Abstract
Extraglottic airway devices (EAD) have become an integral part of anesthetic care since their introduction into clinical practice 25 years ago and have been used safely hundreds of millions of times, worldwide. They are an important first option for difficult ventilation during both in-hospital and out-of-hospital difficult airway management and can be utilized as a conduit for tracheal intubation either blindly or assisted by another technology (fiberoptic endoscopy, lightwand). Thus, the EAD may be the most versatile single airway technique in the airway management toolbox. However, despite their utility, knowledge regarding specific devices and the supporting data for their use is of paramount importance to patient's safety. In this review, number of commercially available EADs are discussed and the reported benefits and potential pitfalls are highlighted.
Keywords: Extraglottic airway devices, laryngeal mask airway, other extraglottic airway devices
INTRODUCTION
Airway management devices that allow gas to enter and exit the airway via an airway tube, which sit above the glottis, are commonly referred to as “supraglottic airways.” However, because a number of device designs incorporate components that are inferior to, but remain outside the glottis, the term “extraglottic airway device” (EAD) is more appropriate.[1] At the time of this writing, there were not less than 25 such devices approved for clinical use in children and adults. For the clinician, the decision of which of these devices to be used can be confusing. Ease of insertion, airway seal pressure, ability to protect against gastric insufflation and regurgitation, ease of intubation through the device, patient comfort, and cost per use are some of the few myriad device characteristics to be considered. The purpose of this review is to provide a comprehensive description of commonly available EADs with particular attention to literature supporting their use. This is of particular importance, as concern exists that increasing number of airway management devices are being introduced into clinical practice without having undergone efficacy and safety examination.[2] The evolution over time, design similarities and differences, and potential advantages and disadvantages of various EADs are also highlighted. For the purposes of uniformity, the term EAD will be used throughout this review.
HOW ARE EADS CLASSIFIED?
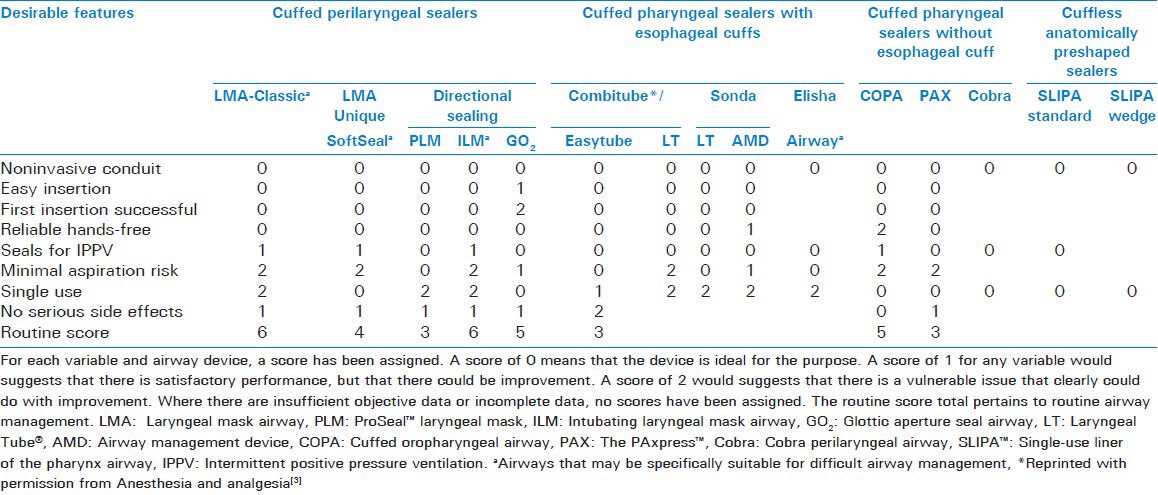
Brimacombe proposed a classification system for EADs based upon: The presence or absence of a cuff, whether the route of insertion was oral or nasal, and the anatomic location of the distal portion of the device in relation to the hypopharynx.[1] However, this proposed classification includes devices that are primarily intended to help clear the airway and/or facilitate tracheal intubation. For EADs whose primary role is airway maintenance during general anesthesia, a more commonly accepted classification is that proposed by Miller.[3] EADs are differentiated by virtue of where in the hypopharynx the cuff of the device (whether it be inflatable or anatomically pre-shaped) provides a seal, whether or not the sealing effect is directional, and whether or not esophageal sealing occurs [Figure 1]. Particular interest has been paid in recent years on devices providing esophageal sealing via the incorporation of an esophageal vent tube due to a perceived benefit in reducing the incidence of gastric regurgitation and pulmonary aspiration during airway maintenance. However, a number of studies have reported extremely low rates of pulmonary aspiration with the use of non-esophageal sealing devices.[4] Thus, little evidence supports the routine widespread use of esophageal sealing devices over their non-esophageal sealing counterparts, particularly, as they are often more costly. Clinical use characteristics of some commonly used extraglottic airway devices are shown in Table 1.
Figure 1.
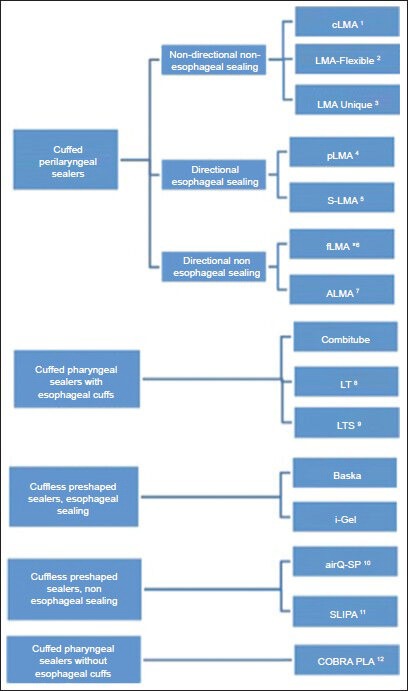
Classification of extraglottic airway devices; (1) Classic laryngeal mask airway, (2) laryngeal mask airway flexible (3) laryngeal mask airway unique (4) lma proseal, (5) lma supreme, (6) fastrachlma, (*) directional only when the handle is engaged, (7) ambu laryngeal mask airway, (8) laryngeal tube (9) laryngeal tube s, (10) airq-self pressurizing, (11) streamlined liner of the pharynx airway, (12) cobra perilaryngeal airway
Table 1.
Clinical use characteristics of some commonly used extraglottic airway devices
SPECIFIC EADS
Cuffed perilaryngeal sealers
Non-directional, non-esophageal sealing
Classic LMA
The original Laryngeal Mask Airway (cLMA, Intavent Direct, Maidenhead, UK, Figure 2) was one of the first EADs introduced into clinical practice. It was invented by Dr. Archie Brain in 1981 and initially introduced to the United Kingdom in 1988 and 1991 in United States. By 1995, it had been introduced in over 80 countries and used in over 100 million patients. Subsequently, a number of other laryngeal masks were introduced; each more specialized than the original.
Figure 2.

The LMA classic
The LMA consists of two parts, the airway tube, and the mask. Reusable devices are constructed of medical grade silicone designed to provide an oval seal around the laryngeal inlet and act as a sleeve joint at the upper esophagus. The mask has a cuff, a pilot tube, and balloon through which the cuff is inflated and maintenance of intracuff pressure may be monitored. The proximal end of the shaft has a standard 15-mm adapter. Two bars at the junction of the shaft and mask prevent the epiglottis from obstructing the ventilating lumen. In contrast to reusable LMAs, the single use devices have a cuff constructed of polyvinyl-chloride, which may be somewhat stiffer and less forgiving during device insertion.
A number of insertion techniques have been described for placement of the cLMA.[5] However, as originally designed, insertion was intended to mimic deglutition. In order to accomplish this, the cuff should be fully deflated to negative pressure and appear as a spoon. The tip of the mask is pressed against the hard palate and advanced toward the larynx until resistance is felt. In this way, the bowl of the mask is encouraged to slide posterior to the epiglottis without deflecting it inferiorly over the glottis opening [Figure 3].[6] When properly positioned the proximal end of the mask abuts the base of the tongue with the tip positioned posteriorly to the cricoid cartilage engaging the proximal esophageal sphincter. The intracuff pressure should not be more than 60 cm H2O (44 mm Hg). This is particularly important when nitrous oxide is used as it can expand the mask volume and cause pressure injuries.[7] Over inflation of the cuff may paradoxically worsen the sealing ability of the device.[8]
Figure 3.
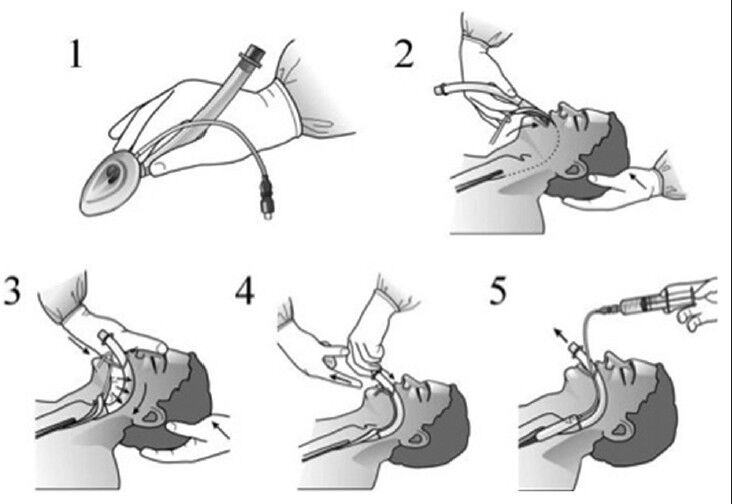
Manufacturer's recommended insertion technique (Courtesy of LMA North America, San Diego, CA)
Advantages of the cLMA
Brimacombe conducted meta-analysis in 1995 to determine whether the cLMA offered any advantages for ventilation over tracheal tube (TT) or face mask (FM) and several were reported. Fewer changes in hemodynamic and intraocular pressures were observed during placement and removal of cLMA compared to tracheal intubation and extubation and when patients were awakened with cLMA in place at the end of procedure, it was reported that there was less coughing, bucking, and hemodynamic changes than waking the patient with an endotracheal tube (ETT) in place.[9] Further, the cLMA has been reported to preserve mucociliary function, laryngeal competence, and be associated with less laryngeal trauma.[10] Following induction of anesthesia, cLMA can be placed quickly without the need for muscle paralysis or laryngoscope.
Compared to placement of a tracheal tube (TT), speed and ease of placement of LMA by inexperienced persons are increased,[11] hemodynamic stability at induction and emergence is improved,[12] the device is tolerated at lighter planes of anesthesia with a lower risk of bronchospasm, laryngospasm, and sore throat.[13] There is a lower frequency of emergence phenomena and oxygen desaturation during emergence. There is a continued debate on the complications of LMA, the frequency of failed placements and other critical complications such as aspiration of gastric contents, particularly as LMA might interfere with lower esophageal sphincter function.[14,15] The Fourth National Audit Project (NAP4) was set up by the Royal College of Anesthetists and Difficult Airway Society to provide an insight into major complications of airway management in the United Kingdom. Thirty percent of all serious airway complications were associated with extubation or removal of LMA at the end of anesthesia.[16] A study compared the quantitative clinical performances of cLMA and endotracheal intubation regarding gastric distension and positive pressure ventilation during laparoscopic cholecystectomy and concluded that the risks of gastric distension and inadequate ventilation during positive pressure ventilation with the LMA have been overestimated.[17] This was in contrast to a study by Owens where the use of the LMA resulted in increased reflux to the level of the mid to upper esophagus, and was associated with a more frequent incidence of multiple reflux events than the use of a face mask.[15]
In addition to the use of LMAs in the OR for routine anesthesia, they are used for airway management in out of OR locations and for the management of difficult or failed endotracheal intubations. Both the American Society of Anesthesiologists and the Difficult Airway Society of the United Kingdom have included the LMA in their airway management algorithms. During failed intubation in the setting of rapid sequence induction the LMA can be used as a rescue device in the event of a ‘cannot intubate cannot ventilate’ situation. While typically used in anesthetized patients, breathing spontaneously, the LMA does allow for controlled mechanical ventilation in complicated operative procedures depending on the specific device used and the airway seal pressure achieved.[18] This underscores the importance of routinely checking the airway seal pressure after insertion to confirm that the device is functioning appropriately for the clinical situation.[19,20]
LMA flexible
As compared to the cLMA, the LMA Flexible (FLMA, LMA North America, San Diego, CA, Figure 4) may facilitate improved surgical access due to its increased flexibility, longer length of the tube, reduced external diameter of the tube with its internal diameter comparable to those found in commonly used endotracheal tubes, and reduced incidence of intra-operative airway obstruction.[21] It acts as a barrier against soiling of the glottis or trachea by blood or secretions from above, making it possible to use the FLMA for intra-oral and nasopharyngeal operations. The FLMA may also be used for head and neck surgeries, ophthalmology and otorhinolaryngology procedures[22] A single use FLMA is available along with the original reusable FLMA both having different physical properties. The former is manufactured from medical grade polyvinyl chloride (PVC) rather than silicone. Both the airway tubes have same length. However, the diameter is slightly greater for the single use device. Both devices are equally easy to insert, with first attempt insertion success rates being comparable to those reported for the cLMA.[23] Due to the flexible nature of the airway tube, insertion may be more challenging and the use of a stylet or introducer has been suggested as a means to overcome this limitation.[24] Increased occurrence of airway trauma from introducer devices have not been reported despite the concern.[25] Use of a direct laryngoscope to perform direct epiglotoscopy or “mini-DL” has also been reported to avoid insertion problems with the FMLA.[22]
Figure 4.

The flexible LMA
Directional, esophageal sealing
LMA proseal
The LMA Proseal (pLMA, LMA North America, San Diego, CA, Figure 5) is a modification of the cLMA with an esophageal drain tube (DT), designed to improve controlled ventilation, airway protection, and diagnosis of misplacement. The pLMA offers an airway that bridges some of the gap between the cLMA and the TT. Compared to the cLMA, the modifications of pLMA include larger and deeper bowl with no grills (bowl lacks the ‘semi rigid shell’ of the cLMA), it incorporates a second tube placed lateral to the airway tube and ending at the tip of the mask, posterior extension of the mask cuff, integral silicone bite block, and an anterior pocket for seating an introducer or finger during insertion. The function of the second tube is to separate the alimentary and respiratory tracts. It allows escape of fluids from the stomach and reduces the risk of gastric insufflation and pulmonary aspiration and can also help determine the correct positioning of the mask. A second posterior cuff is fitted to improve the seal. There is less need for tight occlusion of the upper esophageal sphincter by the mask tip in the event of regurgitation, because of the presence of the DT. Other advantages include opportunity to pass an orogastric tube (OGT) and channeling of regurgitated stomach contents.[26] The pLMA is reusable and recommended product life is 40 sterilizations. pLMA has a shorter airway tube than the cLMA, is wire-reinforced and has similar caliber as the FLMA.
Figure 5.
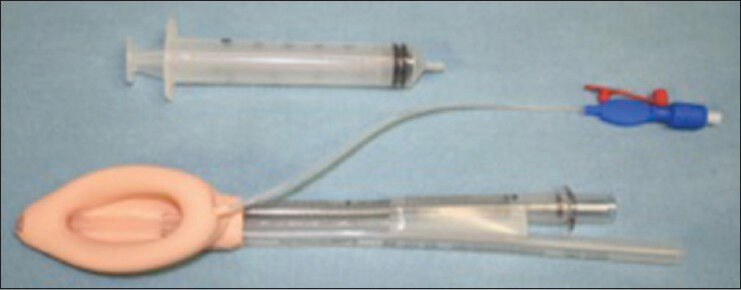
The LMA-proseal
An alternative insertion technique for the pLMA involves placing a gum elastic bougie (GEB) into the esophagus using a laryngoscope and railroading the pLMA DT over the bougie into position in order to preven back-folding of the mask tip and facilitate correct positioning.[27] In 100 anesthetized and paralyzed patients, a 100% first-time insertion success was observed.[28] Compared to two other insertion techniques (digital and introducer), first attempt insertion success was reported to be 84%, 88%, and 100%, respectively (P < 0.05 between railroading and the conventional techniques).[29] A large series of more than 3000 GEB-guided insertions without any reported mask folding confirms these findings.[30] However, GEB-guided insertion requires laryngoscopy with GEB insertion into the esophagus. It is therefore unlikely to be the routine first choice technique, but is useful when difficulties are encountered with conventional methods. The pLMA has been used for laparoscopic and abdominal surgery, obese patients, for management of anticipated or actual difficult airways, and after failed intubation in selected cases. Reported evidence suggests the pLMA reduces aspiration risk compared to the cLMA. Meta-analysis of cLMA use estimates the incidence of clinically detectable regurgitation as 18 in 10,024 and aspiration was one in 4,000 to 11,000 uses.[4] The pLMA may cause airway obstruction,[31,32,33] which is also seen with cLMA but increased frequency is seen with pLMA due to its larger size and softer material. The frequency of pLMA associated airway obstruction was quantified by Brimacombe where 19 cases of partial obstruction during 6,321 pLMA uses (0.3%) in paralyzed patients was reported.[34] Esophageal or gastric inflation during pLMA use has been reported.[35]
LMA-supreme
The LMA-Supreme (S-LMA, The Laryngeal Mask Company Ltd, Wooburn Green Bucks, UK) is easy to insert with rapid insertion time, provides reliable airway with good airway seal pressure. This devise is designed incorporating features of a cLMA, pLMA, and LMA Fastrach (FT-LMA, see below)[36,37,38] The new features are that the airway tube incorporates a DT within its lumen to shorten and straighten its path. It is oval-shaped to match the shape of the mouth and to reduce rotation in the pharynx, and the inner cuff has been strengthened to prevent airway obstruction from in-folding epiglottis. It is directional when the tab on the airway tube is engaged by a dorsally directed force, thus rotating the device against the anterior glottis with what the posterior portion of the proseal LMA cuff does. In contrast to other EAD devices such as the FT-LMA, the S-LMA does not allow the passage of an ETT due to the limited airway tube diameter. It was observed that S-LMA had leak pressures similar to the pLMA, and has been reported to be successful during both spontaneous and positive pressure ventilation [Figure 6].[37]
Figure 6.
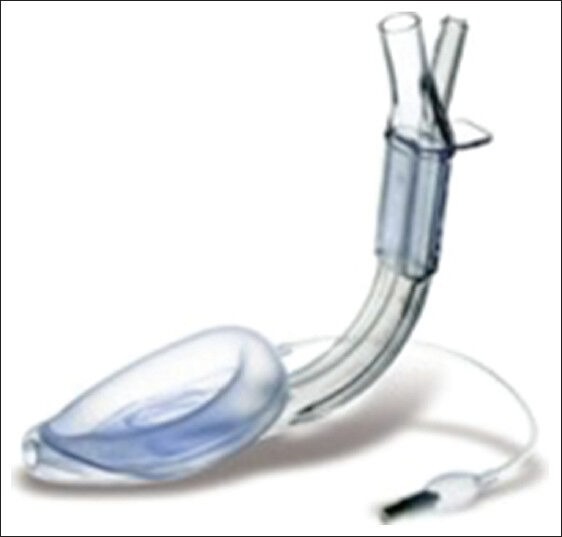
The LMA supreme
Directional, non-esophageal sealing
LMA fastrach
While comparing with cLMA the primary distinguishing features of LMA Fastrach (FT-LMA, LMA North America, Inc., San Diego, CA) include an anatomically curved rigid airway tube, an integrated guiding handle, an epiglottic elevating bar, (replacing the standard cLMA vertical aperture bars) and a guiding ramp built into the floor of the mask aperture.[39] This allows optimal alignment of the mask aperture with the glottic opening and provides a conduit for ETT passage. FT-LMA is generally not considered as directional but when the metal handle is engaged the device becomes directional. The internal diameter of the airway tube is 13 mm, the minimum that is able to accommodate an ETT with an internal diameter up to 8.0 mm. With lubrication, even 8.5 and 9.0 mm ETTs may fit, but generally they are not recommended [Figure 7].
Figure 7.

LMA fastrach
FT-LMAs are available in three sizes. Both reusable and disposable versions are available. Two studies (a single manikin study and a single human subject study) indicate that experienced and novice clinicians achieve equal rates of success in terms of placement, ventilation, and blind intubation with both disposable and non-disposable FT-LMAs[40,41] The Chandy maneuver is recommended routinely and consists of two sequential steps before the technique is performed. The expiratory valve of the breathing circuit is partially closed to ensure high positive pressure. The metal handle is used to rotate the device in the sagittal and/or coronal planes to establish the optimal ventilation with minimal resistance to the bag ventilation and audible “leaks” during manual ventilation. The handle is held in this position and optimizes the passage of the tracheal tube. The second step is to lift the handle without tilting the FTLMA away from the posterior pharyngeal wall. In summary, the first step enables the alignment of the laryngeal aperture and the bowl of the mask, whereas the second facilitates the smooth passage of the ETT.[42] The FT-LMA has a proven role in the airway management of anticipated difficult OR intubations, unanticipated difficult OR intubations, cervical spine injuries, and limited airway access situations. The FT-LMA was used in 257 procedures performed in 254 patients with immobilized cervical spines, with airways distorted by tumors, surgery, or radiation therapy, and patients wearing stereotactic frames. Insertion was accomplished in three attempts or fewer, in all patients. The overall success rates for blind and fiber optically guided intubations were 96.5% and 100%, respectively.[43] The FT-LMA has compared favorably with fiber optic intubation, the cLMA, the laryngeal tube, and the CobraPLA. The FT-LMA airway seal pressures are excellent, serious complications are uncommon, and the FT-LMA figures prominently in most difficult airway guidelines. In a study by Bercker et al.,[44] the FT-LMA was reported to be superior to the pLMA in terms of providing laryngeal seals when the esophagus was pressurized, thus reducing the risk of aspiration. In their study, the FT-LMA withstood up to 115 cm H2O of esophageal pressure versus 48 cm H2O for the cLMA and 71 cm H2O for the pLMA. No clear conclusion can be drawn from these findings regarding the prevention of aspiration. Choyce et al., demonstrated that non-experienced clinicians had a faster and greater success at ventilation with FT-LMA use than cLMA.[45] In another study performed by Asai et al.,[46] it was observed that all 25 FT-LMA patients were ventilated adequately versus 22 cLMA patients. Also, the placement of FT-LMA was easier than cLMA. But FT-LMA has certain limitations like the rigidity of its breathing tube makes it inadvisable for prolonged use as an EAD device, as it might lead to posterior pharyngeal pressure necrosis, and the need for a tracheal tube adding to the overall cost. Also, FT-LMA is not available in pediatric sizes.[47]
ALMA
ALMA (Ambu Laryngeal Masks) has a preformed curve replicating human upper airway anatomy. The internal ribs built in this curve give flexibility to it. It does not require a stylet and is available in both pediatric and adult sizes (sizes 1-5). It has both reusable and disposable versions, and can be used as an intubation conduit. The mask has an extra soft 0.4 mm cuff manufactured from PVC and is claimed to provide a better seal that conforms well to the shape of the airway,[48] hence causing less internal pressure. A disposable ALMA is an acceptable alternative to the reusable cLMA.[48] A study showed that ALMA was as effective as cLMA in maintaining the airway for positive pressure ventilation with all the patients being successfully ventilated without any difficulty. The ease of insertion seen from successful first attempts was also comparable between the groups (cLMA 87% vs. ALMA 83%).[49] Similar results have been reported in other studies.[50,51] It was observed that the time taken for ALMA insertion was shorter than cLMA,[51,52] which could be due to the rigid curve of its main tube that facilitated insertion as compared to the softer main tube of cLMA. In providing effective seal in positive pressure ventilation, ALMA was superior to cLMA.[53,54] The incidence of sore throat was not related to the duration of the operation. This was most likely attributed to the softer material cuff (PVC) and lower cuff pressure asserted by the ALMA.[51] When compared with the original single-use LMA (uLMA, LMA-Unique, LMA North America, San Diego, California, USA), the ALMA supplies a higher oropharyngeal leakage pressure than the uLMA in spontaneously breathing patients undergoing general anesthesia. Both devices showed statistically similar results in terms of overall success rates, insertion times, laryngeal alignment, and postoperative sore throat [Figure 8].[55]
Figure 8.

AMBU laryngeal mask airway
Cuffed Pharyngeal Sealers, non-esophageal sealing
The Cobra Perilaryngeal Airway (Cobra PLA; Pulmodyne, Indianapolis, IN) is a cuffed, disposable EAD. It has a tapered, striated head, a large, circumferential pharyngeal cuff, and a breathing tube and is available in eight sizes for use from neonates through large adults. The ventilatory opening at the junction of the tube and the head is protected from obstruction by the epiglottis by a soft grill on the anterior (laryngeal) aspect of the head. It has been evaluated during spontaneous and positive pressure ventilation in several studies [Figure 9].[56,57,58]
Figure 9.
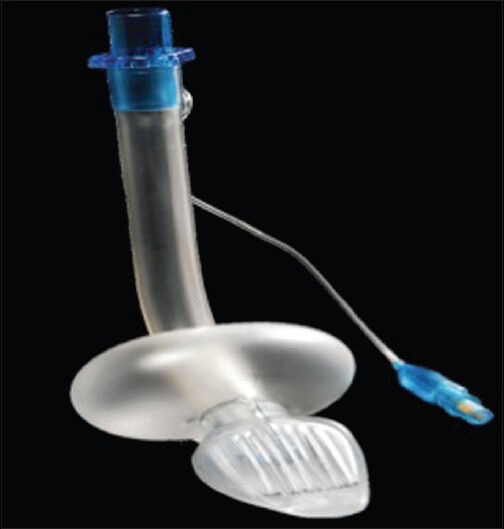
CobraPerilaryngeal airway (PLA)
Cobra PLA is comparable to the cLMA with respect to insertion, incidence, and severity of sore throat but is superior with respect to airway sealing pressure, and has been successfully used in patients with limited mouth opening and limited head extension.[59] While comparing Cobra PLA with cLMA during anesthesia for elective surgery, pulmonary aspiration occurred in two patients with Cobra PLA.[51] Cobra PLA and uLMA are effective in establishing a patent airway in patients who are spontaneously breathing under general anesthesia and have similar insertion and functional characteristics with an infrequent rate of complications.[60] Cobra PLA use resulted in higher airway leakage pressure and a lower cuff pressure compared to LMA, but insertion time was longer.[53] On comparing, S-LMA was found superior to Cobra PLA in terms of ventilation with shorter insertion time and less side effects.[54] Cobra PLA and pLMA devices showed similar insertion characteristics, airway sealing pressure, gastric distension, and adverse effects, and both were successful in providing an adequate airway and effective ventilation during laparoscopic cholecystectomy.[61]
Cuffed Pharyngeal Sealers, esophageal sealing
Combitube
The Combitube (Covidien-Nellcor, Boulder, CO) is a disposable, double-lumen tube that combines the features of a conventional ET with those of an esophageal obturator airway. It has a large proximal oropharyngeal balloon and a distal esophageal (or tracheal), low-pressure small cuff, with eight ventilatory holes between the cuffs and a single ventilatory port at the distal tip. Ventilation is possible with the Combitube regardless of whether the distal tip is in the esophagus or in the trachea.[55] When the distal tip is in the esophagus, the distal cuff seals the esophagus against regurgitation of gastric contents, and a gastric tube can be placed through the esophageal lumen. When the distal tip is in the trachea the device functions like a conventional ET when the distal cuff is inflated. Combitube has a major advantage over conventional ETT as it can be inserted without head and neck movement, which may be an important consideration in trauma patients.[62] Combitube is used for emergent airway control in patients for whom ETT placement is not immediately possible.[63] A potential limitation of the Combitube is that suctioning of tracheal secretions is impossible when the Combitube is in the esophageal position.[64] One study compared the Combitube, the pLMA, and the Laryngeal Tube S (LTS) in 90 patients who underwent general anesthesia for minor gynecologic procedures, Combitube was inferior for the technical aspects of ventilation (time to successful placement and ventilation, failure rate), produced the highest cuff pressures and resulted in the highest incidence of airway morbidity.[59] Another study showed increased airway morbidity with the Combitube compared with the LMA during routine surgery[65] and came to the conclusion that airway management with the Combitube during routine general anesthesia is not recommended [Figure 10].[66,67]
Figure 10.
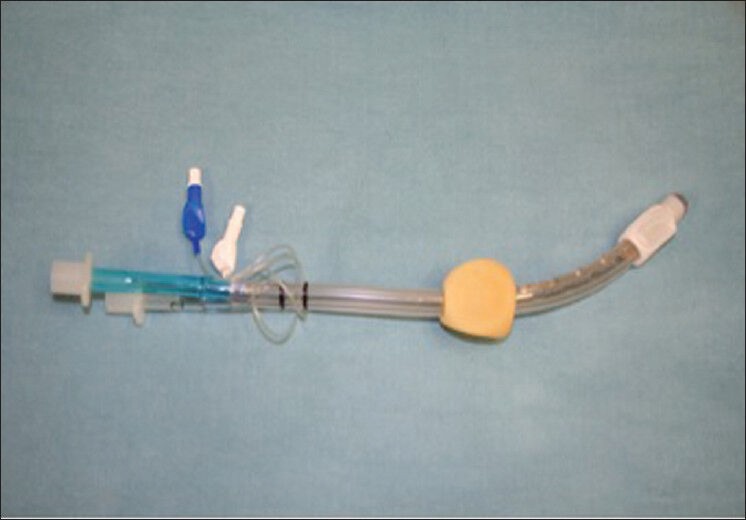
Esophageal-tracheal combitube
Laryngeal tube
The Laryngeal Tube (LT; King Systems, Noblesville, IN) is a multiple-use, single-lumen, silicon tube with an oropharyngeal and esophageal low pressure cuff incorporating a ventilation outlet between these cuffs. The LT can serve as an adjunct to tracheal intubation because its primary ventilating passage accepts a flexible bronchoscope or tube exchanger. It has a ventilating lumen, which is located between the proximal and distal cuffs. LT's insertion time was comparable to LMA.[68] Sufficient ventilation and oxygenation is achieved using the LT similar to the LMA or Combitube. Simple handling of the LT and aspiration protection might be a substantial advantage of this airway device in emergency airway management. Although, the Combitube provides protection from aspiration of gastric contents, it requires extensive regular training. LT might be a simple, alternative device to the Combitube to secure the airway.[60] The LT provides a better seal in the oropharynx than the LMA.[69] However, pLMA offers advantages over LT in most technical aspects of airway management. These include increased success rates, fewer airway interventional requirements, a more effective seal at 50% maximal cuff volume, less airway obstruction, larger expired tidal volumes, lower end tidal CO2, and fewer changes in expired tidal volume with head/neck movement. These advantages are probably related to the anatomical location of each device. The cuff of the pLMA surrounds the laryngeal inlet and forms a seal with the periglottic tissues, whereas the cuff of the LT forms a proximal laryngopharyngeal plug. pLMA offers advantages over the LT in most technical aspects of airway management in paralyzed patients undergoing pressure-controlled ventilation [Figure 11].[70]
Figure 11.
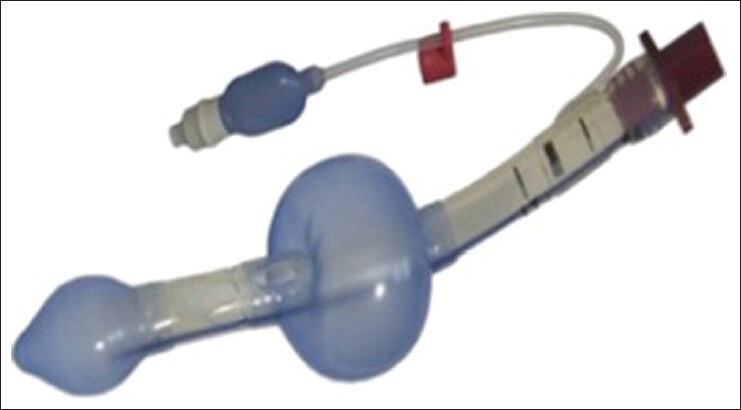
Laryngeal tube
LTS is a double-lumen, silicon tube with an oropharyngeal and esophageal low-pressure cuff and a ventilation outlet between these cuffs thus serving for gastric drainage and suctioning in patients with respiratory arrest.[71]
Cuffless anatomically pre-shaped Sealers, esophageal sealing
Baska mask
The Baska Mask (Proact Medical Systems, Frenchs Forest NSW, Australia) eliminates the need of an orogastric tube and replaces with two drains and a sump. Its oval shaped airway tube is similarly shaped to the mouth thus reducing rotation inside the pharynx. The biggest advantage of Baska mask lies in the fact that cuff deflation or inflation is not required prior to insertion. Neither is monitoring of the cuff pressure before surgical intervention required.[72] Zundert et al., concluded that it improves safety when used in both intermittent positive pressure ventilation (IPPV) and spontaneous breathing. The soft diaphragm Bask mask cuff distends during the inspiratory phase of positive pressure ventilation, increasing the effectiveness of the seal. The Baska Mask was successfully inserted on the first attempt in 76.7% of patients and was relatively very easy to insert. The severity of throat discomfort, dysphagia and dysphonia is very low.[73] Removal of device was also considered easy. The Baska Mask also incorporates a bite block throughout the length of airway tube [Figure 12].
Figure 12.
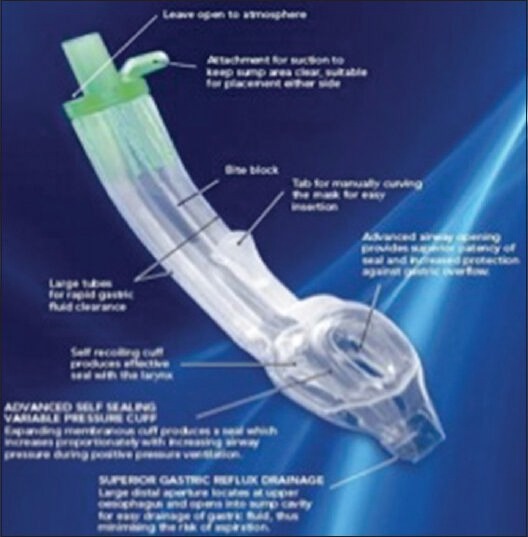
The baska mask
I-Gel
I-Gel (Intersurgical, Wokingham, Berkshire, UK) is a single-use device that has an integral bite block, a gastric tube channel and a soft, non-inflatable cuff that adapts to the hypopharyngeal anatomy after blind placement. With an epiglottic rest and wider and shorter stem, the I-Gel gives an optimal view of the glottis with a fiberoptic scope. A shorter tube is ideal for endotracheal tube placement. Their sizes are chosen depending on the weight and anatomy of patients. It can be left in place or removed using an Aintree intubation catheter.[74] The I-Gel is safe and effective during positive pressure ventilation in adults with a BMI less than 35 kg/m2[66] and in non-obese children,[67] but in another study with 280 uses of the I-Gel yielded three cases of regurgitation, with one resulting in non-fatal aspiration [Figure 13].[68]
Figure 13.
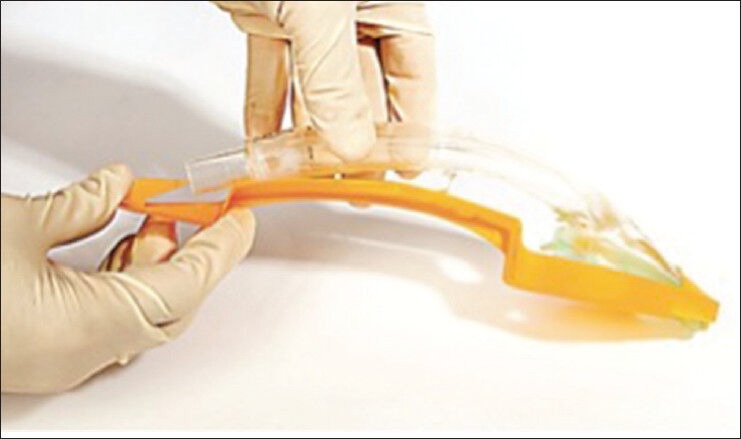
I Gel
In a study it was observed that hemodynamic parameters, ease of insertion and postoperative complications were comparable among the I-Gel, pLMA and cLMA but airway sealing pressure was significantly higher in the I-Gel group.[75]
Cuffless anatomically pre-shaped Sealers, non-esophageal sealing
Self-pressurizing Air-Q intubating laryngeal airway (Air/Q ILA-SP, Cookgas LLC, St Louis, MO, USA) is a self-pressurizing EAD device that incorporates a self-regulating periglottic cuff. The air-Q/ILA is available as a disposable (air-Q) or non-disposable (ILA) device. It has an elliptical, inflatable, cuffed mask and a slightly curved airway tube with a detachable connector. When 100 patients using this device were reviewed retrospectively, a number of observations were reported. Air/Q ILA were successfully inserted in all 100 patients. The median airway seal pressure was observed to be 22 cm H2O. Blind intubation via the air-Q/ILA was successful in 97% patients and 2 failed attempts at blind intubation were rescued by fiberoptic-aid. Complaints of sore throat were seen in 11% of patients postoperatively before discharge. Air Q/ILA's performance was adequate as a primary airway during anesthesia with respect to ease of insertion, adequacy of airway maintenance and as a conduit for intubation in both anticipated and unanticipated difficult airways.[76] Another study showed that the FT-LMA had a higher rate of success (99%) in facilitating blind intubation than did the Air/Q ILA (77%) [Figure 14].[47]
Figure 14.
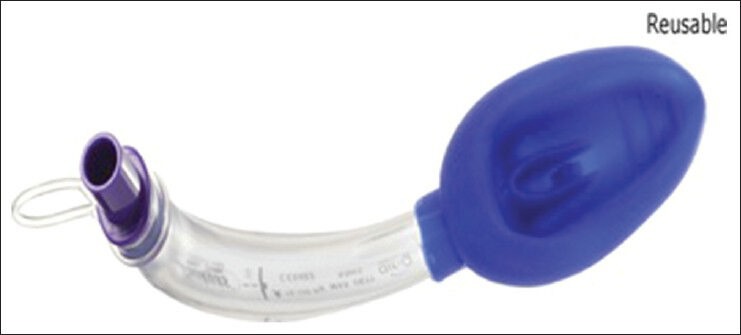
Air-Q Intubating laryngeal airway (airQ-ILA)
In a prospective controlled trial for blind endotracheal intubation, Air Q/ILA was successful in 57% patients. In addition this device was useful for the insertion of fiberoptic scope for the purpose of tracheal intubation.[77] Yet another trial concluded that the Air/Q ILA can be used as the primary supraglottic device of choice.[78]
The streamlined liner of the pharynx airway
The Streamlined Liner of the Pharynx Airway (SLIPA, SLIPA Medical, Douglas, Isle of Man, Figure 15) is a non-cuffed, single use EAD device, made of soft plastic in the shape of a pressurized pharynx. The SLIPA has a hollow, boot-shaped chamber, with a toe bridge that seals at the base of the tongue and a heel that anchors the device in place between the esophagus and the nasopharynx. The hollow chamber can store up to 50 mL of regurgitant gastric liquid. The SLIPA is available in six adult sizes that match the width of the thyroid cartilage. It is used as a primary airway device during general anesthesia of short duration. Its efficacy and complication rate are comparable to those of the cLMA.[57,79] The SLIPA is not recommended for patients placed in positions other than supine or when the risk of aspiration is increased. Insertion times, first insertion success rates, recovery times, and hemodynamic responses, associated with insertion of the SLIPA, are similar to those of the pLMA. Although, the SLIPA has a unique reservoir chamber to contain regurgitated fluid, the extent of its protection against pulmonary aspiration has not been established in the clinical setting.[59]
Figure 15.
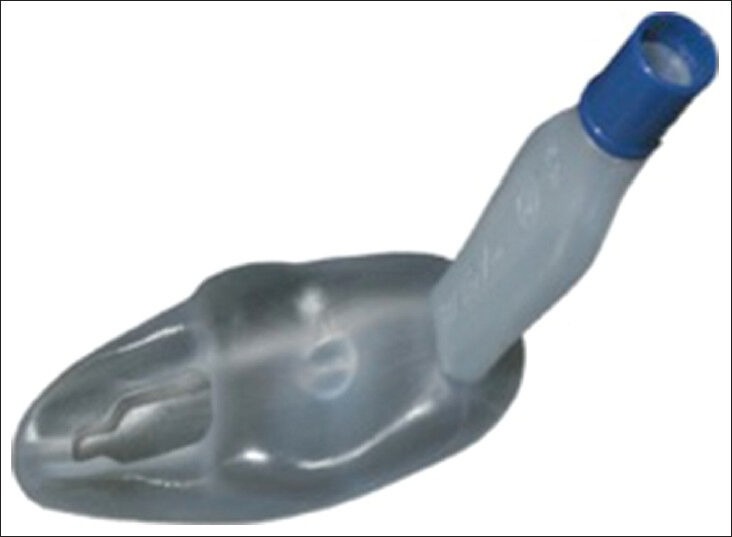
The streamlined liner of the pharynx airway
Uses of EADs for tracheal intubation
Use of introducers or catheters through a supraglottic airway can prove to be a useful alternative intubation technique and also can act as a ventilator device. The Aintree Intubation Catheter (AIC) as an intubation introducer resulted in high success rates in both elective and emergent intubations.[80] Blair et al., did a manikin study using the AIC for tracheal intubation via the LMA Proseal and achieved a 98% success rate.[81] Another technique involves a wire-guided approach with or without an airway exchange catheter. This involves inserting the bronchoscope into the trachea, passing a guidewire through the working channel of the bronchoscope, removing the bronchoscope with the wire in the same relative position, and then passing an exchange catheter over the wire. Intubation is highly successful but the overall insertion time is longer compared to the AIC technique due to the added steps.
CONCLUSION
It has been more than 2 decades since the introduction of the first clinically useful EAD. EADs have been used safely over 200 million times over that period and, as a result, many authorities consider the development of EADs in general, but the LMA in particular, to be the most important development in airway management over the last 50 years.[82,83] EADs continue to evolve and it is incumbent upon clinicians to be intimately familiar with the risk and benefits of any member of this class of airway devices that they will be utilizing. These authors agree with the Difficult Airway Society Airway Device Evaluation Project Team (ADEPT) that evidence-based principles be applied to group or institutional purchasing decisions regarding airway management products. Further, products with documented evidence of efficacy and safety should be preferred to those where evidence is lacking.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Brimacombe J. A proposed classification system for extraglottic airway devices. Anesthesiology. 2004 Aug;101(2):559. doi: 10.1097/00000542-200408000-00054. [DOI] [PubMed] [Google Scholar]
- 2.Pandit JJ, Popat MT, Cook TM, Wilkes AR, Groom P, Cooke H, et al. The Difficult Airway Society ‘ADEPT’ Guidance on selecting airway devices: The basis of a strategy for equipment evaluation. Anaesthesia. 2011;66:726–37. doi: 10.1111/j.1365-2044.2011.06787.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller DM. A proposed classification and scoring system for supraglottic sealing airways: A brief review. Anesth Analg. 2004;99:1553–9. doi: 10.1213/01.ANE.0000134798.00069.2B. [DOI] [PubMed] [Google Scholar]
- 4.Brimacombe JR, Berry A. The incidence of aspiration associated with the laryngeal mask airway: A meta-analysis of published literature. J Clin Anesth. 1995;7:297–305. doi: 10.1016/0952-8180(95)00026-e. [DOI] [PubMed] [Google Scholar]
- 5.Monem A, Khan FA. Laryngeal mask airway insertion anaesthesia and insertion techniques. J Pak Med Assoc. 2007;57:607–11. [PubMed] [Google Scholar]
- 6.Hernandez MR, Klock PA, Jr, Ovassapian A. Evolution of the extraglottic airway: A review of its history, applications, and practical tips for success. Anesth Analg. 2012;114:349–68. doi: 10.1213/ANE.0b013e31823b6748. [DOI] [PubMed] [Google Scholar]
- 7.Ouellette RG. The effect of nitrous oxide on laryngeal mask cuff pressure. AANA J. 2000;68:411–4. [PubMed] [Google Scholar]
- 8.Keller C, Puhringer F, Brimacombe JR. Influence of cuff volume on oropharyngeal leak pressure and fibreoptic position with the laryngeal mask airway. Br J Anaesth. 1998;81:186–7. doi: 10.1093/bja/81.2.186. [DOI] [PubMed] [Google Scholar]
- 9.Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: A meta-analysis. Can J Anaesth. 1995;42:1017–23. doi: 10.1007/BF03011075. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Isono S, Ishikawa T, Sato J, Nishino T. Laryngeal resistance before and after minor surgery: Endotracheal tube versus Laryngeal Mask Airway. Anesthesiology. 2003;99:252–8. doi: 10.1097/00000542-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Baskett PJ. The laryngeal mask in resuscitation. Resuscitation. 1994;28:93–5. doi: 10.1016/0300-9572(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Tanaka H, Toyooka H. Circulatory responses to laryngeal mask airway insertion or tracheal intubation in normotensive and hypertensive patients. Can J Anaesth. 1995;42:32–6. doi: 10.1007/BF03010568. [DOI] [PubMed] [Google Scholar]
- 13.Pennant JH, White PF. The laryngeal mask airway. Its uses in anesthesiology. Anesthesiology. 1993;79:144–63. doi: 10.1097/00000542-199307000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Valentine J, Stakes AF, Bellamy MC. Reflux during positive pressure ventilation through the laryngeal mask. Br J Anaesth. 1994;73:543–4. doi: 10.1093/bja/73.4.543. [DOI] [PubMed] [Google Scholar]
- 15.Owens TM, Robertson P, Twomey C, Doyle M, McDonald N, McShane AJ. The incidence of gastroesophageal reflux with the laryngeal mask: A comparison with the face mask using esophageal lumen pH electrodes. Anesth Analg. 1995;80:980–4. doi: 10.1097/00000539-199505000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Hodzovic I. Airway management disasters-lessons from the United Kingdom. Acta Clin Croat. 2012;51:525–7. [PubMed] [Google Scholar]
- 17.Maltby JR, Beriault MT, Watson NC, Fick GH. Gastric distension and ventilation during laparoscopic cholecystectomy: LMA-classic vs. tracheal intubation. Can J Anaesth. 2000;47:622–6. doi: 10.1007/BF03018993. [DOI] [PubMed] [Google Scholar]
- 18.Taheri A, Hajimohamadi F, Soltanghoraee H, Moin A. Complications of using laryngeal mask airway during anaesthesia in patients undergoing major ear surgery. Acta Otorhinolaryngol Ital. 2009;29:151–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Gil M, Brimacombe J, Keller C. A comparison of four methods for assessing oropharyngeal leak pressure with the laryngeal mask airway in paediatric patients. Paediatr Anaesth. 2001;11:319–21. doi: 10.1046/j.1460-9592.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- 20.Keller C, Brimacombe JR, Keller K, Morris R. Comparison of four methods for assessing airway sealing pressure with the laryngeal mask airway in adult patients. Br J Anaesth. 1999;82:286–7. doi: 10.1093/bja/82.2.286. [DOI] [PubMed] [Google Scholar]
- 21.George JM, Sanders GM. The reinforced laryngeal mask in paediatric outpatient dental surgery. Anaesthesia. 1999;54:546–51. doi: 10.1046/j.1365-2044.1999.00851.x. [DOI] [PubMed] [Google Scholar]
- 22.Choo CY, Koay CK, Yoong CS. A randomised controlled trial comparing two insertion techniques for the Laryngeal Mask Airway Flexible™ in patients undergoing dental surgery. Anaesthesia. 2012;67:986–90. doi: 10.1111/j.1365-2044.2012.07167.x. [DOI] [PubMed] [Google Scholar]
- 23.Flynn P, Ahmed FB, Mitchell V, Patel A, Clarke S. A randomised comparison of the single use LMA Flexible™ with the reusable LMA Flexible™ in paediatric dental day-case patients. Anaesthesia. 2007;62:1281–4. doi: 10.1111/j.1365-2044.2007.05234.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda O, Yoshitake A. Stylet for reinforced laryngeal mask airway. Anaesthesia. 2002;57:1140–1. doi: 10.1046/j.1365-2044.2002.288110.x. [DOI] [PubMed] [Google Scholar]
- 25.William A, Chambers NA, Erb TO, von Ungern-Sternberg BS. Incidence of sore throat in children following use of flexible laryngeal mask airways-impact of an introducer device. Pediatr Anesth. 2010;20:839–43. doi: 10.1111/j.1460-9592.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- 26.Brain AI, Verghese C, Strube PJ. The LMA ‘ProSeal’-a laryngeal mask with an oesophageal vent. Br J Anaesth. 2000;84:650–4. doi: 10.1093/bja/84.5.650. [DOI] [PubMed] [Google Scholar]
- 27.Howath A, Brimacombe J, Keller C, Kihara S. Gum elastic bougie-guided placement of the ProSeal laryngeal mask. Can J Anaesth. 2002;49:528–9. doi: 10.1007/BF03017942. [DOI] [PubMed] [Google Scholar]
- 28.Howath A, Brimacombe J, Keller C. Gum-elastic bougie-guided insertion of the ProSeal laryngeal mask airway: A new technique. Anaesth Intensive Care. 2002;30:624–7. doi: 10.1177/0310057X0203000514. [DOI] [PubMed] [Google Scholar]
- 29.Brimacombe J, Keller C, Judd DV. Gum elastic bougie-guided insertion of the ProSeal laryngeal mask airway is superior to the digital and introducer tool techniques. Anesthesiology. 2004;100:25–9. doi: 10.1097/00000542-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Pons V, Madrid V. Ease placement of LMA ProSeal with a gastric tube inserted. Anesth Analg. 2004;98:1816–7. doi: 10.1213/01.ANE.0000120096.49638.FF. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor CJ, Jr, Davies SR, Stix MS, Dolan RW. Gastric distention in a spontaneously ventilating patient with a ProSeal laryngeal mask airway. Anesth Analg. 2002;94:1656–8. doi: 10.1097/00000539-200206000-00055. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor CJ, Jr, Stix MS. Bubble solution diagnoses ProSeal insertion into the glottis. Anesth Analg. 2002;94:1671–2. doi: 10.1097/00000539-200206000-00065. [DOI] [PubMed] [Google Scholar]
- 33.Braun U, Zerbst M, Füllekrug B, Gentzel I, Hempel V, Leier M, et al. A comparison of the Proseal laryngeal mask to the standard laryngeal mask on anesthesized, non-relaxed patients. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:727–33. doi: 10.1055/s-2002-35911. [DOI] [PubMed] [Google Scholar]
- 34.Brimacombe J, Richardson C, Keller C, Donald S. Mechanical closure of the vocal cords with the laryngeal mask airway ProSeal. Br J Anaesth. 2002;88:296–7. doi: 10.1093/bja/88.2.296. [DOI] [PubMed] [Google Scholar]
- 35.Stix MS, Rodriguez-Sallaberry FE, Cameron EM, Teague PD, O’Connor CJ., Jr Esophageal aspiration of air through the drain tube of the ProSeal laryngeal mask. Anesth Analg. 2001;93:1354–7. doi: 10.1097/00000539-200111000-00065. [DOI] [PubMed] [Google Scholar]
- 36.van Zundert A, Brimacombe J. The LMA Supreme-a pilot study. Anaesthesia. 2008;63:209–10. doi: 10.1111/j.1365-2044.2007.05421.x. [DOI] [PubMed] [Google Scholar]
- 37.Hosten T, Gurkan Y, Ozdamar D, Tekin M, Toker K, Solak M. A new supraglottic airway device: LMA-Supreme™, comparison with LMA-Proseal™. Acta Anaesthesiol Scand. 2009;53:852–7. doi: 10.1111/j.1399-6576.2009.01986.x. [DOI] [PubMed] [Google Scholar]
- 38.Cook TM, Gatward JJ, Handel J, Hardy R, Thompson C, Srivastava R, et al. Evaluation of the LMA Supreme™ in 100 non-paralysed patients*. Anaesthesia. 2009;64:555–62. doi: 10.1111/j.1365-2044.2008.05824.x. [DOI] [PubMed] [Google Scholar]
- 39.Brain AI, Verghese C, Addy EV, Kapila A. The intubating laryngeal mask. I: Development of a new device for intubation of the trachea. Br J Anaesth. 1997;79:699–703. doi: 10.1093/bja/79.6.699. [DOI] [PubMed] [Google Scholar]
- 40.Haardt V, Lenfant F, Cailliod R, Freysz M. Tracheal intubation through the intubating laryngeal mask airway training on manikin: Comparison of single use and reusable devices from the same manufacturer. Ann Fr Anesth Reanim. 2008;27:297–301. doi: 10.1016/j.annfar.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Teoh WH, Lim Y. Comparison of the single use and reusable intubating laryngeal mask airway. Anaesthesia. 2007;62:381–4. doi: 10.1111/j.1365-2044.2007.04980.x. [DOI] [PubMed] [Google Scholar]
- 42.Verghese C. Anesthesiology News; 2010. Laryngeal mask airway devices: Three maneuvers for any clinical situation; p. 36. [Google Scholar]
- 43.Ferson DZ, Rosenblatt WH, Johansen MJ, Osborn I, Ovassapian A. Use of the Intubating LMA-Fastrach™ in 254 Patients with Difficult-to-manage Airways. Anesthesiology. 2001;95:1175–81. doi: 10.1097/00000542-200111000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Bercker S, Schmidbauer W, Volk T, Bogusch G, Bubser HP, Hensel M, et al. A comparison of seal in seven supraglottic airway devices using a cadaver model of elevated esophageal pressure. Anesth Analg. 2008;106:445–8. doi: 10.1213/ane.0b013e3181602ae1. [DOI] [PubMed] [Google Scholar]
- 45.Choyce A, Avidan MS, Patel C, Harvey A, Timberlake C, McNeilis N, et al. Comparison of laryngeal mask and intubating laryngeal mask insertion by the naive intubator. Br J Anaesth. 2000;84:103–5. doi: 10.1093/oxfordjournals.bja.a013363. [DOI] [PubMed] [Google Scholar]
- 46.Asai T, Wagle AU, Stacey M. Placement of the intubating laryngeal mask is easier than the laryngeal mask during manual in-line neck stabilization. Br J Anaesth. 1999;82:712–4. doi: 10.1093/bja/82.5.712. [DOI] [PubMed] [Google Scholar]
- 47.Karim YM, Swanson DE. Comparison of blind tracheal intubation through the intubating laryngeal mask airway (LMA Fastrach) and the Air-Q. Anaesthesia. 2011;66:185–90. doi: 10.1111/j.1365-2044.2011.06625.x. [DOI] [PubMed] [Google Scholar]
- 48.Sudhir G, Redfern D, Hall JE, Wilkes AR, Cann C. A comparison of the disposable Ambu AuraOnce Laryngeal Mask with the reusable LMA Classic laryngeal mask airway. Anaesthesia. 2007;62:719–22. doi: 10.1111/j.1365-2044.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- 49.Suzanna AB, Liu CY, Rozaidi SW, Ooi JS. Comparison between LMA-Classic and AMBU AuraOnce laryngeal mask airway in patients undergoing elective general anaesthesia with positive pressure ventilation. Med J Malaysia. 2011;66:304–7. [PubMed] [Google Scholar]
- 50.Shariffuddin II, Wang CY. Randomised crossover comparison of the Ambu AuraOnce Laryngeal Mask with the LMA Classic laryngeal mask airway in paralysed anaesthetised patients. Anaesthesia. 2008;63:82–5. doi: 10.1111/j.1365-2044.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 51.Cook TM, Lowe JM. An evaluation of the Cobra Perilaryngeal Airway: Study halted after two cases of pulmonary aspiration. Anaesthesia. 2005;60:791–6. doi: 10.1111/j.1365-2044.2005.04261.x. [DOI] [PubMed] [Google Scholar]
- 52.Francksen H, Bein B, Cavus E, Renner J, Scholz J, Steinfath M. Comparison of LMA Unique, Ambu laryngeal mask and Soft Seal laryngeal mask during routine surgical procedures. Eur J Anaesthesiol. 2007;24:134–40. doi: 10.1017/S0265021506001219. [DOI] [PubMed] [Google Scholar]
- 53.Andrews DT, Williams DL, Alexander KD, Lie Y. Randomised comparison of the classic laryngeal mask airway with the cobra perilaryngeal airway during anaesthesia in spontaneously breathing adult patients. Anaesth Intensive Care. 2009;37:85–92. doi: 10.1177/0310057X0903700107. [DOI] [PubMed] [Google Scholar]
- 54.Turkmen A, Ali M, Akkaya, et al. Supreme-LMA vs Cobra PLA: 19AP1-1. Eur J Anaesthesiol. 2010;27:244. [Google Scholar]
- 55.Agro F, Frass M, Benumof JL, Krafft P. Current status of the Combitube™: A review of the literature. J Clin Anesth. 2002;14:307–14. doi: 10.1016/s0952-8180(02)00356-2. [DOI] [PubMed] [Google Scholar]
- 56.Galvin EM, van Doorn M, Blazquez J, Ubben JF, Zijlstra FJ, Klein J, et al. A randomized prospective study comparing the cobra perilaryngeal airway and laryngeal mask airway-classic during controlled ventilation for gynecological laparoscopy. Anesth Analg. 2007;104:102–5. doi: 10.1213/01.ane.0000246812.21391.d1. [DOI] [PubMed] [Google Scholar]
- 57.Gaitini L, Carmi N, Yanovski B, Tome R, Resnikov I, Gankin I, et al. Comparison of the CobraPLA (Cobra Perilaryngeal Airway) and the Laryngeal Mask Airway Unique in children under pressure controlled ventilation. Paediatr Anaesth. 2008;18:313–9. doi: 10.1111/j.1460-9592.2008.02449.x. [DOI] [PubMed] [Google Scholar]
- 58.Hooshangi H, Wong DT. Brief review: The Cobra Perilaryngeal Airway (CobraPLA and the Streamlined Liner of Pharyngeal Airway (SLIPA) supraglottic airways. Can J Anaesth. 2008;55:177–85. doi: 10.1007/BF03016093. [DOI] [PubMed] [Google Scholar]
- 59.Bein B, Carstensen S, Gleim M, Claus L, Tonner PH, Steinfath M, et al. A comparison of the proseal laryngeal mask airway (TM), the laryngeal tube S (R) and the oesophageal-tracheal combitube (TM) during routine surgical procedures. Eur J Anaesthesiol. 2005;22:341–6. doi: 10.1017/s026502150500058x. [DOI] [PubMed] [Google Scholar]
- 60.Dörges V, Ocker H, Wenzel V, Schmucker P. The laryngeal tube: A new simple airway device. Anesth Analg. 2000;90:1220–2. doi: 10.1097/00000539-200005000-00042. [DOI] [PubMed] [Google Scholar]
- 61.Chung CJ, Lee KH, Choi SR, Kim DC, Lee SC. Comparison of the CobraPLA and the LMA Classic airway devices during volume-controlled ventilation in children. Korean J Anesthesiol. 2008;55:145–9. [Google Scholar]
- 62.Mercer MH, Gabbott DA. Insertion of the Combitube airway with the cervical spine immobilised in a rigid cervical collar. Anaesthesia. 1998;53:971–4. doi: 10.1046/j.1365-2044.1998.00561.x. [DOI] [PubMed] [Google Scholar]
- 63.Calkins MD, Robinson TD. Combat trauma airway management: Endotracheal intubation versus laryngeal mask airway versus combitube use by Navy SEAL and Reconnaissance combat corpsmen. J Trauma. 1999;46:927–32. doi: 10.1097/00005373-199905000-00025. [DOI] [PubMed] [Google Scholar]
- 64.Krafft P, Röggla M, Fridrich P, Locker GJ, Frass M, Benumof JL. Bronchoscopy via a redesigned Combitube in the esophageal position. A clinical evaluation. Anesthesiology. 1997;86:1041–5. doi: 10.1097/00000542-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Oczenski W, Krenn H, Dahaba AA, Binder M, El-Schahawi-Kienzl I, Kohout S, et al. Complications following the use of the Combitube, tracheal tube and laryngeal mask airway. Anaesthesia. 1999;54:1161–5. doi: 10.1046/j.1365-2044.1999.01107.x. [DOI] [PubMed] [Google Scholar]
- 66.Uppal V, Fletcher G, Kinsella J. Comparison of the I-gel with the cuffed tracheal tube during pressure-controlled ventilation. Br J Anaesth. 2009;102:264–8. doi: 10.1093/bja/aen366. [DOI] [PubMed] [Google Scholar]
- 67.Beylacq L, Bordes M, Semjen F, Cros AM. The I-gel, a single-use supraglottic airway device with a non-inflatable cuff and an esophageal vent: An observational study in children. Acta Anaesthesiol Scand. 2009;53:376–9. doi: 10.1111/j.1399-6576.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 68.Gibbison B, Cook TM, Seller C. Case series: Protection from aspiration and failure of protection from aspiration with the I-gel airway. Br J Anaesth. 2008;100:415–7. doi: 10.1093/bja/aem396. [DOI] [PubMed] [Google Scholar]
- 69.Asai T, Kawashima A, Hidaka I, Kawachi S. The laryngeal tube compared with the laryngeal mask: Insertion, gas leak pressure and gastric insufflation. Br J Anaesth. 2002;89:729–32. [PubMed] [Google Scholar]
- 70.Brimacombe J, Keller C, Brimacombe L. A comparison of the laryngeal mask airway ProSeal™ and the laryngeal tube airway in paralyzed anesthetized adult patients undergoing pressure-controlled ventilation. Anesth Analg. 2002;95:770–6. doi: 10.1097/00000539-200209000-00045. [DOI] [PubMed] [Google Scholar]
- 71.Dorges V, Ocker H, Wenzel V, Steinfath M, Gerlach K. The Laryngeal Tube S: A modified simple airway device. Anesth Analg. 2003;96:618–21. doi: 10.1097/00000539-200302000-00057. [DOI] [PubMed] [Google Scholar]
- 72.Zundert TV, Gatt S. The Baska Mask®-A new concept in self-sealing membrane cuff extraglottic airway devices, using a sump and two gastric drains: A critical evaluation. J Obstet Anaesth Crit Care. 2012;2:23–30. [Google Scholar]
- 73.Alexiev V, Salim A, Kevin LG, Laffey JG. An observational study of the Baska (R) mask: A novel supraglottic airway. Anaesthesia. 2012;67:640–5. doi: 10.1111/j.1365-2044.2012.07140.x. [DOI] [PubMed] [Google Scholar]
- 74.Emmerich M, Tiesmeier J. The I-gel supraglottic airway: A useful tool in case of difficult fiberoptic intubation. Minerva Anestesiol. 2012;78:1169–70. [PubMed] [Google Scholar]
- 75.Das B, Mitra S, Jamil SN, Varshney RK. Comparison of three supraglottic devices in anesthetised paralyzed children undergoing elective surgery. Saudi J Anaesth. 2012;6:224–8. doi: 10.4103/1658-354X.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galgon RE, Schroeder K, Joffe AM. The self-pressurising air-Q® Intubating Laryngeal Airway for airway maintenance during anaesthesia in adults: A report of the first 100 uses. Anaesth Intensive Care. 2012;40:1023–7. doi: 10.1177/0310057X1204000614. [DOI] [PubMed] [Google Scholar]
- 77.Erlacher W, Tiefenbrunner H, Kästenbauer T, Schwarz S, Fitzgerald RD. CobraPLUS and Cookgas air-Q versus Fastrach for blind endotracheal intubation: A randomised controlled trial. Eur J Anaesthesiol. 2011;28:181–6. doi: 10.1097/EJA.0b013e328340c352. [DOI] [PubMed] [Google Scholar]
- 78.Whyte SD, Cooke E, Malherbe S. Usability and performance characteristics of the pediatric air-Q (R) intubating laryngeal airway. Can J Anaesth. 2013;60:557–63. doi: 10.1007/s12630-013-9918-6. [DOI] [PubMed] [Google Scholar]
- 79.Hein C, Plummer J, Owen H. Evaluation of the SLIPA (streamlined liner of the pharynx airway), a single use supraglottic airway device, in 60 anaesthetized patients undergoing minor surgical procedures. Anaesth Intensive Care. 2005;33:756–61. doi: 10.1177/0310057X0503300609. [DOI] [PubMed] [Google Scholar]
- 80.Wong DT, Yang JJ, Mak HY, Jagannathan N. Use of intubation introducers through a supraglottic airway to facilitate tracheal intubation: A brief review. Can J Anaesth. 2012;59:704–15. doi: 10.1007/s12630-012-9714-8. [DOI] [PubMed] [Google Scholar]
- 81.Blair EJ, Mihai R, Cook TM. Tracheal intubation via the Classic and Proseal laryngeal mask airways: A manikin study using the Aintree Intubating Catheter. Anaesthesia. 2007;62:385–7. doi: 10.1111/j.1365-2044.2007.04994.x. [DOI] [PubMed] [Google Scholar]
- 82.Hagberg C. 3rd ed. Philadelphia: Saunders; 2013. Benumof and Hagberg's Airway Management. [Google Scholar]
- 83.Verghese C, Smith TG, Young E. Prospective survey of use of Laryngeal mask airway in 2359 patients. Anaesthesia. 1993 Jan;48:58–60. doi: 10.1111/j.1365-2044.1993.tb06794.x. [DOI] [PubMed] [Google Scholar]


