Abstract
Securing an airway is a vital task for the anesthesiologist. The pediatric patients have significant anatomical and physiological differences compared with adults, which impact on the techniques and tools that the anesthesiologist might choose to provide safe and effective control of the airway. Furthermore, there are a number of pathological processes, typically seen in the pediatric population, which present unique anatomical or functional difficulties in airway management. The presence of one of these syndromes or conditions can predict a “difficult airway.” Many instruments and devices are currently available which have been designed to aid in airway management. Some of these have been adapted from adult designs, but in many cases require alterations in technique to account for the anatomical and physiological differences of the pediatric patient. This review focuses on assessment and management of pediatric airway and highlights the unique challenges encountered in children.
Keywords: Airway anatomy, congenital syndromes, difficult intubation, pediatric airway
INTRODUCTION
One of the fundamental skills of an anesthesiologist is management of the airway. To be successful at this task, it is important for the provider to have knowledge of the important anatomical, physiological, and pathological features related to the airway as well as knowledge of the various tools and methods that have been developed for this purpose. In this vein most anesthetic providers are very familiar and skilled at managing the adult airway successfully. However, children are not merely small adults. There are important differences that occur during development that require a different approach or technique. Forewarned is forearmed. Therefore, this review article will highlight some of the important anatomical and physiological differences and their implication. It will then briefly describe some of the pathological conditions which present particular concern for airway management. Finally, an overview of techniques and tools for managing the pediatric airway will be discussed.
AIRWAY ANATOMY
The airway of the pediatric patient differs in many ways which impact the anesthesiologist's management of the airway. Predictably, these differences are most pronounced at birth and the most unfamiliar (non-adult like) airway is encountered in neonates and infants under 1 year of age. Observational data bears out this point as laryngoscopy is more likely to yield suboptimal views in this age group.[1]
The first anatomical difference between the pediatric and adult patient becomes important when positioning the child prior to or immediately after the induction of anesthesia. The head of a pediatric patient is larger relative to body size, with a prominent occiput. This predisposes to airway obstruction in asleep children, because the neck is in flexed when they lie on a flat surface. A folded towel is often required as a shoulder roll to achieve a neutral position of the neck and open up the airway. This is demonstrated visually in Figure 1. The larger occiput combined with a shorter neck makes laryngoscopy relatively more difficult by providing obstacles to the alignment of the oral, laryngeal, and tracheal axes.[2]
Figure 1.
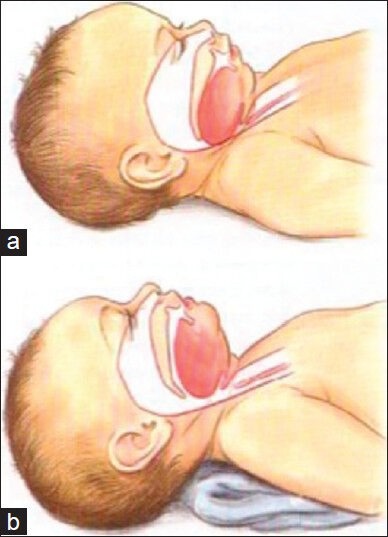
Artistic rendering of infant airway. (a) In image a note the large occiput which has caused flexion of the head and subsequently caused the base of the tongue to obstruct the upper airway. This obstruction has been relieved (b) by placing a towel under the shoulders and neck allowing more extension of the head and an opening of the upper airway
The tongue is larger and the mandible shorter in the young child. In infancy, the child is an obligate nasal breather until 5 months of age. Prominent adenoids and tonsils are frequently found in preschool age children and are a frequent reason to present for elective ENT surgery.[3] These factors all contribute to loss of upper airway space which can lead to difficulty with mask ventilation, obstruction during spontaneous ventilation, and can make laryngoscopy more difficult. In addition, sedatives, hypnotic, and anesthetic drugs cause loss of tone of upper airway muscles which can itself result in potential upper airway obstruction.[4]
The hypopharynx of the pediatric patient is relatively shorter in height and narrower in width. On cross section, the airway of an adult is more elliptical than that of the child.[5] This has implication for supraglottic airway placement.
The larynx is relatively higher in the neck in children. In some positions, the mandible may lie in line with the upper glottic structures. The cricoid ring is located approximately at the level of the C4 vertebrae at birth, C5 at age 6, and C6 as adult.[6] Vocal cords are not typically found at a right angle (90°) to the trachea. They are angled in an anterior-inferior to posterior-superior fashion.[2] While this typically does not affect laryngoscopic view, it can make insertion of the endotracheal tube more challenging or more traumatic. Especially in suboptimal views or with indirect video laryngoscopy, the endotracheal tube will have a higher tendency to collide with or become obstructed on the anterior commissure of the vocal folds.
The epiglottis in children is more “U” shaped (compared to flat in adults) and it is less in line with the trachea and may lie across the glottic opening.[6] This feature makes many anesthesiologists prefer semi-straight laryngoscope blades such as a Miller which are designed to directly lift the epiglottis out of view compared to a curved Macintosh blade which relies on ligamentous connection from the vallecula with the epiglottis to lift it out of view [Figure 2]. The hyoid bone is the first airway structure to ossify. The cartilaginous portions of the airway are soft and compliant. Calcification of the larynx and trachea typically does not occur until the teenage years.[7] The flexible cartilaginous rings of the trachea can predispose to dynamic obstruction with negative pressure ventilation, especially when any partial airway obstruction exists.[6,8]
Figure 2.
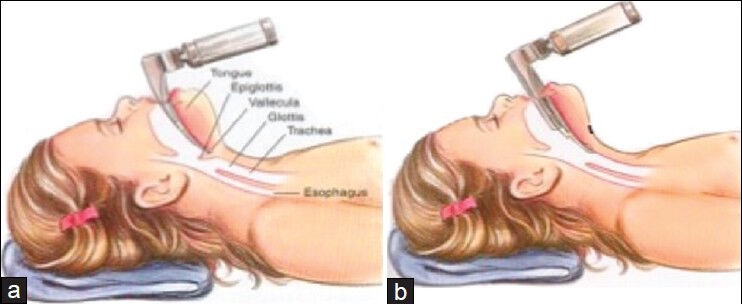
Artistic rendering demonstrating proper technique of Macintosh (a) and Miller (b) laryngoscope blades
Based on cadaveric studies, it has been a commonly held belief that the pediatric airway is funnel-shaped with the narrowest portion of the airway being found at the level of the cricoid. This was contrasted to the adult airway, where the narrowest portion is the glottis and the airway is described as cylindrical. These refer to the more rigid structures of the larynx as outlined by the laryngeal and tracheal cartilages.[6] Recent in vivo measurements taking into account the functional location of the softer tissues, specifically the vocal folds present a somewhat different picture. Measurements of the size of the airway of children using bronchoscopic images as well as magnetic resonance imaging images were consistently found to have glottic openings smaller than at the cricoid.[9,10,11] However, the distensibilty of the glottic tissues and the relatively nondistensible cricoid cartilage may still lead to the effect of the cricoid being functionally the narrowest part of the airway. This cartilaginous ring is the only circumferential complete structure in the airway and an endotracheal tube that passes easily though the vocal cords may not pass through the cricoid ring. The cricoid ring in an infant is elliptical, not circular, being oflarger diameter in the Antero-posterior dimension.[12] This affects the seal of cuffed and uncuffed endotracheal tubes and may guide selection of tracheal tubes.
PHYSIOLOGY
The pediatric patient has a number of physiological challenges which can predispose him/her to hypoxemia. Oxygen consumption of an infant is relatively greater than an adult with some authors quoting differences at rest of 6 mL/kg/min vs. 3 mL/kg/min.[8] This combined with a somewhat lower functional residual capacity can lead to rapid desaturation during apnea, such as during laryngoscopy or a rapid sequence induction, despite best efforts at preoxygenation. CO2 production is likewise increased, on the order of 100-150 mL/kg/min compared to the 60 mL/kg/min of an adult. Since the tidal volume (per kg body weight) is relatively consistent with that of an adult, the respiratory rate in children is higher to achieve this need for higher minute ventilation to eliminate the CO2.[13]
The resistance to flow in the airway is governed by Poiseulle's law: R =8ƞL/πr4. The resistance to flow is inversely related to the radius of the airway raised to the fourth power, a small amount of narrowing (due to edema, inflammation, etc.,) in the already small pediatric airway could have severe consequences on respiratory function. A number of disease processes that could result in such narrowing of the airway include growths within the airway such as hemangiomas or papillomas, aberrant embryological development such as tracheomalacia, laryngomalacia, and laryngeal clefts, iatrogenic causes like vocal cord paralysis and subglottic stenosis, or compression of the airway structures by a mass located outside the airway.[14] The management of these processes is unique to the disease itself but common themes exist, such as the desire to avoid further trauma to the tissues of the airway in order to avoid edema and further narrowing of the already compromised airway.
AIRWAY ASSESSMENT
The initial airway assessment starts with a good history. Questions are directed toward eliciting indications of a potentially difficult airway. This would include any complications of birth or delivery, any history of prior trauma or surgery to the airway or adjacent structures, or of prior anesthetics. Additionally, one should inquire about current or recent symptoms suggesting upper respiratory infection (URI), difficulty in speaking, difficulty breathing, difficulty feeding, hoarseness, and noisy breathing. Questions such as a history of snoring, day time drowsiness, or stopping breathing during sleep, may help to identify children with obstructive sleep apnea. Many syndromes are associated with potentially difficult airway management. A nonexhaustive list of syndromes with potential airway complications is summarized in Table 1.[12,15]
Table 1.
List of possible factors complicating airway management in a number of congenital syndromes
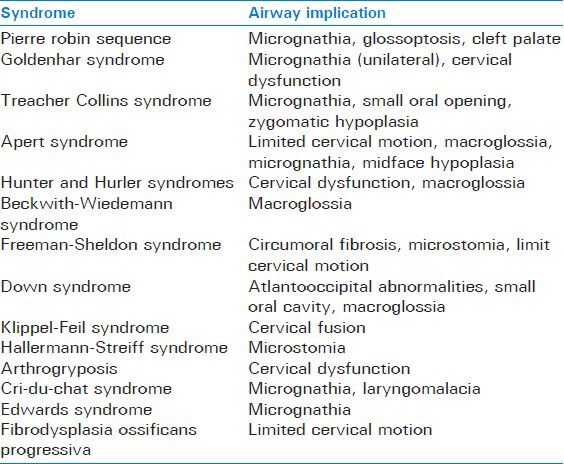
Many physical exam findings which may be well-known in the adult difficult airway literature also apply to children. Limited head extension, reduced mandibular space, and increased tongue thickness have been shown to be the most reliable predictors of difficult intubation.[16] Some studies have attempted to use scores calculated from various facial measurements to predict difficult laryngoscopic views. However, these scoring systems can be difficult to use in clinical practice. Some series of cases have demonstrated a relationship between mandible length and lip to chin distance being associated with Cormack and Lehane view classification.[17] In one study, bilateral microtia was associated with a 42% incidence of difficult laryngoscopy.[18] However, even if no specific diagnosis is known severity of disease or certain types of surgery are associated with increased risk for airway management complications. In one large case series the rate of difficult laryngoscopy, as defined as Cormack and Lehane grade III or IV, was found to be 1.35%. Some factors that increased likelihood of difficult visualization included age <1 year, cardiac surgery, ASA status III and IV, Mallampati III or IV, and low body mass index.[1]
MANAGEMENT TOOLS AND TECHNIQUES
The fundamental maneuver in airway management is properly performed mask ventilation. As in adults, there are one- and two-hand techniques. Upper airway obstruction which may be encountered during simple mask ventilation is often relieved by head tilt, chin lift, jaw thrust, and the application of continuous positive airway pressure.[19] Additionally, the lateral position may also improve airway patency, particularly when combined with chin lift and jaw thrust. This has been demonstrated in children undergoing surgery for adenotonsillary hypertrophy, a group that is more prone to upper airway obstruction.[20] It is important to note that face mask ventilation increases dead space compared to ventilation via an endotracheal tube. In smaller children, this increase in volume becomes more significant due to the low absolute volumes of ventilation.[13]
The use of an oral airway during spontaneous or positive pressure ventilation with a face mask helps to relieve the obstruction that may be caused by posterior displacement of the tongue in the anesthetized child. The appropriate size of airway can be approximated by the distance from the anterior gum line to the angle of the mandible.[13]
Nasopharyngeal airways may also be used to relive upper airway obstruction during mask ventilation. Their use has also been described as a means of providing anesthetic gas, oxygen, and End tidal CO2 monitoring while performing fiber optic tracheal intubation in the child with a difficult airway.[21]
A relatively recent advancement is the development of the supraglottic airway. Many devices exist. The specifics of each device provide a unique set of pros and cons for each device. The variables to consider includematerial of construction, ability to adjust seal pressure, reusability, gastric port, ability to use as intubating conduit, and ease of insertion among other factors. Two of the more popular supraglottic devices, the classic laryngeal mask airway (LMA) and proseal LMA have good data in the pediatric population to support their safety and efficacy.[22] The classic LMA has a 95%-98% success rate in achieving adequate ventilation in children.[23] When studied in a series of 1400 patients, there is also reported a low overall rate of problems (11.5%) with none of the problems resulting in major morbidity.[24] Some authors report successful use of proseal LMAs for laparoscopic procedures in children age 6 months to 8 years.[25]
The supraglottic airway may hold some advantages over endotracheal intubation for anesthesia in children with recent URI. In one randomized trial comparing LMA to endotracheal tube, the LMA group had significantly fewer respiratory complications compared to the endotracheal tube group.[26] However, the LMA is still associated with an increased incidence of respiratory complications when used in children with a recent URI as compared to healthy children.[27]
It has been recommended that a manometer be used to gauge the inflation pressure of the cuff of the LMA. In one observational study following routine insertion of proseal or classic LMAs using slight elevation of the device as a clinical endpoint guiding inflation, inflation pressures significantly exceeded the 60 cm H2O recommended by the manufacturer. Higher intracuff pressure may expose the patient to increased risk for mucosal damage by exceeding mucosal perfusion pressures.[28] Once a supraglottic airway has been chosen and inserted, it is recommended that the inflation pressure be kept to less than 40 cm H2O. This appears to improve the oropharyngeal leak pressure and reduce the incidence of throat pain postoperatively.[12]
There are dozens of tools to facilitate tracheal intubation. The gold standard is still direct laryngoscopy. Within this practice, there are a multitude of different laryngoscope blade styles and sizes from which to choose. Many anesthesiologists prefer the Macintosh blade, adapted from adult sizes, for older children. This blade is designed to be utilized with an indirect elevation of the epiglottis by placing the tip of the blade in the vallecula. Though this blade was originally envisioned as a device to facilitate laryngoscopy in the preneuromuscular blocking drug era in more lightly anesthetized patients, its popularity has continued to this day.[29] As mentioned before, in younger children the orientation of the epiglottis in a more anterior-posterior plane this indirect method of elevating the epiglottis may be less effective. Therefore, many anesthesiologists prefer the use of a straight or semi-curved blade designed to directly elevate the epiglottis in patients under the age of 4 or 5 years.[6,30]
It is increasingly common to use cuffed endotracheal tubes in neonates, infants, and young children. Whereas historically uncuffed endotracheal tubes were used in this population in an effort to minimize resistance of the endotracheal tube while also minimizing pressure trauma to the subglottis, it is now believed that cuffed tubes can provide better ventilating conditions while also minimizing trauma to the delicate airway of pediatric patients. The elliptical shape of the cricoid leads to the possibility that an uncuffed tube with an acceptable leak pressure may still be causing pressure trauma to the subglottic mucosa.[12] Additionally, the use of uncuffed endotracheal tubes may be associated with a higher incidence of laryngospasm.[12,31]
The utility of using an audible air leak at an airway pressure of less than 20 cm H2O has been called into question as being inadequate to prevent over inflation of endotracheal tube cuffs. In one observational study median cuff pressures were found to be 40-60 cm H2O, exceeding the commonly accepted limit of 20-30 cm H2O.[28]
There are multiple options for induction of anesthesia in the anticipated difficult intubation. The use of inhalational induction tends to maintain spontaneous respirations but also depresses upper airway musculature and may worsen upper airway obstruction.[4,32] Remifentanil has the advantage of being short-acting opioid which can produce reasonable intubating conditions, while muscle relaxant rocuroniumhas the advantage of being reversible by sugammadex. Experience with sugammadex is limited however and has limitations of not reversing anesthetic agents, and may not be sufficient for a patient to regain upper airway muscular tone in a failed airway situation due to potential trauma and edema of the attempted airway as is suggested by a recent case report.[33] In adults, awake fiberoptic intubation is often considered the gold standard method for the known or predicted difficult airway. It allows for the maintenance of spontaneous respirations until the trachea is intubated.[12] In the pediatric population, this option is usually not feasible due to the need for significant cooperation on the part of the patient. In one survey of pediatric anesthesiologist in Canada, the use of inhalational induction was the preferred method approaching a predicted difficult airway.[34]
Many techniques for managing the challenging airway take advantage of the relatively good positioning of a supraglottic device above the glottic opening. One method is to place a supraglotic airway and then use a fiberoptic bronchoscope through an airway exchange catheter to intubate the trachea. The fiberscope and the supraglottic airway are then removed and an endotracheal tube can be advanced over the exchange catheter.[35] Another method involves placement of the supraglottic airway, followed by introduction of the fiberscope into the trachea through the supraglottic airway. Once the fiberscope is in the trachea a J-wire can be placed through the operating port of the scope. The scope can then be removed and an airway exchange catheter advanced over the wire. Following the removal of the wire and supraglottic device an endotracheal tube can be advanced over the exchange catheter.[36] More recently, supraglottic devices have been designed specifically for use as conduits for intubation. These devices do not have fenestrations and allow for direct placement of endotracheal tubes through the supraglottic airway.[37] In cases series, excellent success rates, 96% first attempt, have been reported.[38]
There are many newer devices designed to aid laryngoscopy. These include pediatric sizes of Glidescope video larygoscope, a Karl-Storz video laryngoscope on what is essentially a Miller 1 blade, Airtraq optical laryngoscope, among others.[30] Though rigorous prospective trials of many of these devices have yet to be done, there is some early evidence and experience that they may provide improved glottic views and therefore provide viable alternatives to direct laryngoscopy when direct laryngoscopy is predicted to be or found to be challenging.[39]
The difficulty of airway management is usually due to either difficulty in either performing adequate mask ventilation or in successfully achieving tracheal intubation. The intersection of these two scenarios is the “cannot ventilate, cannot intubate” situation. An often recommended strategy for anesthesiologists in the failed airway to provide oxygenation is the needle cricoidotomy. In the pediatric population, however, this may be more challenging. Owing to the flexible and compressible nature of the cricoid and trachea, it may be more likely to pierce through-and-through the airway with the needle and penetrate the esophagus. It has been suggested that risk of complications to successful cannulation of the airway with the needle cricothyroidotomy is too high in children under age 5 or 6.[40] In light of this, there is some suggestion that a direct surgical approach to cricothyroidotomy is likely to be more successful.[40] However, there are a number of anatomical differences in the pediatric airway which complicate any potential transcutaneous approach to the airway. First of all the neonatal cricothyroid membrane is small, measuring only 3 mm wide and 2.6 mm tall.[41] Second, in infants, the hyoid bone by overlap the usually prominent thyroid cartilage, thereby making identification of crucial anatomy more difficult.[42] Some experts have, therefore, suggested that in these youngest patients the preferred approach may be direct puncture of the trachea below the level of the cricoid.[42]
CONCLUSION
Airway management is a key skill for the anesthesiologist. The airway of the pediatric patient has a number of significant differences when compared to the adult airway and presents some unique challenges. Awareness of anatomical and physiological differences, important pathological conditions affecting children, and a knowledge of the available airway techniques and tools will allow the anesthesiologist to formulate and execute safe and effective management of the pediatric airway.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Heinrich S, Birkholz T, Ihmsen H, Irouschek A, Ackermann A, Schmidt J. Incidence and predictors of difficult laryngoscopy in 11,219 pediatric anesthesia procedures. Paediatr Anaesth. 2012;22:729–36. doi: 10.1111/j.1460-9592.2012.03813.x. [DOI] [PubMed] [Google Scholar]
- 2.Carr RJ, Beebe DS, Belani KG. The difficult pediatric airway. Sem Anesth Perioper Med Pain. 2001;20:219–27. [Google Scholar]
- 3.Sunder RA, Haile DT, Farrell PT, Sharma A. Pediatric airway management: Current practices and future directions. Paediatr Anaesth. 2012;22:1008–15. doi: 10.1111/pan.12013. [DOI] [PubMed] [Google Scholar]
- 4.Litman RS, McDonough JM, Marcus CL, Schwartz AR, Ward DS. Upper airway collapsibility in anesthetized children. Anesth Analg. 2006;102:750–4. doi: 10.1213/01.ane.0000197695.24281.df. [DOI] [PubMed] [Google Scholar]
- 5.Abramson Z, Susarla S, Troulis M, Kaban L. Age-related changes of the upper airway assessed by 3-dimensional computed tomography. J Craniofac Surg. 2009;20(Suppl 1):657–63. doi: 10.1097/SCS.0b013e318193d521. [DOI] [PubMed] [Google Scholar]
- 6.Adewale L. Anatomy and assessment of the pediatric airway. Paediatr Anaesth. 2009;19(Suppl 1):1–8. doi: 10.1111/j.1460-9592.2009.03012.x. [DOI] [PubMed] [Google Scholar]
- 7.Hudgins PA, Siegel J, Jacobs I, Abramowsky CR. The normal pediatric larynx on CT and MR. AJNR Am J Neuroradiol. 1997;18:239–45. [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen A, Lenz K, Abildstrøm H, Lauritsen TL. Anesthetizing the obese child. Paediatr Anaesth. 2011;21:623–9. doi: 10.1111/j.1460-9592.2011.03559.x. [DOI] [PubMed] [Google Scholar]
- 9.Dalal PG, Murray D, Feng A, Molter D, McAllister J. Upper airway dimensions in children using rigid video-bronchoscopy and a computer software: Description of a measurement technique. Paediatr Anaesth. 2008;18:645–53. doi: 10.1111/j.1460-9592.2008.02533.x. [DOI] [PubMed] [Google Scholar]
- 10.Dalal PG, Murray D, Messner AH, Feng A, McAllister J, Molter D. Pediatric laryngeal dimensions: An age-based analysis. Anesth Analg. 2009;108:1475–9. doi: 10.1213/ane.0b013e31819d1d99. [DOI] [PubMed] [Google Scholar]
- 11.Litman RS, Weissend EE, Shibata D, Westesson PL. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology. 2003;98:41–5. doi: 10.1097/00000542-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Sims C, von Ungern-Sternberg BS. The normal and the challenging pediatric airway. Paediatr Anaesth. 2012;22:521–6. doi: 10.1111/j.1460-9592.2012.03858.x. [DOI] [PubMed] [Google Scholar]
- 13.Brambrink AM, Braun U. Airway management in infants and children. Best Pract Res Clin Anaesthesiol. 2005;19:675–97. doi: 10.1016/j.bpa.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Bruce IA, Rothera MP. Upper airway obstruction in children. Paediatr Anaesth. 2009;19(Suppl 1):88–99. doi: 10.1111/j.1460-9592.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 15.Nargozian C. The airway in patients with craniofacial abnormalities. Paediatr Anaesth. 2004;14:53–9. doi: 10.1046/j.1460-9592.2003.01200.x. [DOI] [PubMed] [Google Scholar]
- 16.Frei FJ, Ummenhofer W. Difficult intubation in paediatrics. Paediatr Anaesth. 1996;6:251–63. doi: 10.1111/j.1460-9592.1996.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 17.Mirghassemi A, Soltani AE, Abtahi M. Evaluation of laryngoscopic views and related influencing factors in a pediatric population. Paediatr Anaesth. 2011;21:663–7. doi: 10.1111/j.1460-9592.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 18.Uezono S, Holzman RS, Goto T, Nakata Y, Nagata S, Morita S. Prediction of difficult airway in school-aged patients with microtia. Paediatr Anaesth. 2001;11:409–13. doi: 10.1046/j.1460-9592.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 19.Meier S, Geiduschek J, Paganoni R, Fuehrmeyer F, Reber A. The effect of chin lift, jaw thrust, and continuous positive airway pressure on the size of the glottic opening and on stridor score in anesthetized, spontaneously breathing children. Anesth Analg. 2002;94:494–9. doi: 10.1097/00000539-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Arai YC, Fukunaga K, Hirota S, Fujimoto S. The effects of chin lift and jaw thrust while in the lateral position on stridor score in anesthetized children with adenotonsillar hypertrophy. Anesth Analg. 2004;99:1638–41. doi: 10.1213/01.ANE.0000135637.95853.1C. [DOI] [PubMed] [Google Scholar]
- 21.Holm-Knudsen R, Eriksen K, Rasmussen LS. Using a nasopharyngeal airway during fiberoptic intubation in small children with a difficult airway. Paediatr Anaesth. 2005;15:839–45. doi: 10.1111/j.1460-9592.2004.01566.x. [DOI] [PubMed] [Google Scholar]
- 22.White MC, Cook TM, Stoddart PA. A critique of elective pediatric supraglottic airway devices. Paediatr Anaesth. 2009;19(Suppl 1):55–65. doi: 10.1111/j.1460-9592.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 23.Mason DG, Bingham RM. The laryngeal mask airway in children. Anaesthesia. 1990;45:760–3. doi: 10.1111/j.1365-2044.1990.tb14449.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Gil M, Brimacombe J, Alvarez M. Safety and efficacy of the laryngeal mask airway. A prospective survey of 1400 children. Anaesthesia. 1996;51:969–72. doi: 10.1111/j.1365-2044.1996.tb14968.x. [DOI] [PubMed] [Google Scholar]
- 25.Sinha A, Sharma B, Sood J. ProSeal as an alternative to endotracheal intubation in pediatric laparoscopy. Paediatr Anaesth. 2007;17:327–32. doi: 10.1111/j.1460-9592.2006.02127.x. [DOI] [PubMed] [Google Scholar]
- 26.Tait AR, Pandit UA, Voepel-Lewis T, Munro HM, Malviya S. Use of the laryngeal mask airway in children with upper respiratory tract infections: A comparison with endotracheal intubation. Anesth Analg. 1998;86:706–11. doi: 10.1097/00000539-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 27.von Ungern-Sternberg BS, Boda K, Schwab C, Sims C, Johnson C, Habre W. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology. 2007;107:714–9. doi: 10.1097/01.anes.0000286925.25272.b5. [DOI] [PubMed] [Google Scholar]
- 28.Ong M, Chambers NA, Hullet B, Erb TO, von Ungern-Sternberg BS. Laryngeal mask airway and tracheal tube cuff pressures in children: Are clinical endpoints valuable for guiding inflation? Anaesthesia. 2008;63:738–44. doi: 10.1111/j.1365-2044.2008.05486.x. [DOI] [PubMed] [Google Scholar]
- 29.Scott J, Baker PA. How did the Macintosh laryngoscope become so popular? Paediatr Anaesth. 2009;19(Suppl 1):24–9. doi: 10.1111/j.1460-9592.2009.03026.x. [DOI] [PubMed] [Google Scholar]
- 30.Doherty JS, Froom SR, Gildersleve CD. Pediatric laryngoscopes and intubation aids old and new. Paediatr Anaesth. 2009;19(Suppl 1):30–7. doi: 10.1111/j.1460-9592.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 31.von Ungern-Sternberg BS, Boda K, Chambers NA, Rebmann C, Johnson C, Sly PD, et al. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet. 2010;376:773–83. doi: 10.1016/S0140-6736(10)61193-2. [DOI] [PubMed] [Google Scholar]
- 32.Nandi PR, Charlesworth CH, Taylor SJ, Nunn JF, Doré CJ. Effect of general anaesthesia on the pharynx. Br J Anaesth. 1991;66:157–62. doi: 10.1093/bja/66.2.157. [DOI] [PubMed] [Google Scholar]
- 33.Curtis R, Lomax S, Patel B. Use of sugammadex in a ‘can’t intubate, can’t ventilate’ situation. Br J Anaesth. 2012;108:612–4. doi: 10.1093/bja/aer494. [DOI] [PubMed] [Google Scholar]
- 34.Brooks P, Ree R, Rosen D, Ansermino M. Canadian pediatric anesthesiologists prefer inhalational anesthesia to manage difficult airways. Can J Anaesth. 2005;52:285–90. doi: 10.1007/BF03016065. [DOI] [PubMed] [Google Scholar]
- 35.Thomas PB, Parry MG. The difficult paediatric airway: A new method of intubation using the laryngeal mask airway, Cook airway exchange catheter and tracheal intubation fibrescope. Paediatr Anaesth. 2001;11:618–21. doi: 10.1046/j.1460-9592.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- 36.Walker RW. The laryngeal mask airway in the difficult paediatric airway: An assessment of positioning and use in fibreoptic intubation. Paediatr Anaesth. 2000;10:53–8. doi: 10.1046/j.1460-9592.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 37.Sinha R, Chandralekha, Ray BR. Evaluation of air-Q intubating laryngeal airway as a conduit for tracheal intubation in infants-a pilot study. Paediatr Anaesth. 2012;22:156–60. doi: 10.1111/j.1460-9592.2011.03710.x. [DOI] [PubMed] [Google Scholar]
- 38.Barch B, Rastatter J, Jagannathan N. Difficult pediatric airway management using the intubating laryngeal airway. Int J Pediatr Otorhinolaryngol. 2012;76:1579–82. doi: 10.1016/j.ijporl.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Nagler J, Bachur RG. Advanced airway management. Curr Opin Pediatr. 2009;21:299–305. doi: 10.1097/MOP.0b013e32832b112c. [DOI] [PubMed] [Google Scholar]
- 40.Weiss M, Engelhardt T. Proposal for the management of the unexpected difficult pediatric airway. Paediatr Anaesth. 2010;20:454–64. doi: 10.1111/j.1460-9592.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- 41.Navsa N, Tossel G, Boon JM. Dimensions of the neonatal cricothyroid membrane-how feasible is a surgical cricothyroidotomy? Paediatr Anaesth. 2005;15:402–6. doi: 10.1111/j.1460-9592.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- 42.Coté CJ, Hartnick CJ. Pediatric transtracheal and cricothyrotomy airway devices for emergency use: Which are appropriate for infants and children? Paediatr Anaesth. 2009;19(Suppl 1):66–76. doi: 10.1111/j.1460-9592.2009.02996.x. [DOI] [PubMed] [Google Scholar]


