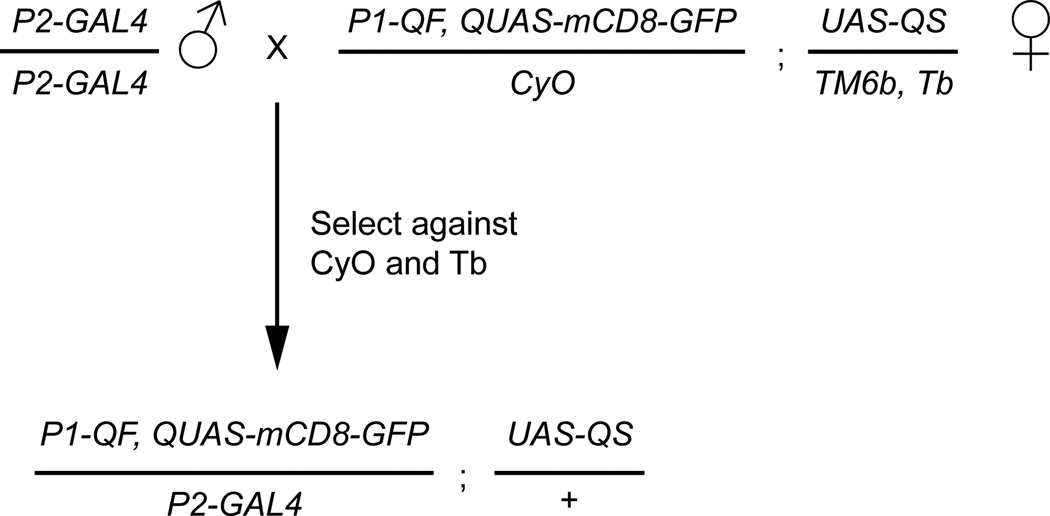
Figure 7. Crossing scheme for QF NOT GAL4 intersectional experiments.
For the NOT intersectional strategy to work, four components (P1-QF, P2-GAL4, QUAS-geneX, and UAS-QS) need to be combined into a fly. In this example, a QF NOT intersectional ready female fly is diagramed. This fly contains a P1-QF line recombined with a QUAS-mCD8-GFP marker, as well as the UAS-QS transgene on the third chromosome. Crossing this stock to any GAL4 line and selecting against the balancers will result in progeny that have reduced QF expression based on the GAL4 expression pattern. This simplifies the experimental setup for testing the intersectional results for many different GAL4 lines.
TROUBLESHOOTING
Troubleshooting advice is provided in Table 2.
Table 2.
Troubleshooting
| Step | Problem | Possible reason | Possible solution |
|---|---|---|---|
| 2 | No reporter expression with promoter-QF line. | QF line not expressed. | Try promoter-QF insertion at different genomic locus. |
| QF killing expressing cells. | Verify cells are dying by co-labeling cells with antibody marker or GAL4/UAS marker. Try weaker promoter-QF line. | ||
| Reporter expression is low. | Use two copies of reporter or promoter-QF line. Use different reporter. | ||
| 6A | QS expression can not inhibit QF | QS expression too low. | Use extra copies of QS transgenic lines. |
| 6B | QS not expressed in same cells as QF. | Use different QS transgene. | |
| 6C | Quinic acid not inhibiting QS | Quinic acid solution too old. | Make fresh quinic acid solution. |
| 6D | Quinic acid solution not concentrated enough. | Make saturated 300 mg/mL quinic acid solution. | |
| QS expression too high. | Try different QS transgenic line (e.g., tubP-QS#9B, Bloomington Stock # 30022). | ||
| 7C | Intersection of QF AND GAL4 shows no expression. | FRT-STOP-FRT reporter weak | Use extra copies of the FRT-STOP-FRT reporter. |
| 7D | QF and GAL4 are not expressed in the same cells. | Try a different QF or GAL4 line. | |
| Expression of QFor GAL4 is weak at examined stage | There are two approaches for the “AND” intersectional. They differ by which transcription factor is the final readout, and which is the developmental readout. The final readout might be weak at the examined stage. Try the alternative method. | ||
| All four required genetic components are not in same fly | Check crossing strategy to ensure that selected progeny contain all four components. | ||
| 7C | Intersection of QF AND GAL4 shows stochasticity in labeled tissues. | Low FLPase expression. | Use extra copy of FLPase or reporter. |
| 7D | Use codon optimized FLPase for higher expression. | ||
| Low expression of GAL4 enhancer trap. | Perform intersection using different components (e.g., different UAS-FLPase line; or use UAS-FLPase, QUAS>stop>reporter instead of QUAS-FLPase, UAS>stop>reporter). | ||
| BOX 1 | Little or no Q MARCM clones | MARCM stocks broken down | Check that all components (e.g., hsFLP, FRT sites) are still present. |
| Heat shock time at wrong developmental period or too short. | Try heat shocking at earlier developmental time points. Try heat shocking for longer (e.g., 1.5–2 hr at 37°C) | ||
• TIMING
Step 1, ~10 days (1 fly generation)
Step 2, ~5 days for immunohistochemistry and imaging
Step 3, 1 fly generation
Step 4, 2 fly generations (~20 days)
Step 5, Variable depending on experimental design; ~5 days if staining and imaging are required
Step 6A, 1 generation for cross; ~ 5 days if staining and imaging are required
Step 6B, 1 generation for cross; ~5 days if staining and imaging are required
Step 6C, 1 generation for cross; variable depending on extent of quinic acid feeding during development.
Step 6D, 1 generation for cross; Adult feeding of quinic acid can continue as long as necessary for the experiment.
Step 7A, ~ 4 fly generations to generate required stocks; 1 fly generation to perform intersectional experiment; ~ 5 days for staining and imaging if required.
Step 7B, Variable depending on necessity to generate appropriate fly stocks: 1–5 fly generations, and ~ 5 days for immunohistochemistry and imaging if required.
Step 7C, Variable: 1–5 fly generations, and ~ 5 days for imaging.
Step 7D, Variable: 1–5 fly generations, and ~ 5 days for imaging.
BOX 1, Variable: 1–5 fly generations, and ~ 5 days for imaging.
BOX2, Variable: 1–5 fly generations, and ~ 5 days for imaging.
ANTICIPATED RESULTS
When a promoter-QF and QUAS-geneX are combined in the same fly, there will be induced expression of geneX. However, when the QUAS-geneX is alone, there will be no efector expression. Figure 1c shows adult flies that contain the QUAS-mtDT-3xHA reporter alone, or when combined with a QF enhancer trap line. When the QS suppressor is also introduced, this will block QF activity and keep QUAS-geneX reporters silent. Figure 1c also shows adult flies whose broad QF induced expression of QUAS-mtdT-3xHA has been silenced by ubiquitous expression of QS. QS mediated suppression can itself be inhibited by treating flies with quinic acid. Quinic acid can be fed to developing animals by supplementing their food with quinic acid, and larvae will ingest enough quinic acid for efficient QS suppression in many tissues. Figure 1c shows an adult fly that was previously suppressed by ubiquitous QS but was relieved from such QS suppression by developing on fly food containing quinic acid. Similar quinic acid mediated re-expression of QF induced genes can also be performed in adult animals.
By combining the GAL4 and Q systems together, more refined expression patterns can be achieved (Fig. 5, Fig. 11). These are called intersectional expression experiments since the final expression pattern depends on the intersection between the QF and GAL4 expression domains. Such intersectional expression experiments could be used to target expression of an effector to a carefully defined target tissue, bypassing confounding effects due to more widespread expression. The outcome of the intersectional experiment depends on the additional genetic components that are used with the promoter1-QF and promoter2-GAL4 lines. By using a UAS-QS transgene, GAL4 expression can be used to effectively limit a QF expression pattern. An example of this QF NOT GAL4 intersection is shown in Figure 11d. Similarly, by using a QUAS-GAL80 transgene, QF expression can be used to effectively limit a GAL4 expression pattern. An example of this GAL4 NOT QF intersection is shown in Figure 11e. A powerful expressional refinement approach is to limit effector expression only to tissues that express both QF and GAL4. An example of this GAL4 AND QF intersection is shown in Figure 11f. This approach can effectively limit effector expression to a very small subset of cells. Since the expression pattern of promoter1-QF and promoter2-GAL4 can be easily determined, targeting expression to a desired population of cells only requires picking and choosing the right intersectional combination of GAL4 and QF lines.
Ubiquitous QS expression can effectively silence QF induced reporter expression. By using mitotic recombination to differentially segregate a tubP-QS transgene, one population of cells will no longer have the tubP-QS transgene and so will be released from QS suppression. These cells that are positively labeled (e.g., marked by a QUAS-CD8-GFP reporter) can also be made homozygous mutant for a gene of interest. This technique is called Q MARCM and is a powerful approach to genetically manipulate and label a small number, or even single, cells. An example of a Q MARCM clone that labels a single olfactory projection neuron in shown in Figure 9c and 9d.
The MARCM technique was originally developed for the GAL4 system3. In this case, ubiquitous expression of the GAL4 suppressor, GAL80, is differentially segregated to cell progeny based on a mitotic recombination event. Since the GAL4 system and the Q system function independently, these two mosaic labeling techniques can be combined together in coupled MARCM (Fig. 10). An example of a coupled MARCM clone in the wing imaginal disc is shown in Figure 10c and 10d. A QF marked clone could be homozygous mutant for a gene of interest and/or express an effector gene. Similarly, the GAL4 marked clone could be homozygous for a different gene of interest, and/or express a different effector gene. Such experiments could prove useful in addressing cell-cell communication or cell non-autonomous effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mimi Russler for the image in Figure 11e, and Chun-Chieh Lin and Sonia Chin for critical reading of the manuscript. C.J.P. is supported by a startup fund from The Center for Sensory Biology at Johns Hopkins University School of Medicine. L.L. is a Howard Hughes Medical Institute investigator.
Footnotes
AUTHOR CONTRIBUTIONS
C.J.P designed and performed the experiments and generated the figures and tables; C.J.P and L.L. wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JB. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 4.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum JA, Geever R, Giles NH. Expression of qa-1F activator protein: identification of upstream binding sites in the qa gene cluster and localization of the DNA-binding domain. Mol Cell Biol. 1987;7:1256–1266. doi: 10.1128/mcb.7.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geever RF, et al. DNA sequence, organization and regulation of the qa gene cluster of Neurospora crassa. J Mol Biol. 1989;207:15–34. doi: 10.1016/0022-2836(89)90438-5. [DOI] [PubMed] [Google Scholar]
- 7.Giles NH, Geever RF, Asch DK, Avalos J, Case ME. The Wilhelmine E. Key 1989 invitational lecture. Organization and regulation of the qa (quinic acid) genes in Neurospora crassa and other fungi. J Hered. 1991;82:1–7. doi: 10.1093/jhered/82.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Huiet L. Molecular analysis of the Neurospora qa-1 regulatory region indicates that two interacting genes control qa gene expression. Proc Natl Acad Sci USA. 1984;81:1174–1178. doi: 10.1073/pnas.81.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel VB, Giles NH. Autogenous regulation of the positive regulatory qa-1F gene in Neurospora crassa. Mol Cell Biol. 1985;5:3593–3599. doi: 10.1128/mcb.5.12.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JS, Luo L. A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat Protoc. 2006;1:2583–2589. doi: 10.1038/nprot.2006.320. [DOI] [PubMed] [Google Scholar]
- 11.Berdnik D, Fan AP, Potter CJ, Luo L. MicroRNA processing pathway regulates olfactory neuron morphogenesis. Curr Biol. 2008;18:1754–1759. doi: 10.1016/j.cub.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jefferis GSXE, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuldiner O, et al. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai S-L, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 15.Yagi R, Mayer F, Basler K. Refined LexA transactivators and their use in combination with the Drosophila Gal4 system. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1005957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer BD, et al. Refinement of Tools for Targeted Gene Expression in Drosophila. Genetics. 2010 doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- 18.Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai S-L, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Chen C, Shi L, Huang Y, Lee T. Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nat Neurosci. 2009 doi: 10.1038/nn.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H-H, et al. A Complete Developmental Sequence of a Drosophila Neuronal Lineage as Revealed by Twin-Spot MARCM. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin R, et al. The twin spot generator for differential Drosophila lineage analysis. Nat Methods. 2009;6:600–602. doi: 10.1038/nmeth.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Fishilevich E, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Ang L-H, et al. Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev Biol. 2006;293:178–190. doi: 10.1016/j.ydbio.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venken K, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009 doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan W, Ashburner M, Scott Hawley R. Drosophila protocols. 2000:697. [Google Scholar]
- 31.Wu JS, Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc. 2006;1:2110–2115. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- 32.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 33.LaJeunesse DR, et al. Three new Drosophila markers of intracellular membranes. Bio Techniques. 2004;36:784–788. 790. doi: 10.2144/04365ST01. [DOI] [PubMed] [Google Scholar]
- 34.Nicolai LJ, et al. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc Natl Acad Sci USA. 107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- 36.Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 37.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Rao Y, Rao Y. A Subset of Octopminergic Neurons Play Important Roles in Drosophila Aggression. Nat Neurosci. 2008:34. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 39.White K, et al. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 40.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 41.Chen P, Nordstrom W, Gish B, Abrams JM. grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- 42.Seelig JD, et al. Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat Methods. 7:535–540. doi: 10.1038/nmeth.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prober DA, Edgar BA. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 2002;16:2286–2299. doi: 10.1101/gad.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 45.Shi L, Yu HH, Yang JS, Lee T. Specific Drosophila Dscam juxtamembrane variants control dendritic elaboration and axonal arborization. J Neurosci. 2007;27:6723–6728. doi: 10.1523/JNEUROSCI.1517-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, Ge, Wang A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur J Neurosci. 2007 doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
- 48.Peabody NC, et al. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29:3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;13 doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 51.Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 52.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 53.Suster ML, Martin J-R, Sung C, Robinow S. Targeted expression of tetanus toxin reveals sets of neurons involved in larval locomotion in Drosophila. J Neurobiol. 2003;55:233–246. doi: 10.1002/neu.10202. [DOI] [PubMed] [Google Scholar]
- 54.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 55.Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS ONE. 2007;2:el62. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.