Abstract
Our recent study demonstrated miR-15a/16-1 downregulation in mantle cell lymphoma (MCL). Here, we investigated mechanisms of miR-15a/16-1 transcriptional repression and its epigenetic regulation by c-Myc and histone deacetylase (HDAC) in MCL. c-Myc expression was detected in MCL cell lines and in the primary MCL samples, and pri-miR-15a/16-1 mRNAs were significantly upregulated in Mino and Jeko-1 cells with c-Myc knockdown by small interfering RNAs (siRNAs). Our co-immunoprecipitation analysis showed that c-Myc interacted with HDAC3. Moreover, using chromatin immunoprecipitation, we demonstrated that both c-Myc and HDAC3 co-localized to the two promoters of the miR-15a/16-1 cluster gene, DLEU2, and inhibition of HDAC3 increased histone acetylation of the DLEU2 promoters. Luciferase reporter assay confirmed the dependence of Myc-mediated DLEU2 transcriptional repression on HDAC3. Treatment with the pan-HDAC inhibitor, suberoylanilide hydroxamic acid and HDAC3 siRNA resulted in increased miR-15a/16-1 expression. The regulatory mechanism of miR-15a/16-1 was further demonstrated in Burkitt lymphoma and Myc overexpressing cell lines. These findings highlight the role of HDAC3 in Myc-induced miR-15a/16-1 changes and reveal novel mechanisms for c-Myc-driven microRNA suppression and malignant transformation in aggressive B-cell malignancies.
Keywords: Myc, miR15a/miR-16-1, lymphoma
Introduction
The deregulation of microRNA (miRNA) has been frequently identified in a variety of tumors and has been linked to tumorigenesis. However, the underlying mechanism responsible has been challenging to ascertain and has not been well elucidated. Cluster 15a/16-1 miRNAs (miR-15a/16-1), located at 13q14.3, were the first demonstrated tumor suppressor miRNA gene (Calin et al., 2002). Expression of these miRNAs inhibits the cell proliferation, promotes apoptosis of cancer cells and suppresses tumorigenicity by targeting multiple oncogenes (Aqeilan et al., 2010). Loss or downregulation of these miRNAs has been reported in a variety of hematopoietic and solid tumors (Calin et al., 2002; Bottoni et al., 2005; Bonci et al., 2008; Bandi et al., 2009; Davidson-Moncada et al., 2010). Our recent study demonstrated that miR-15a/16-1 is downregulated in the majority of patients with mantle cell lymphoma (MCL) (Zhao et al., 2010). However, the mechanism responsible for miR-15a/16-1 suppression is unknown.
c-Myc (hereafter Myc) is an oncogenic transcription factor that promotes tumorigenesis by activating and repressing its target genes that control the cell growth and proliferation (Meyer and Penn, 2008). Besides direct regulation of the target genes involved in the proliferation and growth, Myc has been recently implicated in controlling the complex networks of miRNAs (O'Donnell et al., 2005; Chang et al., 2008). Myc was reported to suppress the expression of a host of miRNAs in B-cell lymphomas. Among these repressed miRNAs are several putative tumor suppressors, such as the miR-15a/16-1, miR-34a and let-7 family members (Chang et al., 2008; Sotillo et al., 2011). Although the mechanisms by which Myc activates transcription have been extensively studied, less is known about how Myc represses transcription of the target genes as well as miRNAs. It was recently reported that Myc induced transcriptional repression of the target genes Id2 and Gadd153, by recruitment of histone deacetylase 3 (HDAC3) (Kurland and Tansey, 2008). Moreover, N-Myc had been demonstrated to act as a transrepressor by recruiting HDAC1 and HDAC2 (Liu et al., 2007; Marshall et al., 2010). These findings define a novel mechanism of miRNA transcriptional repression by Myc and shed light on the poorly understood mechanism for wide and general miRNA suppression in B-cell lymphomas.
Results and discussion
Myc interacts with HDAC3 in MCL
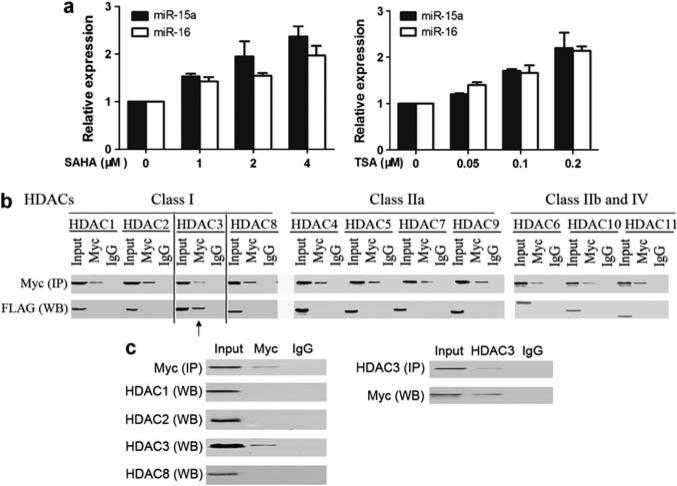
We recently reported a set of dysregulated miRNAs in MCL and revealed no correlation between the levels of miR-15a and miR-16-1 expression with corresponding cytogenetic abnormalities, 13q14 deletion (Zhao et al., 2010). This suggests that additional mechanisms, alternative to amplification and deletion of various miRNA loci, may result in miRNA deregulation. There is increasing evidence showing association between aberrant expression of miRNAs and epigenetic regulation (Croce, 2009; Garzon et al., 2010). It has been reported that some miRNAs are controlled by epigenetic alterations, such as DNA methylation and histone modification in human cancer cells (Han et al., 2007; Lee et al., 2009). This prompted us to study the epigenetic regulation of miRNAs in MCL. We therefore examined the effects of chromatin-modifying drugs such as inhibitors of HDAC on the expression of miRNAs in MCL. The expression profile of miRNAs from the MCL cell line Jeko-1 after 24-h suberoylanilide hydroxamic acid (SAHA) (1 μm) treatment was determined by miRNA microarray analysis. Indeed, SAHA induced gene expression of a set of miRNAs, including miR-29c, let-7, miR-30 and miR-15a/16-1 (Supplementary Table S1). As miR-15a/16-1 was reported to be downregulated in MCL, in other hematologic tumors and identified as a tumor suppressor gene, here we chose to focus on studying the mechanisms of miR-15a/16-1 gene repression. Quantitative real-time RT–PCR was performed to evaluate the effect of SAHA on miR-15a/16-1 expression. Figure 1a shows that SAHA caused a dose-dependent increase in miR-15a/16-1 expression in Jeko-1 cells, validating the miRNA array result. Moreover, trichostatin A, another pan-HDAC inhibitor, also induced miR-15a/16-1 expression. This suggests the role of HDAC in miR-15a/16-1 gene expression, supporting the notion that miR-15a/16-1 is subjected to epigenetic control in MCL.
Figure 1.
Myc interacts with HDAC3 and inhibition of Myc or HDAC3 increases miR-15a/16-1 gene expression in MCL. (a) Inhibition of HDAC induced dose-dependent miRNA expression by quantitative RT–PCR. Hsa-miR-15a and hsa-miR-16-1 expression levels were measured in Jeko-1 cells treated with different doses of SAHA or trichostatin A (TSA) for 24 h and compared with those that had no treatment. Data were normalized to RNU44. (b) Interaction of HDAC3 and Myc. HEK 293 cells were co-transfected with Myc plasmid and each plasmids with FLAG-HDAC1-11. The whole-cell lysates were immunoprecipitated (IP, Pierce co-immunoprecipitation kit, Thermo Scientific, Rockford, IL, USA) using an antibody against Myc or control IgG, followed by western blotting with an antibody against FLAG (for HDACs). (c) Reciprocal co-IP assays in Jeko-1 cells. Whole-cell extracts of Jeko-1 cells were subjected to IP with anti-Myc antibody followed by western blotting for HDACs (1,2,3,8), and similar whole-cell extracts were subjected to IP with anti-HDAC3, followed by western blotting with anti-Myc. Input is equivalent to 10% of the lysate used for the co-IP. All experiments were performed three independent times.
We next studied the mechanism of downregulation of miR-15a/16-1 in MCL and how HDAC represses its expression. HDAC has been shown to be recruited to gene promoters to induce histone hypoacetylation and transcriptional repression (Bhalla, 2005; Haberland et al., 2009). Recently, several lines of evidence have shown that Myc regulates expression of up to 60 miRNAs in human lymphoma cells and that the majority of identified Myc-regulated miRNAs are repressed by Myc. Among these repressed miRNAs, several are putative tumor suppressors, like miR-15a/16–1, miR-34a and the let-7 family members (Chang et al., 2008; Robertus et al., 2010). A more recent study suggests that DLEU2, a single genomic locus that encodes miR-15a/16, is repressed by the transcription factor Myc (Lerner et al., 2009). Remarkably, most of the miRNAs known to be downregulated by Myc have been shown to be underexpressed in MCL (Chang et al., 2008; Robertus et al., 2010; Zhao et al., 2010). We therefore reasoned that Myc may have a critical role in miRNA dysregulation of MCL and that Myc acts as a transcriptional repressor, at least through recruitment of HDAC. Next, we determined whether all HDACs or a specific HDAC is associated with Myc to regulate miR-15a/16-1 gene expression. We first determined the interaction between Myc and HDACs by using a transfection and co-immunoprecipitation approach. Mammalian HEK293 cells were co-transfected with each vector expressing full-length HDACs (1–11) of all classes tagged with FLAG and full-length Myc. After co-transfection, the cell lysates were immunoprecipitated using an antibody against Myc, followed by western blotting with an antibody against FLAG (for HDACs). Interestingly, we found that Myc only co-immunoprecipitated with HDAC3 (Figure 1b) and was not associated with other HDACs. The association was specific because it was not observed when control IgG was used for mock immunoprecipitation. These findings are in agreement with a recent report that Myc repressed target genes Id2 and Gadd153 through interaction with HDAC3 (Kurland and Tansey, 2008). Furthermore, the interaction between Myc and HDAC3 was identified in an untransfected MCL cell line, Jeko-1 cells (Figure 1c). In this experiment, immunoprecipitate obtained with Myc-specific antibody was shown to contain HDAC3, but not HDAC1, HDAC2 or HDAC8. Under control conditions, using an IgG antibody, no precipitation of Myc was observed. The reverse endogenous co-immunoprecipitation of Myc with HDAC3 was also observed, supporting the direct association between Myc and HDAC3 in MCL.
Myc and HDAC3 are tethered to the miR-15a/16-1 promoter regions, and Myc downregulates the transcription activity of miR-15a/16-1 promoter through histone acetylation
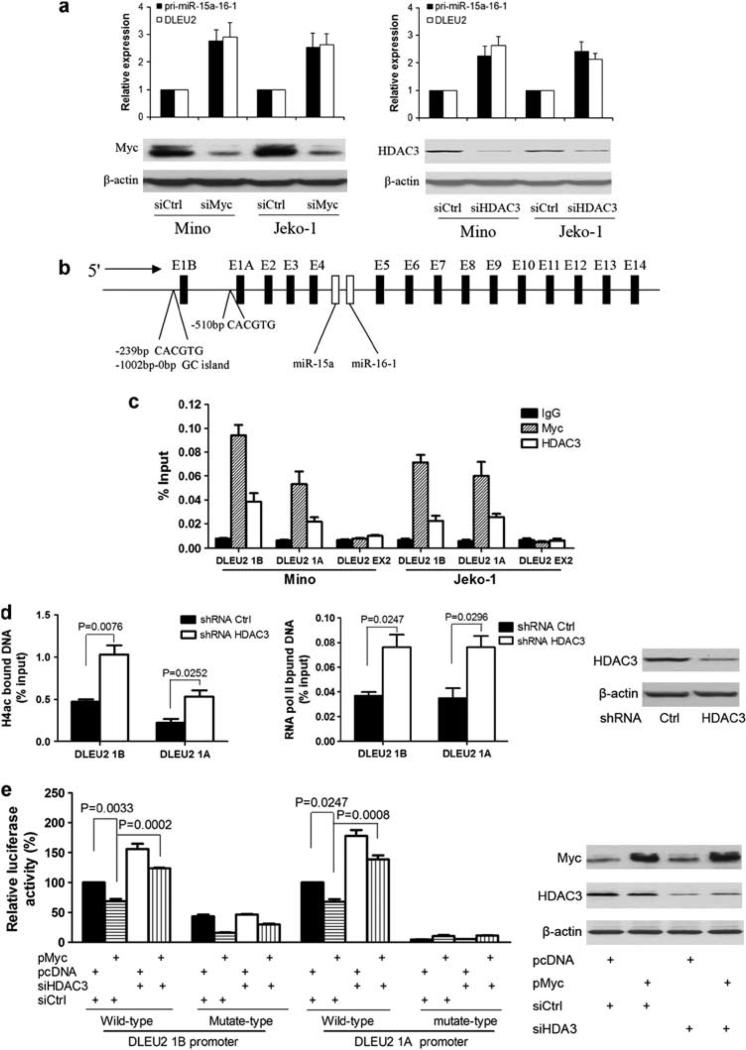
The interaction of Myc and HDAC3 led us to speculate that Myc and HDAC3 act together to silence the miR-15a/16-1 gene expression. We next examined the putative role of Myc and HDAC3 in the transcriptional regulation of miR-15a/16-1 expression. Given that miR-15a and miR-16-1 are located in intron 4 of DLEU2 and pri-miR-15a/16-1 was cleaved from DLEU2, we assessed the role of Myc and HDAC3 in miR-15a/16-1 gene expression by silencing the expression of Myc and HDAC3, respectively, using small interfering RNAs (siRNAs). We analyzed the levels of DLEU2 and pri-miR-15a/16-1 in Mino and Jeko-1 cells after Myc or HDAC3 was knocked down by their siRNAs. In agreement with our earlier results of HDAC inhibition by SAHA, depletion of HDAC3 significantly enhanced DLEU2 and pri-miR-15a-16-1 gene expression (Figure 2a). Moreover, knockdown of Myc also markedly increased DLEU2 and pri-miR-15a/16-1 gene expression. Taken together, these data support the role of Myc and HDAC3 in the repression of miR-15a/16-1 gene expression.
Figure 2.
Myc and HDAC3 are tethered to the miR-15a/16-1 promoter regions and Myc downregulates the transcription activity of miR-15a/16-1 promoter through HDAC3. (a) Knockdown of Myc or HDAC3 increased miR-15a/16-1 gene expression. Myc or HDAC3 was knocked down by siRNA (Dharmacon RNA Technologies, Lafayette, CO, USA) in Mino or Jeko-1 cells transfected by using Nucleofector (Amaxa, Cologne, Germany), and DLEU2 (primers sequence seen in Supplementary Table 2) and pri-miR-15a/16-1 expression levels (primers purchased from Applied Biosystems, Foster City, CA, USA) were measured by quantitative RT–PCR. Data were normalized to GAPDH. For relative mRNA expression, mRNA expression levels of cells treated with siCtrl were arbitrarily set as 1. (b) Schematic representation of miR-15a/16-1 host gene, DLEU2 promoter regions. (c) ChIP assay was performed using Myc or HDAC3 antibody to detect binding at the DLEU2 promoter, E1A and E1B regions. DLEU2 EX2, 6.3 kb distal from transcription start of promoter A, was used as a negative control. Pecentage input was calculated with 2(Ct [1% of input]–Ct [ChIP]). (d) ChIP assay using acetylated histone 4 (H4ac) antibody or RNA polymerase II antibody to detect binding of DLEU1A and 1B sites after HDAC3 was knocked down by shRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in Jeko-1 cells. Jeko-1 cells treated with 2 μg/ml puromycin and selected for 4 days after 72 h of transduction were used to perform ChIP. (e) HDAC3 is involved in Myc-induced miR-15a/16-1 transcriptional regression. HEK 293 cells were transfected with either 200 ng of wild-type or mutant-type of DLEU1A or DLEU1B promoter luciferase reporter, 20 ng pcDNA3-cmyc or control pCDNA, 50 nm HDAC3 siRNA (Dharmacon RNA Technologies) or non-targeting siRNA, or 30 ng pCMV-β-galactosidase per well in 24-well plates. At 48 h posttransfection, cells were subjected to luciferase reporter assay using the luciferase assay system (Promega, Madison, WI, USA) and normalized to β-galactosidase activities. Each experiment was repeated three times in triplicate. ChIP, chromatin immunoprecipitation.
Next, we performed chromatin immunoprecipitation experiments to determine whether Myc and HDAC3 can bind to DLEU2/miR-15a/16-1 promoters in the MCL cells. We used primers located within the DLEU2 proximal promoter regions because it is the region recognized by Myc (Figure 2b). A purified rabbit IgG was used as a negative control. As shown in Figure 2c, antibodies against Myc and HDAC3 both efficiently immunoprecipitated the two regions of the DLEU2 promoter carrying Myc binding sites. This approach revealed that both Myc and HDAC3 can bind to two alternative DLEU2 promoters in Mino and Jeko-1 cells. These bindings are specific as no signal was detected at DLEU2 exon2, 6.3 kb distal from the transcription start of promoter A. These findings suggest that HDAC3-mediated histone hypoacetylation contributes to Myc-induced miR-15a/16-1 gene repression. Histone H4 acetylation is a means through which Myc activates transcription, and Myc-driven Gadd153 and Id2 repression correlates with reduced histone H4 acetylation (Frank et al., 2001; Kurland and Tansey, 2008). Thus, we performed chromatin immunoprecipitation analysis to probe acetylated histone H4 of DLEU2 promoters and RNA polymerase II binding to DLEU2 promoters when HDAC3 was knocked down by short hairpin RNA. Chromatin immunoprecipitation was performed to measure accumulation of RNA polymerase II, which is considered a hallmark of active transcription. We found that specific inhibition of HDAC3 increased H4 acetylation and RNA polymerase II recruitment to DLEU2 promoters (Figure 2d). This result indicates that Myc-induced miR-15a/16-1 gene repression occurs through an inhibition of RNA polymerase II recruitment and transcription initiation by histone acetylation modification.
Given that Myc regulates DLEU2 at the transcription level and to confirm that Myc transcriptional suppression of DLEU2 was directly mediated by HDAC3, we performed experiments using luciferase reporter constructs carrying DLEU2 promoter to assess whether HDAC3 is required for repression. We cloned the two alternative promoters of DLEU2 (Figure 2b, E1A and E1B), and their mutated types were separately cloned into luciferase reporter plasmid (Figure 2e). The mutants were constructed to harbor mutation in the CACGTG of Myc binding site (E-box). These wild type and mutant plasmids were transfected into HEK293 cells, and the luciferase activity was measured. Figure 2e shows that the luciferase activity of wild-type DLEU2 promoters was significantly reduced in the presence of Myc overexpression using Myc plasmid and significantly increased by HDAC3 knockdown by siRNA. Furthermore, knockdown of HDAC3 by siRNA reversed Myc-mediated repression, supporting that HDAC3 is involved in Myc-driven miRNA repression (Figure 2e). Compared with wild-type promoters, luciferase activity of mutated-type promoters was greatly inhibited. Of note, unlike E1A promoter, mutated E1B promoter still contains some activity, and its activity was further inhibited by Myc over-expression. This is likely related to the presence of another Myc noncanonical binding site (CACGAG, -335-330). These results indicate that HDAC3 has a critical role in the ability of Myc-induced repression of DLEU2 expression and is E-box element-specific.
Myc repressed miR-15a/16-1 expression in primary MCL and other Myc-expressing B-cell lymphomas
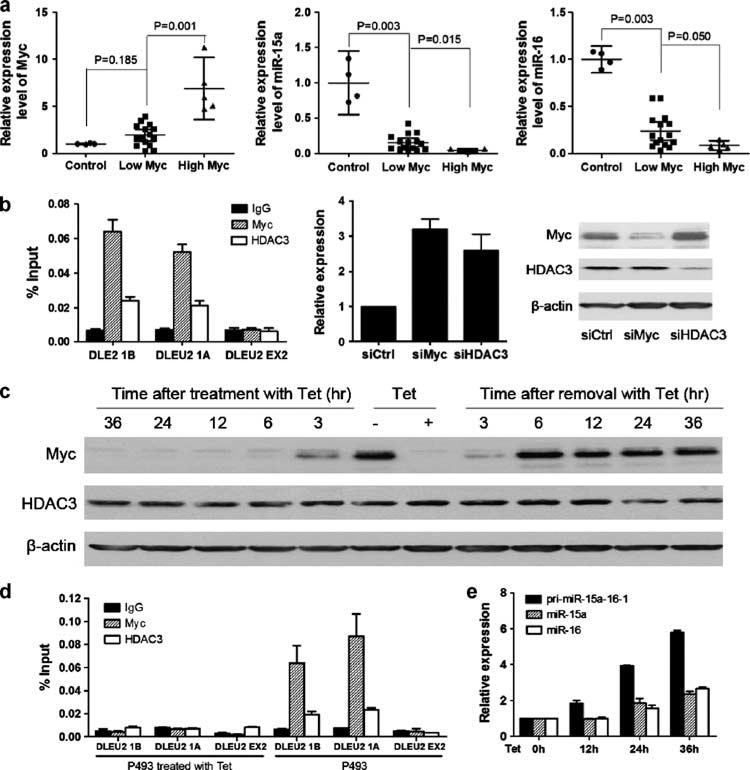
The results above revealed a novel mechanism for regulation of miR-15a/16-1 expression. We next determined if this regulation mechanism observed in MCL cell lines is operative in primary MCL and other Myc-expressing lymphomas. We analyzed miR-15a/16-1 and Myc expression in purified CD19+ lymphoma or lymphocytes from a set of MCL patients and four normal healthy donors. We investigated the relation of miR-15a/16-1 and Myc expression in these MCL patients. As shown in Figure 3a, compared with normal CD19+ peripheral blood lymphocytes, miR-15a/16-1 was consistently downregulated in these MCL samples. Furthermore, an inverse relation existed between miR-15a/16-1 and Myc in the patients.
Figure 3.
Myc downregulated miR-15a/16-1 expression in primary MCL and other Myc-expressing B-cell lymphomas. (a) miR-15a/16-1 expression reversely correlated with Myc expression in primary MCL cells. The expression levels of miR-15a, miR-16 and Myc in normal B lymphocytes (n = 4) and primary MCL samples (n = 20) were measured by quantitativeRT–PCR. The 75th percentile of Myc was used as the cutoff for patients with high- and low-levels of Myc. (b) Myc downregulated miR-15a/16-1 expression in Ramos cells. Left: ChIP assay for Myc and HDAC3 bindings to DLEU2. Middle: quantitative RT–PCR. Right: western blot. Knockdown of Myc or HDAC3 with siRNAs increased miR-15a/16-1 gene expression. (c) Myc and HDAC3 expression in P493-6 cells with Myc-on (removed with tetracycline, Tet) or Myc-off (treated with Tet). Tet (+) and Tet (−) represent cells maintained in medium with or without Tet for 72 h, respectively. (d) ChIP assay for Myc and HDAC3 bindings to DLEU2 in P493-6 with Myc-on or Myc-off. (e) miR-15a/16-1 and pri-miR-15a/16-1 change in P493-6 after treatment with Tet for 12, 24 and 36 h. For relative mRNA expression, miR-15a/16-1 and pri-miR-15a/16-1 expression levels in P493-6 cells not with Tet were set as 1. All experiments were performed at least three independent times. ChIP, chromatin immunoprecipitation.
To generalize these observations beyond MCL cells, we further investigated the role of Myc and HDAC3 in miR-15a/16-1 expression in other Myc-expressing lymphomas. As shown in Figure 3b, antibodies against Myc and HDAC3 both efficiently immunoprecipitated the two regions of the DLEU2 promoter carrying Myc binding sites in a Burkitt lymphoma cell line (Ramos). When Myc or HDAC3 was knocked down by siRNAs, increased miR-15a/16-1 expression was observed (Figure 3c).
We then used a human B-cell line, P493-6, featuring a tetracycline-repressible c-myc gene, to determine whether and how Myc influences miR-15a/16-1 expression in B cells. This P493-6 cell line is particularly useful because proliferation can also be driven by an estrogen-inducible Epstein–Barr virus EBNA-2 gene (Pajic et al., 2000), so that Myc expression can potentially be turned on or off without altering the survival of these cells. This cell line allowed us to study the detailed mechanism involving Myc suppression of miR-15a/16-1 expression. P493-6 cells grown in tetracycline express very low-Myc levels (Myc-off), whereas cells grown in the absence of tetracycline express high-Myc levels (Myc-on), as shown in Figure 3d. Moreover, we found that Myc-on or Myc-off does not alter HDAC3 expression levels in P493-6 cells (Figure 3d). Next, we next investigated whether HDAC3 was recruited to DLEU2 promoters and whether this step was dependent on Myc. Our chromatin immunoprecipitation results showed that HDAC3 cannot bind to DLEU2 promoters in P493-6 cells with Myc-off, whereas HDAC3 can bind to DLEU2 promoters in P493-6 cells with Myc-on (Figure 3e), supporting that Myc is required for HDAC3 recruitment of DLEU2 promoters. In agreement with results shown in MCL and Ramos cells, pri-miR-15a/16-1 and mature miR-15a/16-1 expression levels increased in P493-6 cells after treatment with tetracycline to suppress Myc expression. Taken together, these results are consistent with our hypothesis that Myc hyperactivity contributes to downregulation of miR-15a/16-1 expression through recruitment of HDAC3.
Although Myc has been described as a defining feature and the driving oncogene for Burkitt lymphoma, overexpression of Myc has also been recognized in other non-Hodgkin lymphomas. For example, rearrangement of Myc was detected in 9–14% of diffuse large B-cell lymphomas, which has been associated with an adverse prognosis with chemoresistance and shortened survival (Savage et al., 2009; Barrans et al., 2010). In MCL, increased expression of Myc has been found to be associated with poor prognosis and associated with an aggressive type, blastic variant of MCL (Hernandez et al., 1999; Nagy et al., 2003; Hartmann et al., 2008). However, the mechanisms underlying the clinical aggressiveness of lymphoma with Myc aberrations are unclear. A recent study revealed that activation of Myc results in a widespread direct repression of miRNA expression, indicating that Myc-induced miRNA repression may contribute to lymphoma with aggressive progression (O'Donnell et al., 2005; Chang et al., 2008). Our findings provide insight into the mechanism by which Myc represses miRNA expression and underlies its oncogenic role. To our knowledge, this is the first example that Myc regulates miRNA expression through recruitment of HDAC and histone acetylation modification. This work provides a target, HDAC3, and an inhibitor of HDAC could be used to reverse Myc-regulated miRNA expression in patients with MCL and with other aggressive lymphomas.
Supplementary Material
Acknowledgements
We are grateful to the Tissue Procurement, Molecular Core Laboratory and Flow Cytometry Core Facilities at H Lee Moffitt Cancer Center for providing specimens and molecular analysis. We thank Rasa Hamilton for editorial assistance. This work was supported by grants from the National Cancer Institutes (R01 CA137123, to JT), Maher Fund (to JT) and Moffitt Cancer Center Foundation (to JT).
Footnotes
Author contributions: XZ and XC designed and performed all of the experiments and contributed to manuscript preparation; ES and KW provided essential reagents and intellectual support; JL, TL and GW contributed to miRNA and ChIP analysis. ES, LM, WD, KW and JT provided clinical samples, information and intellectual support; XZ and JT designed experiments, interpreted data and wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010;17:215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L, et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009;69:5553–5559. doi: 10.1158/0008-5472.CAN-08-4277. [DOI] [PubMed] [Google Scholar]
- Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann NY Acad Sci. 2010;1183:183–194. doi: 10.1111/j.1749-6632.2009.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SR, Schroeder M, Fernandez P, Taubert S, Amati B. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 2001;15:2069–2082. doi: 10.1101/gad.906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Witmer PD, Casey E, Valle D, Sukumar S. DNA methylation regulates microRNA expression. Cancer Biol Ther. 2007;6:1284–1288. doi: 10.4161/cbt.6.8.4486. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Fernandez V, Moreno V, Valls J, Hernandez L, Bosch F, et al. Five-gene model to predict survival in mantle-cell lymphoma using frozen or formalin-fixed, paraffin-embedded tissue. J Clin Oncol. 2008;26:4966–4972. doi: 10.1200/JCO.2007.12.0410. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hernandez S, Bea S, Pinyol M, Ferrer A, Bosch F, et al. c-myc mRNA expression and genomic alterations in mantle cell lymphomas and other nodal non-Hodgkin's lymphomas. Leukemia. 1999;13:2087–2093. doi: 10.1038/sj.leu.2401599. [DOI] [PubMed] [Google Scholar]
- Kurland JF, Tansey WP. Myc-mediated transcriptional repression by recruitment of histone deacetylase. Cancer Res. 2008;68:3624–3629. doi: 10.1158/0008-5472.CAN-07-6552. [DOI] [PubMed] [Google Scholar]
- Lee EM, Shin S, Cha HJ, Yoon Y, Bae S, Jung JH, et al. Suberoylanilide hydroxamic acid (SAHA) changes microRNA expression profiles in A549 human non-small cell lung cancer cells. Int J Mol Med. 2009;24:45–50. doi: 10.3892/ijmm_00000204. [DOI] [PubMed] [Google Scholar]
- Lerner M, Harada M, Loven J, Castro J, Davis Z, Oscier D, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp Cell Res. 2009;315:2941–2952. doi: 10.1016/j.yexcr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Liu T, Tee AE, Porro A, Smith SA, Dwarte T, Liu PY, et al. Activation of tissue transglutaminase transcription by histone deacetylase inhibition as a therapeutic approach for Myc oncogenesis. Proc Natl Acad Sci USA. 2007;104:18682–18687. doi: 10.1073/pnas.0705524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GM, Gherardi S, Xu N, Neiron Z, Trahair T, Scarlett CJ, et al. Transcriptional upregulation of histone deacetylase 2 promotes Myc-induced oncogenic effects. Oncogene. 2010;29:5957–5968. doi: 10.1038/onc.2010.332. [DOI] [PubMed] [Google Scholar]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Nagy B, Lundan T, Larramendy ML, Aalto Y, Zhu Y, Niini T, et al. Abnormal expression of apoptosis-related genes in haematological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br J Haematol. 2003;120:434–441. doi: 10.1046/j.1365-2141.2003.04121.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Pajic A, Spitkovsky D, Christoph B, Kempkes B, Schuhmacher M, Staege MS, et al. Cell cycle activation by c-myc in a Burkitt lymphoma model cell line. Int J Cancer. 2000;87:787–793. doi: 10.1002/1097-0215(20000915)87:6<787::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Robertus JL, Kluiver J, Weggemans C, Harms G, Reijmers RM, Swart Y, et al. MiRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphoma. Br J Haematol. 2010;149:896–899. doi: 10.1111/j.1365-2141.2010.08111.x. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- Sotillo E, Laver T, Mellert H, Schelter JM, Cleary MA, McMahon S, et al. Myc overexpression brings out unexpected antiapoptotic effects of miR-34a. Oncogene. 2011;30:2587–2594. doi: 10.1038/onc.2010.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.