Abstract
Background:
Calprotectin is a 36 kDa protein present in the cytoplasm of the neutrophil has antimicrobial and apoptosis inducing activities. In vitro studies have shown that calprotectin inhibits the growth of various microorganisms. Necrotizing enterocolitis (NEC) remains one of the leading causes of morbidity and mortality in neonatal intensive care units (NICU), affecting up to 5% of premature infants. Fecal calprotectin is resistant to degradation and has been proposed as a useful marker of gastrointestinal inflammation.
Objective:
The objective of the present study is to evaluate fecal calprotectin concentrations in NEC.
Study Design:
Fifteen neonates with a clinical diagnosis of NEC were studied; they admitted at NICU of Zagazig University Hospital. In addition, 20 age sex matched neonates fed all caloric requirement served as the control group. All neonates were subjected to history taking, clinical examination, laboratory investigations (complete blood count, C-reactive protein) and determination of stool calprotectin.
Results:
There was a highly significant increase in fecal calprotectin in patients than control and there was a highly significant increase in its fecal level in died patients than living one. Also significant increase in fecal calprotectin level with increasing severity of NEC.
Conclusion:
Fecal calprotectin measurements could be a valuable tool for the investigation of preterm and full term infants suspected of having NEC.
Keywords: Stool calprotectin, necrotizing enterocolitis, neonates
INTRODUCTION
Necrotizing enterocolitis (NEC) is a leading cause of morbidity and mortality in preterm infants born prior to 32 weeks gestation or has a birth weight <1500 g.[1] Mild NEC is adequately treated by cessation of enteral feeding, empiric antibiotics and supportive care. Approximately, 50% of affected infants will develop progressive intestinal necrosis requiring urgent operation.[2] Despite advances in the care of critically ill neonates, the mortality rates of NEC have remained unchanged in the past three decades ranging from 15% to 30% respectively.[3] In the group of infants characterized by pan intestinal involvement the mortality rate approaches 100%. Surviving patients with NEC are facing significant morbidity, including neurodevelopmental impairment, feeding problems, failure to thrive, dependence on parental nutrition and short bowel syndrome.[4] Although the pathogenesis of NEC remains elusive, a deregulated inflammatory response by the neonatal intestine to luminal bacteria is a unifying hypothesis.[5] Intestinal epithelial injury is caused by different initiating events including intestinal ischemia, formula feeding and colonization by opportunistic pathogens leading to activation of the mucosal innate immune system and further damage of the epithelial barrier.[6] Calprotectin is a 36.5 kD calcium binding protein consisting of three polypeptide chains. In the presence of Ca2+, calprotectin is remarkably resistant toward proteolysis[7] and is abundant in neutrophil granulocytes, monocytes, macrophages and submucosal epithelial cells.[8] Calprotectin exerts antimicrobial properties in vitro, both bactericidal and fungicidal.[9] The protein also appears to have a regulatory function in the inflammatory process.[10] Several studies have described the clinical relevance of measuring calprotectin. Elevated concentrations of calprotectin in feces have been measured in patients with inflammatory bowel diseases and bacterial infections in the gastrointestinal (GI) tract. The highest levels of calprotectin in plasma have been found in patients with acute bacterial infections such as sepsis and meningococcal disease.[11] There are several studies on fecal calprotectin levels in NEC, but none of these studies discussed its relationship to the seriousness of the disease and also to deaths of patients.
Aim of the work
The aim of the following study is to evaluate fecal calprotectin concentrations in NEC.
MATERIALS AND METHODS
This case-control study included 15 neonates with a combination of 9 male and 6 female with a clinical diagnosis of NEC and were admitted at intensive care unit, Zagazig University Hospital during the year 2012. They were non-randomly selected. Twenty age sex matched neonates (10 male and 10 female) fed all caloric requirement served as a control group. Ethical approval was obtained from the local research ethics committee and parents of all neonates gave an informed written consent prior to the study.
All the neonates were subjected to the following:
History taking including prenatal, natal and postnatal
Clinical examination including signs of prematurity, neonatal reflexes, vital signs, abdominal examinations, staging of NEC,[12] other systems examinations
Laboratory investigations including (complete blood count, C-reactive protein [CRP], CRP levels ≥6 mg/L were considered as increased)[13]
Radiological examination including (X-ray chest and abdomen erect, abdominal ultrasonography)
Measurement of stool calprotectin.
Stool collection
Stools were collected in plastic containers and immediately stored frozen at −20°C.
Sample preparation
Fecal samples (50-100 mg) were collected with a disposable, breakable inoculation loop (10 μl, sterile, firm loop, T572-B, Technical Service Consultants, Lancaster, UK) and entered into a 14-ml disposable screw cap tube (Greiner tube, Greiner GmbH, Labortechnik, Frickenhausen, Germany). The feces weight was measured and the loop handle was broken off, leaving the loop and 4-6 cm of the handle inside the tube. Extraction solution containing urea and citrate (Nycomed Pharma AS) was added in a weight: volume ratio of 1:50. After 30 s agitation on a mixer (WhirliMixer™, Fisons Scientific Equipment, UK) followed by homogenization for 20 min at 1400 rpm on a shaker (Ika-Vibrax-VXR, IKA Werke, Janke and Kunkel GmbH, KG, Germany), 1 ml of the homogenate was transferred to an Eppendorf tube and centrifuged for 20 min at 10,000 × g. The supernatant (0.5 ml) was collected and analyzed immediately or frozen at −20°C for later analysis. The original sample preparation was performed according to the method described by Røseth et al.,[14] and in the original PhiCal enzyme linked immunosorbent assay (ELISA) kit (Nycomed Pharma AS). Stools were thawed and 5 g feces were suspended in 10 ml of extraction solution (1:3 dilution) and homogenized at room temperature for 1 min at 20,000 rpm using a rod mixer (Ultra Turrax, IKA Werke, Janke and Kunkel GmbH). The homogenate was centrifuged and supernatants were collected for analysis.
Determination of fecal calprotectin
Supernatants from the fecal sample extraction procedures were assayed in a calprotectin ELISA (PhiCal ELISA kit, Nycomed Pharma AS) with standards and controls included and performed according to the manufacturer's instructions. The supernatants were diluted 12-2000 fold in the kit dilution liquid. Diluted supernatants (100 μl) were added to the microtiter plate wells, incubated at room temperature for 45 min on a shaker (600 rpm) and washed four times with washing solution. Affinity purified rabbit anti-calprotectin antibodies conjugated with alkaline phosphatase (100 μl/well) were added and incubated for 45 min at room temperature on a shaker (600 rpm). After washing the wells four times, enzyme substrate was added and optical densities read at 405 nm. Calprotectin concentrations were calculated from the standard curve obtained with the kit standards.
Statistical analysis
Data were presented as mean ± standard deviation (X ± SD) or percentage (%). The means of two groups were compared using student t-test. Linear correlation and regression were used to test the correlation between the measured parameters and the studied groups. Data were tabulated and statistically analyzed with the statistical package for Social Sciences (SPSS), version 10 software. Fissure exact test was used when expected cell is <5. P < 0.05 were considered to be significant.[15]
RESULTS
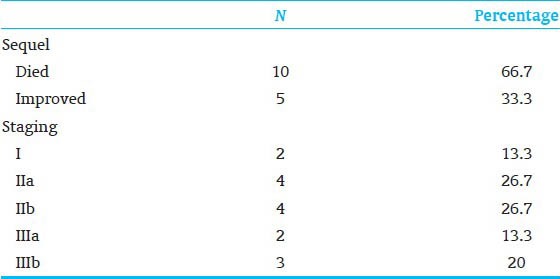
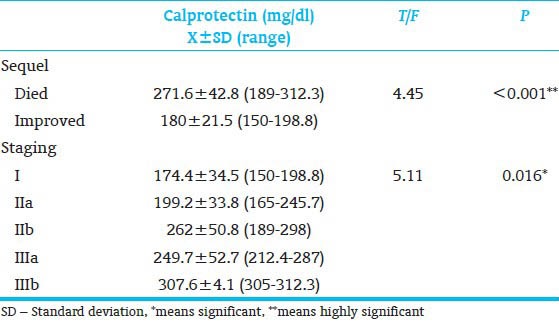
The characteristics of the two study groups are shown in Table 1. The two groups were similar in gestational age (GA), gender, type of feeding and birth weight. A highly significant difference was observed in hemoglobin level, platelet count and fecal calprotectin between patients and control. Furthermore, significant difference was observed in white blood cells (WBC) and neutrophil counts between patients and control [Table 2]. Table 3 shows that 33.3% of our patients improved and 66.7% died respectively. Furthermore, stage I, IIa, IIb, IIIa and IIIb represented 13.3, 26.7, 26.7, 13.3 and 20% of our patients respectively. Regarding the level of calprotectin there was a highly significant increase in its fecal level in died patients than living one. Also significant increase in fecal calprotectin level as the severity of NEC increased.
Table 1.
Demographic data of the studied groups
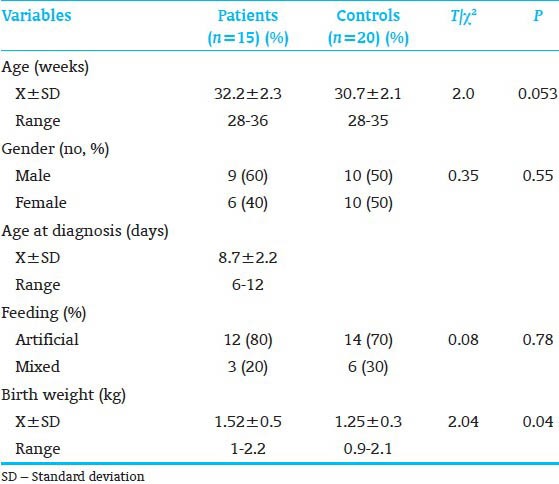
Table 2.
The laboratory investigations of patients and controls
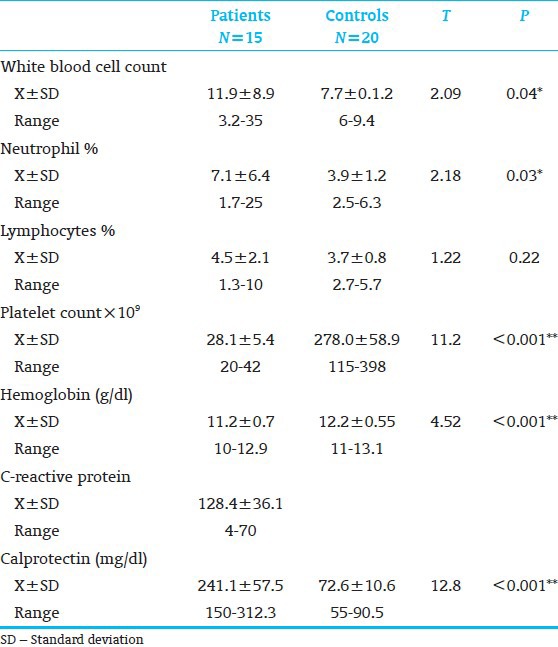
Table 3.
Sequel and staging of the patient group
DISCUSSION
NEC remains a potentially fatal disease in premature infants despite the recent advances in neonatal care. It is a disease with a multifactorial etiology leading to common final pathway of necrosis and inflammation of the neonatal intestine.[16] Calprotectin is an abundant calcium binding protein found in neutrophilic granulocytes, comprising up to 60% of the total cytosolic protein content of neutrophils. It is extremely stable in faces even during storage for 7 days at room temperature and easy to measure using a commercially available assay.[14] Intestinal mucosal defects or increased permeability of the mucosal barrier causes migration of large numbers of granulocytes into the gut lumen. In extensive mucosal disease this leads to calprotectin levels 10-20 times above the upper reference limit of 30 mg/L in adults.[17] Our patients had a mean GA of 32 weeks, a mean birth weight of 1.5 kg and a mean age at diagnosis of 9 days, 9 were boys and 6 were girls, 12 patients were fed artificially and 3 were fed mixed. Study of Carroll et al.,[18] had a mean GA of 30 weeks, mean age at diagnosis of 12 days, four were boys and three were girls. In the study of Zoppelli et al.,[19] mean GA was 28.5 weeks and birth weight 1,057 g. Zoppelli et al.,[19] declared that diagnosis of NEC is made on the basis of clinical criteria since there are no specific diagnostic tests but our study showed a significant increase in WBC and neutrophils counts and a highly significant decrease in hemoglobin and platelet counts in patient group. Two of our patients (14%) had suspect NEC (bell's stage I), 8 (53%) had definite NEC (bell's stage IIa or IIb), 5 (33%) had advanced NEC (bell's stage IIIa or IIIb). Nearly 53% of our patients died and 67% improved. Carroll et al.,[18] had 43% of their patients (bell's stage Ib), 14% (bell's stage IIb) and 43% (bell's stage IIIa or IIIb) respectively. Zoppelli et al.,[19] declared that 19 (9.2%) of his patients developed NEC stage II+, of whom 5 had fulminant NEC.
We found a highly significant increase in fecal calprotectin level in patients with NEC and the level of fecal calprotectin reach maximum level in patients with bell's stage IIIb (307.6 ± 4.1). Also as regards sequel of NEC, our study showed highly significant increase in fecal calprotectin level in died than improved patients [Table 4]. Carroll et al.,[18] found marked increase in fecal calprotectin in patients with NEC than controls. Aydemir et al.,[20] found that fecal calprotectin increases in infants with NEC and serial measurements may be useful as a noninvasive prognostic marker for progression of disease. Nissen et al.,[21] declared that because NEC primarily affects preterm infants, fecal calprotectin measurements could be a valuable tool for the investigation of preterm infants suspected of having NEC. Reisinger et al.,[22] detected that the combination of noninvasive measurement of intestinal fatty acid-binding protein and fecal calprotectin seems promising for diagnosing NEC at an early time point. As regards sequel of NEC, our study showed highly significant increase in fecal calprotectin level in died than improved patients. A study by Aydemir et al.,[16] found that infants with NEC had increased fecal calprotectin concentrations and there was a correlation between calprotectin concentrations and severity of NEC and concluded that fecal calprotectin is a useful marker for diagnosis and severity of NEC in preterm infants. In the other study, Zoppelli et al.,[19] declared that fecal calprotectin levels showed significant GA and postnatal age dependent dynamics with particularly low levels in extremely premature infants. Fecal calprotectin levels depend on GA and postnatal age and in contrast to adults, there is a lower limit in premature infants. Fecal calprotectin can be a useful marker in identifying premature infants with GI distress and NEC in particular. In contrast Selimoğlu et al.,[23] found that mean fecal lactoferrin and fecal calprotectin were not different between preterm and full-term newborns (P = 0.235 and P = 0.845, respectively), or those who were diagnosed with NEC or not (P = 0.545 and P = 0.968, respectively). This may be due to the large number of the control group reaching 63. In conclusion, fecal calprotectin measurements could be a valuable tool for the investigation of preterm and full term infants suspected of having NEC. Hence, we recommend further studies to detect the role of fecal calprotectin or others fecal markers in the diagnosis of NEC.
Table 4.
Relation of fecal calprotectin to the severity of necrotizing enterocolitis
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Song R, Subbarao GC, Maheshwari A. Haematological abnormalities in neonatal necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):22–5. doi: 10.3109/14767058.2012.715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol. 2013;40:135–48. doi: 10.1016/j.clp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: Changing pathology over 2 decades. J Pediatr. 2012;160:49–531. doi: 10.1016/j.jpeds.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–51. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–8. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berntzen HB, Fagerhol MK. L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Lab Invest. 1990;50:769–74. doi: 10.1080/00365519009091071. [DOI] [PubMed] [Google Scholar]
- 8.Dale I, Brandtzaeg P. Expression of the epithelial L1 antigen as an immunohistochemical marker of squamous cell carcinoma of the lung. Histopathology. 1989;14:493–502. doi: 10.1111/j.1365-2559.1989.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 9.Sohnle PG, Collins-Lech C, Wiessner JH. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–92. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 10.Sorg C. The calcium binding proteins MRP8 and MRP14 in acute and chronic inflammation. Behring Inst Mitt. 1992;91:126–37. [PubMed] [Google Scholar]
- 11.Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–80. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 12.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–5. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16:735–46. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–8. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 15.Vaeth M. A note on the calculation of expected survival, illustrated by survival of liver transplant patients by B.L. Thomsen, N. Keiding and D.G. Altman, Statistics in Medicine, 10, 733-738 (1991) Stat Med. 1992;11:1527–30. doi: 10.1002/sim.4780111111. [DOI] [PubMed] [Google Scholar]
- 16.Aydemir G, Cekmez F, Tanju IA, Canpolat FE, Genc FA, Yildirim S, et al. Increased fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab. 2012;58:841–4. [PubMed] [Google Scholar]
- 17.Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783–4. doi: 10.1016/S0140-6736(00)03224-4. [DOI] [PubMed] [Google Scholar]
- 18.Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361:310–1. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 19.Zoppelli L, Güttel C, Bittrich HJ, Andrée C, Wirth S, Jenke A. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology. 2012;102:68–74. doi: 10.1159/000337841. [DOI] [PubMed] [Google Scholar]
- 20.Aydemir O, Aydemir C, Sarikabadayi YU, Emre Canpolat F, Erdeve O, Biyikli Z, et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25:2237–41. doi: 10.3109/14767058.2012.684172. [DOI] [PubMed] [Google Scholar]
- 21.Nissen AC, van Gils CE, Menheere PP, Van den Neucker AM, van der Hoeven MA, Forget PP. Fecal calprotectin in healthy term and preterm infants. J Pediatr Gastroenterol Nutr. 2004;38:107–8. doi: 10.1097/00005176-200401000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Reisinger KW, Van der Zee DC, Brouwers HA, Kramer BW, van Heurn LW, Buurman WA, et al. Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. 2012;47:1640–5. doi: 10.1016/j.jpedsurg.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Selimoğlu MA, Temel I, Yıldırım Ç, Özyaln F, Aktaş M, Karabiber H. The role of fecal calprotectin and lactoferrin in the diagnosis of necrotizing enterocolitis. Pediatr Crit Care Med. 2012;13:452–4. doi: 10.1097/PCC.0b013e3182388ae9. [DOI] [PubMed] [Google Scholar]


