Abstract
Hyperglycemia upregulates intracellular angiotensin II production in cardiac myocytes, effects of which are blocked more effectively by renin inhibition than angiotensin receptor blockers (ARBs) or ACE inhibitors. Here we determined whether renin inhibition is more effective at preventing diabetic cardiomyopathy than an ARB or ACE inhibitor. Diabetes was induced in adult mice for 10 wks by streptozotocin. Diabetic mice were treated with insulin, aliskiren (renin inhibitor), benazeprilat (ACE inhibitor), or valsartan (ARB) via subcutaneous minipumps. Significant impairment in diastolic and systolic cardiac function was observed in diabetic mice, which was completely prevented by all three RAS inhibitors. Hyperglycemia significantly increased cardiac oxidative stress and circulating inflammatory cytokines, which were blocked by aliskiren and benazeprilat, while valsartan was partially effective. Diabetes increased cardiac (pro)renin receptor (PRR) expression and nuclear translocation of promyelocytic zinc finger protein (PLZF), which was completely prevented by aliskiren and valsartan, and partially by benazeprilat. Renin inhibition provided similar protection of cardiac function as ARBs and ACE inhibitors. Activation of PLZF by PRR represented a novel mechanism in diabetic cardiomyopathy. Differential effects of the three agents on oxidative stress, cytokines, and PRR expression suggested subtle differences in their mechanism of action.
Keywords: Diabetic cardiomyopathy, intracrine, prorenin receptor
INTRODUCTION
The renin angiotensin system (RAS)1 has been significantly associated with diabetes-induced organ damage, including diabetic cardiomyopathy. The RAS consists of two major subcomponents, the circulating and the local tissue system. Recently, we presented evidence that the tissue RAS can be further divided into extracellular and intracellular systems (1, 2). The intracellular RAS is distinguished from the local extracellular RAS in that the synthesis of angiotensin (Ang) II occurs inside the cells and Ang II actions are not mediated by plasma membrane type 1 (AT1) and type 2 (AT2) Ang receptors (3, 4). The local RAS has been demonstrated to have a role in hypertrophy, fibrosis, inflammation, oxidative stress, and thrombosis independent of systemic Ang II (5). Local Ang II levels in the heart are increased in pathological conditions such as myocardial infarction and diabetes (5, 6). Recently, our laboratory demonstrated that the intracellular RAS constituted the major part of the local RAS in hyperglycemic conditions (1, 7–11). We reported a several fold increase in intracellular Ang II levels in cultured cardiac myocytes, when grown in high-glucose medium or from the hearts of diabetic rats. The observation of increased cardiac intracellular Ang II levels had previously been described in diabetic patients (12). We reported that the intracellular Ang II was biologically active and produced cardiac hypertrophy in mice (4). Significantly, growth effects of intracellular Ang II in cultured cardiac myocytes and in the heart were not prevented by AT1 receptor antagonists. Further, high glucose-stimulated cardiac myocyte production of Ang II was chymase-dependent, in contrast to ACE-dependent conversion in cardiac fibroblasts (13, 14). These observations suggested that treatment with an ACE inhibitor or ARB may be only partially protective in diabetic cardiomyopathy, since the former would inhibit Ang II production only by cardiac fibroblasts and the latter would block actions of only extracellular Ang II, without affecting intracellular Ang II production and actions in cardiac myocytes. Accordingly, we showed that renin inhibition proved more effective than an ARB or ACE inhibitor, in preventing cardiomyocyte superoxide production and fibrosis after one wk of diabetes (5).
Several studies have been performed to compare the relative efficacy of aliskiren with ACE inhibitors or ARBs in hypertensive cardiovascular diseases, mainly renal function (15–17). These studies have shown a similar therapeutic profile of the three classes of drugs. A comparative effect of all three RAS blockers on cardiac function in diabetes has not been reported. The latter is important, due to the changes in the characteristics of the cardiac RAS in diabetes, i.e., from an extracellular to an intracellular system, ACE-dependent to largely chymase-dependent system, and possibly an AT1-dependent to an AT1-independent system (11). Diabetic patients remain at an increased risk of cardiovascular events compared to non-diabetics, despite the use of ACE inhibitors and ARBs, suggesting insufficient RAS inhibition as one of the possible explanations, in addition to other mechanisms (16, 18). In this context, a renin inhibitor might provide more complete inhibition of the RAS in diabetes. The objective of this study was to determine whether direct renin inhibition, which blocks both the intracellular and extracellular RAS, is more effective in preventing diabetic cardiomyopathy in a mouse model of type I diabetes, than an ARB or an ACE inhibitor, which block only extracellular Ang II.
MATERIALS AND METHODS
All protocols were approved by the Institutional Animal Care and Use Committee and conformed to the NIH guidelines. The renin inhibitor aliskiren, the AT1 receptor blocker valsartan, and the ACE inhibitor benazeprilat were obtained from Novartis (Cambridge, MA); and insulin (Humulin N) was from Eli Lily (Indianapolis, IN).
Animals
Male C57bl6/J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and fed ad libitum. At 12 wks of age, animals were randomized into 6 groups (n=10): 1) control, 2) Streptozotocin (STZ), 3) STZ + Saline (STZ-Veh), 4) STZ + Aliskiren (20 mg/kg, STZ-Alsk), 5) STZ + Valsartan (2 mg/kg, STZ-Vals), 6) STZ + Benazeprilat (10 mg/kg, STZ-Benz). STZ (50 mg/kg/day; zanosar) was injected intraperitoneally (i.p.) for 5 consecutive days. Doses of the RAS inhibitors were based on results of a preliminary study described in the Supplement. Control groups received 0.1 M sodium citrate buffer (pH 4.5). After 2 wks, all STZ-injected mice reached a blood glucose value of ≥ 250 mg/dl and were considered diabetic. At this point, diabetic mice in treatment groups were implanted with osmotic minipumps (ALZET 1004, 0.11 μl/hr), containing one of the aforementioned agents, for 10 wks (Fig. 1). Minipumps were replaced every 4 wks. An insulin group was included to verify that the cardiac effects observed in the diabetic group were due to hyperglycemia. The insulin group received 0.7U/day of insulin (Humulin N) by subcutaneous injection during the 10 wks of the treatment period.
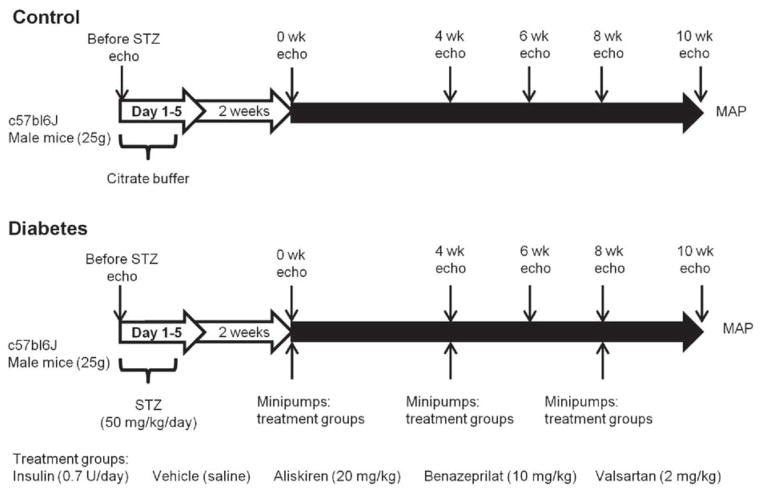
Fig. 1. Study design.
Ten to twelve wk old male c57bl6 mice were injected with either 0.1 M citrate buffer (pH 4.5) or streptozotocin (50 mg/kg) for five days. After 2 wks, animals with blood glucose levels of ≥ 250 mg/dl were considered diabetic and osmotic minipumps containing one of the drugs were implanted subcutaneously. Echocardiographic measurements were taken before STZ injections (Before STZ) and at two wk intervals, beginning at four wks after minipump installation. After conclusion of the study at 10 wks, mean arterial pressure (MAP) was measured and tissues were collected.
Echocardiographic measurements
Transthoracic echocardiography was performed on anesthetized mice, using a VisualSonicVevo 2100 and a 35-MHz probe, before injection with STZ (Before STZ) and 0, 4, 6, 8, and 10 wks after establishment of diabetes. Briefly, mice were anesthetized with 3–5% isoflurane that was reduced to 1.5% to maintain the heart rate between 400–500 beats per minute. The heart was imaged in the 2-dimensional, short-axis and 4 chamber view. Left ventricular fractional shortening (FS), ejection fraction (EF), cardiac output (CO), heart rate (HR), LV internal dimension at end-diastole and end-systole (LVIDd and LVIDs), isovolumic relaxation time (IVRT), peak velocity of early (E) and late (A) filling waves, and mitral deceleration time were measured.
Mean arterial pressure (MAP)
Arterial pressure was measured at the end of the study (10 wks of diabetes) in anesthetized animals. The right carotid artery was cannulated and arterial pressure was measured with a blood pressure analyzer (BPA 400, Micro-Med, Louisville, KY). MAP was calculated as follows: 1/3 systolic pressure + 2/3 diastolic pressure.
Isolation of cardiac myocytes
Adult mouse cardiac myocytes were isolated using Langendorff’s perfusion system, as previously described (19). Briefly, hearts were perfused retrogradely for 5 min, followed by digestion with 0.1% (W/V) collagenase II (Worthington Biochemical Corp) for 10 min at a rate of 3 ml/min. After stopping digestion with a calcium-containing buffer, cells were washed with PBS and collected by centrifugation (180g, 1 min). Myocytes were stored in RNAlater solution for RNA analysis.
Reactive oxygen species staining
Hearts were fixed in 4% paraformaldehyde and frozen in O.C.T. compound (Tissue-Tek). Frozen sections (20 μm) were incubated with 10 μM dihydroethidium (DHE, Sigma-Aldrich), at 37°C, for 30 min in a humidified chamber protected from light. Fluorescent images (60X) were obtained with a Leica TCS SP5X and analyzed using ImageJ. Mean DHE fluorescence was calculated by subtracting integrated density of the background signal from the integrated density of the fluorescent staining for 10 fields/heart, 5 hearts/group, and normalized to control.
Histological analysis
Heart sections (5 μm) were fixed with 4% formaldehyde and permeablized using 0.1% Triton X-100. After blocking, the sections were incubated with anti-PRR (1:30; Everest Biotech) and anti-promyelocytic zinc finger protein (PLZF; 1:50; Santa Cruz) antibodies, followed by incubation with respective secondary antibodies (1:500). Specificity of staining was determined by using secondary antibody alone. The sections were co-stained with Phalloidin (1:100). Images (60X) were acquired with a confocal fluorescence microscope (Leica TCS SP5X). Fluorescence intensities were determined using ImageJ after subtracting background fluorescence and normalizing to control.
Real-time PCR
Gene expression of (Pro)renin receptor (PRR) was determined using a Taqman assay (Applied Biosystems). RNA was extracted using an RNeasy Fibrous Tissue Kit (Qiagen). cDNA was made using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Real-time PCR was performed in 20 μl reaction containing cDNA, Taqman Universal PCR master Mix, and 20X specific gene expression assay mix (Applied Biosystem). Data were normalized to 18s mRNA. Each sample was run in duplicate, and the threshold cycle, ΔCt, was calculated as Ct (target gene) - Ct (18s). The relative changes in target gene in different treatment groups were determined by the formula 2−ΔΔCt, where ΔΔ Ct = ΔCt (control) - ΔCt (treatment group).
Plasma Cytokines
Blood was collected in tubes containing EDTA and centrifuged at 1600g, for 15 min, at 4°C. Plasma was collected and stored at −20°C. Milliplex Mag Mouse Cytokine/Chemokine kit (Millipore, TX) was used to determine plasma levels of interleukin-1 β (IL-1β), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and monocyte chemotactic protein-1 (MCP-1).
Fibrosis
For characterization of fibrosis in the ventricular tissue, picrosirius red, which specifically binds to collagen, and Fast Green, which stains non-collagen proteins, were used. Briefly, paraffin embedded sections were de-paraffinized with xylene (60°C for 15 min), rehydrated through graded concentrations of ethanol (100%, 95%, 85%, 70%), washed, and stained with 0.1% picrosirius red/0.1% Fast Green overnight. Stained slides were washed, rapidly dehydrated through graded ethanol concentrations, and mounted with Permount. For quantification of fibrosis, sections were air dried for 5 min following staining, as described by others (20). One ml dye extraction solution (0.1N NaOH + pure methanol, 1:1) was used to extract dyes from the stained section, which was read at 356 and 605 nm (Sirius red and Fast Green) by spectrophotometer. The ratio of optical density (356/605 nm) determined the amount of collagen to total protein ratio.
Statistical analysis
All data were expressed as the mean ± SEM. One-way ANOVA with Tukey’s post hoc test or multiple comparisons using two-way ANOVA with the Bonferroni post hoc test were used for statistical analysis where appropriate (GraphPad San Diego, CA). P <0.05 was considered statistically significant.
RESULTS
We had previously reported that one wk of STZ-induced hyperglycemia in rats significantly upregulated intracellular Ang II levels in cardiac myocytes and was associated with increased oxidative stress, cardiac myocyte apoptosis, and fibrosis (5). The latter conditions were corrected more efficiently by a renin inhibitor, which completely reduced intracellular Ang II levels, in comparison to an ARB or ACE inhibitor. In the present study, we evaluated the comparative efficacy of the three RAS blocking agents on diabetes-induced cardiac dysfunction after 10 wks.
STZ-injected mice have a similar degree of hyperglycemia and MAP, regardless of treatment
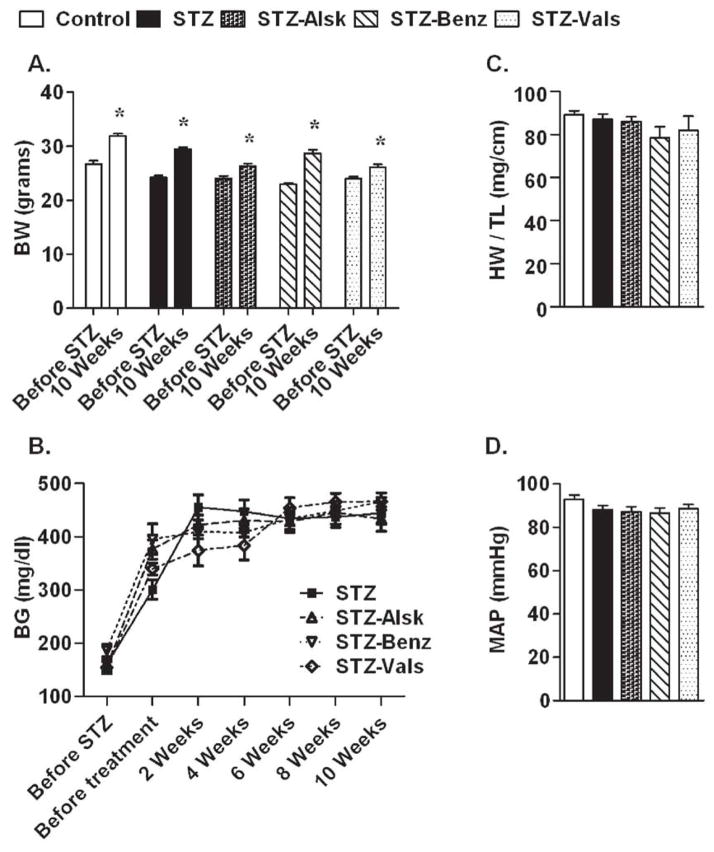
Body weight and blood glucose were monitored on a bi-weekly basis to determine whether aliskiren, benazeprilat, or valsartan have an effect on these parameters. As shown in Fig. 2A, mice in STZ, STZ-Alsk, STZ-Benz, and STZ-Vals groups had weights similar to the control group after 10 wks in the study. Mice injected with STZ had an increase in blood glucose two wks after injection, which remained elevated throughout the study, regardless of treatment (Fig. 2B). Heart weight to tibia length ratio was not significantly different between the groups (Fig. 2C). Similarly, no significant change in the MAP was observed as a result of hyperglycemia or treatments (Fig. 2D). These observations suggested that the effect of the three RAS blockers on cardiac function were not modified by the degree of hyperglycemia or blood pressure.
Fig. 2.
Effect of hyperglycemia, with and without treatment with RAS inhibitors, on body weight (A), blood glucose (B), heart weight normalized to tibia length at the end of the study (C), and mean arterial pressure at the end of the study (D). Values are expressed as the mean ± SEM. *P < 0.05 vs. before STZ in each group.
Hyperglycemia-induced diastolic dysfunction is prevented by aliskiren, benazeprilat, and valsartan
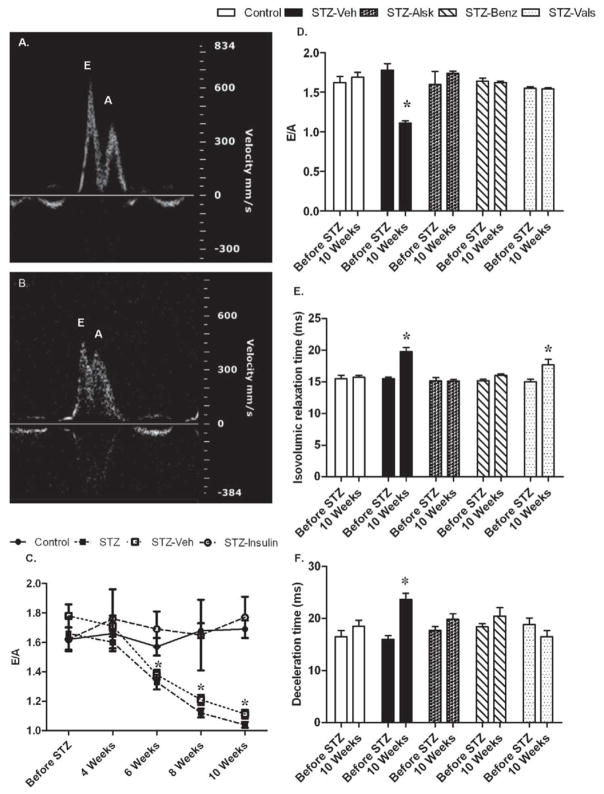
Diabetic cardiomyopathy is correlated with early diastolic dysfunction independent of changes in MAP (21). We used echocardiography to non-invasively monitor diastolic function in control mice, diabetic mice, and diabetic mice treated with an ARB, ACE inhibitor, or renin inhibitor. Diastolic function was evaluated by measuring the ratio of mitral valve flow velocities (E/A ratio), isovolumic relaxation time (IVRT), and mitral valve deceleration time (Fig. 3). Representative echocardiograms of diastolic parameters from control and STZ-Veh mice are presented in Fig. 3 A and B, respectively. The STZ-Veh group, which received saline-containing osmotic minipumps, was included to control for the impact of surgery on the study parameters. Hyperglycemia caused a progressive decrease in the E/A ratio, which reached statistical significance following six wks of diabetes and continued to decrease through 10 wks (Fig. 3C). A similar decline in cardiac function was observed in the STZ and STZ-Veh groups; therefore, data from only the STZ-Veh group have been shown in subsequent figures. Insulin reversed all parameters of the STZ induced decrease in cardiac function (Supplement Fig. 2S, only E/A ratio shown in Fig. 3A), indicating that the cardiac effects observed in the diabetic group were due to hyperglycemia. Compared to before STZ, hyperglycemia led to a significant decrease in E/A at 10 wks, which was completely prevented by aliskiren, benazeprilat, and valsartan (Fig. 3D). IVRT was significantly increased in the STZ-Veh group after 10 wks, which was prevented by aliskiren and benazeprilat. Valsartan partially prevented the increase in IVRT by hyperglycemia (Fig. 3E). Mitral valve deceleration time was significantly increased in the 10 wk STZ-Veh group and was prevented by all treatments (Fig. 3F).
Fig. 3. Measurement of diastolic function by echocardiography.
Representative pulsed-wave Doppler images of Mitral valve flow of Control and STZ-Veh mice at 10 wks of diabetes (A and B, respectively). Mitral valve flow velocity (E/A) in Control, STZ-injected (STZ), STZ-injected with saline minipumps (STZ-Veh), and STZ-injected mice treated with insulin (STZ-Insulin), before STZ injection and 4, 6, 8, and 10 wks after becoming diabetic (C). Mitral valve flow velocity (D), isovolumic relaxation time (E), and deceleration time of early mitral inflow (F) of Control, STZ-Veh, and those treated with aliskiren (STZ-Alsk), benazeprilat (STZ-Benz), and valsartan (STZ-Vals), before STZ treatment and at 10 wks after becoming diabetic. Values are expressed as the mean ± SEM. *P < 0.05 vs. control.
Hyperglycemia-induced decrease in systolic function is prevented by aliskiren, benazeprilat, and valsartan
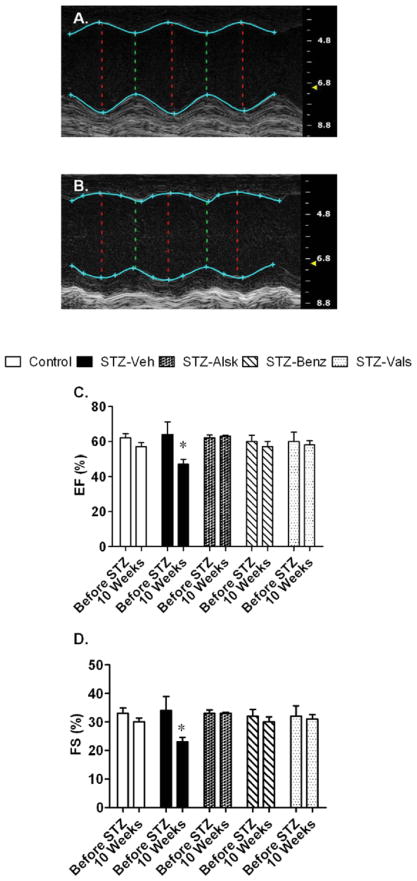
In addition to diastolic dysfunction, hyperglycemia can cause modest systolic impairment (22). To determine the extent by which systolic function was impaired in this study, we evaluated EF and FS using echocardiography. Representative M-mode echocardiograms of control and STZ-Veh mice are presented in Fig. 4 A and B, respectively. After 10 wks of diabetes, STZ-Veh mice had a significantly decreased EF and FS, which was prevented by all treatments (Fig. 4 C and D). There were no differences in cardiac output, stroke volume, or heart rate between groups (data not shown).
Fig. 4. Measurement of systolic function by echocardiography.
Representative M-mode short axis views of Control and STZ-Veh mice at 10 wks of diabetes (A and B, respectively). Ejection fraction (C) and fractional shortening (D) of Control, STZ-Veh, STZ-Alsk, STZ-Benz, and STZ-Vals, before STZ treatment and at 10 wks after becoming diabetic. Values are expressed as the mean ± SEM. *P < 0.05 vs. control.
Aliskiren, benazeprilat, and valsartan prevent hyperglycemia-induced oxidative stress in the heart
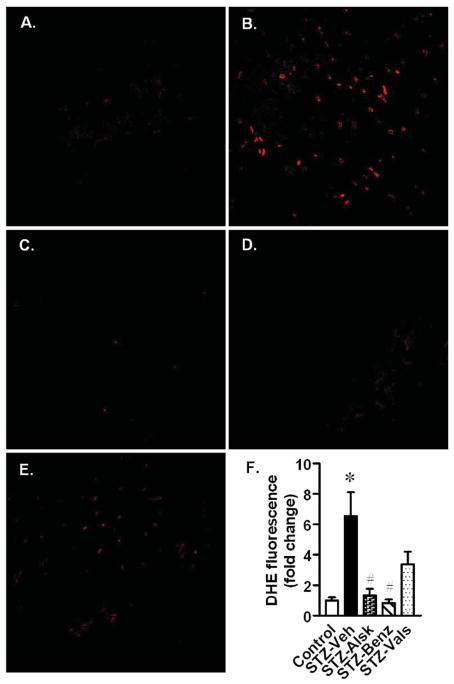
Oxidative stress is a well documented mechanism that contributes to cardiac remodeling and diabetic cardiomyopathy (23, 24). Microscopic analysis of frozen heart sections stained with dihydroethidium (DHE) was used to assess oxidative stress. Diabetic mice had a significant increase in DHE staining, compared to control mice, which was completely prevented by aliskiren and benazeprilat, whereas valsartan was only partially protective (Fig. 5). A quantitative analysis of the oxidative stress is presented in Fig. 5F.
Fig. 5. Detection of oxidative stress in heart sections by DHE staining.
Representative images of DHE-stained heart sections from Control (A), and diabetic mice, treated with vehicle (B), aliskiren (C), benazeprilat (D), or valsartan (E). Magnification X60. DHE fluorescence intensity was calculated from five images per heart and three hearts per group (F). Values are expressed as the mean ± SEM. *P < 0.05 vs. control, # P < 0.05 vs. STZ-Veh.
Fibrosis is not significantly increased in STZ-injected mice after 10 wks of diabetes
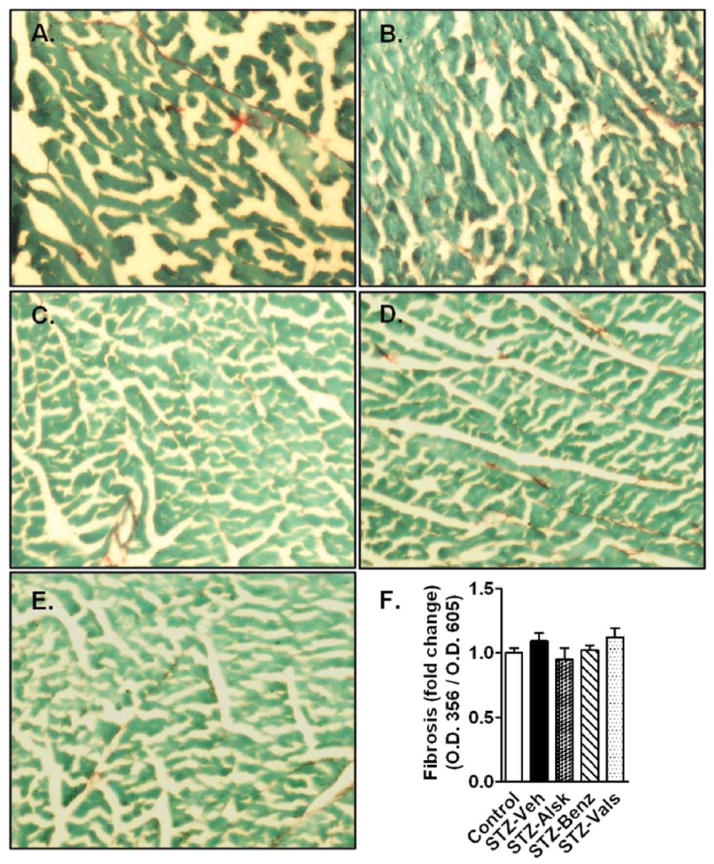
Fig. 6 A–E shows representative images of heart sections from different groups stained with picrosirius red and fast green. There was no significant increase in collagen content in STZ-Veh mice compared to controls. Similarly, there was no change in collagen staining in aliskiren, benazeprilat, or valsartan treated mice. A semi-quantitative analysis of fibrosis is presented as the ratio of O.D. 356/O.D. 605, which represents the ratio of the amount of collagen to total protein, and confirmed similar collagen content in all groups of animals (Fig. 6F).
Fig. 6. Detection of cardiac fibrosis by picrosirius red staining.
Representative images from Control and STZ-Veh mice (A), and diabetic mice treated with vehicle (B), aliskiren (C), benazeprilat (D), or valsartan (E). Semi-quantitative calculation of picrosirius staining represented as a ratio of absorbance of OD356 (Sirius red)/OD605 (Fast green), after normalization to control (F).
Hyperglycemia-induced increase in plasma cytokines is prevented by aliskiren and benazeprilat, but only partially attenuated by valsartan
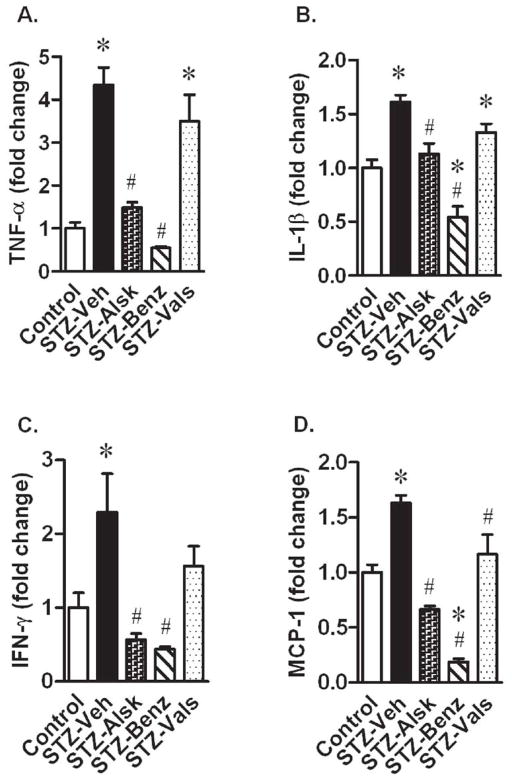
Ang II mediates cytokine production observed in diabetes (25, 26). STZ-Veh mice had significantly elevated plasma levels of TNF-α, IL-β, IFN-γ, and MCP-1. Treatment with aliskiren and benazeprilat completely prevented the observed increases in these inflammatory cytokines. However, valsartan only partially prevented the increase in all four of these cytokines, in which TNF-α, and IL-β levels remained significantly elevated, as compared to control (Fig. 7).
Fig. 7. Measurement of plasma cytokine levels.
Tumor necrosis factor-α (TNF-α) (A), interleukin-1 β (IL-1β) (B), interferon-γ (IFN-γ) (C), and monocyte chemotactic protein-1 (MCP-1) (D) in Control, STZ-Veh, STZ-Alsk, STZ-Benz, and STZ-Vals, after 10 wks of diabetes and normalization to control. Values are expressed as the mean ± SEM. *P < 0.05 vs. control, # P < 0.05 vs. STZ-Veh.
PRR expression and nuclear translocation of PLZF is significantly increased by hyperglycemia
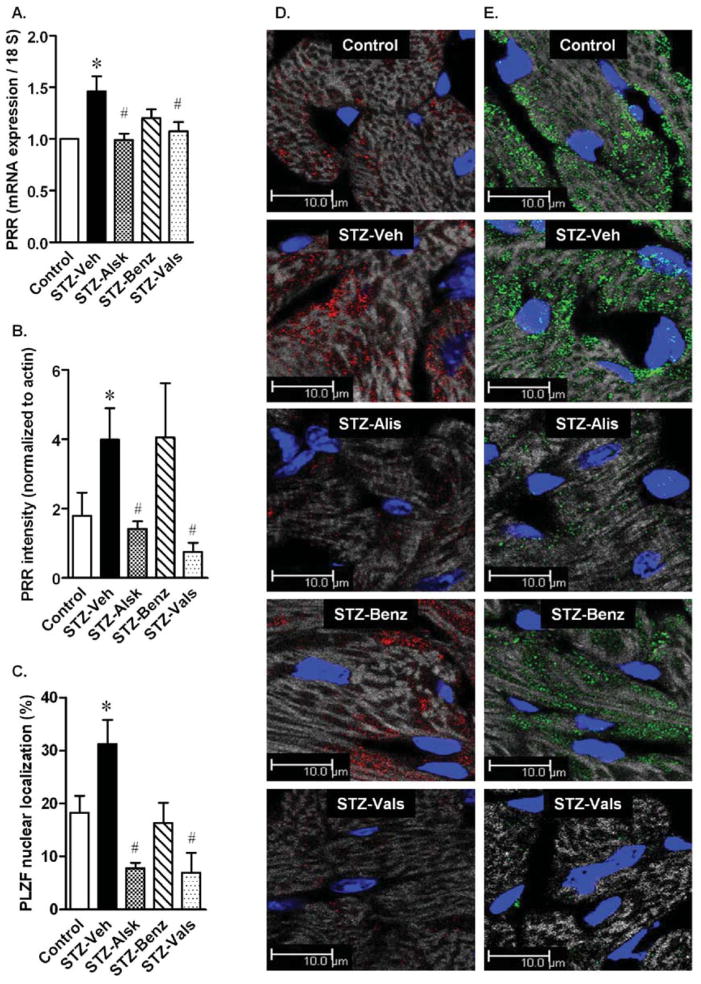
PRR expression has been shown to be increased in high glucose conditions (27). In the present study, the gene expression of PRR was increased in isolated cardiac myocytes of STZ-Veh mice (Fig. 8A). This increase was prevented by aliskiren, benazeprilat, and valsartan. Fig. 8D shows representative images of PRR immunostaining in frozen heart sections, which was significantly increased in the STZ-Veh group, compared to Control. Aliskiren and valsartan completely prevented the increase, whereas the benazeprilat effect was non-significant. A quantification of PRR from immunostained sections is presented in Fig. 8B. PLZF, a transcription factor that directly binds to and mediates the effects of the PRR (28), showed increased translocation to the nucleus (Fig. 8C), suggesting enhanced transcriptional activity of PLZF. Treatment with all three RAS blockers prevented the increased expression of PRR, PLZF, and translocation of the latter to the nucleus.
Fig. 8. Cardiomyocyte expression of (pro)renin receptor and nuclear translocation of PLZF.
(Pro)renin receptor (PRR) gene expression in cardiac myocytes of control, STZ-Veh, STZ-Alsk, STZ-Benz, and STZ-Vals, after 10 wks and normalization to control (A). Quantification of PRR immunostaining after normalization to actin staining (B). Quantification of nuclear localization of PLZF, represented as percent nuclei showing nuclear PLZF staining (C). Representative images of PRR immunostaining in the heart (D). Representative images of PLZF immunostaining in the heart (E). Grey: actin filaments, Blue: Nuclei, Red: PRR, Green: PLZF. Values are expressed as the mean ± SEM. *P < 0.05 vs. control (A–C), # P < 0.05 vs. STZ-Veh.
DISCUSSION
This study provides, for the first time, a direct comparison between a renin inhibitor, an ARB, and an ACE inhibitor in the context of diabetic cardiomyopathy. We observed that renin inhibition completely prevented diastolic and systolic cardiac dysfunction in diabetic animals. An ARB and ACE inhibitor were similarly effective. However, there were differences in the effect of these drugs on oxidative stress, plasma pro-inflammatory cytokines, and PRR expression. Further, we demonstrated increased nuclear localization of PLZF, in association with increased PRR expression, which represented a novel cellular mechanism operating in diabetic cardiomyopathy.
Diabetic cardiomyopathy has been described as ventricular dysfunction, including early diastolic dysfunction, in diabetic patients, without coronary artery disease or hypertension (21, 29). We demonstrated that diabetic mice had impaired diastolic function, as well as a modest decrease in systolic function, determined by echocardiography, after 10 wks of hyperglycemia. These effects were independent of blood pressure, as STZ-induced diabetes in C57bl6 mice is a normotensive model (30). Previously, we had shown that the intracellular Ang II levels were significantly increased in cardiac myocytes in diabetes (13). A renin inhibitor was more effective than an ACE inhibitor in reducing Ang II levels and associated cardiac fibrosis, oxidative stress, and apoptosis in rats, independent of AT1 (5, 13). In another study on cardioprotective effects of the three different classes of drugs in Spontaneously Hypertensive Rats (SHR), aliskiren improved coronary endothelial function and cardiac hypertrophy to the same degree as an ACE inhibitor and ARB; however, provided better long-term cardiac Ang II suppression (31). It is noteworthy that aliskiren accumulates in tissues, including cardiac myocytes (5, 32, 33), whereas there are no data regarding ARBs. Based on these previous findings, we anticipated that renin inhibition would more completely block the cardiac RAS; thus, preventing diastolic impairment more effectively than an ARB or ACE inhibitor, which block only cardiac fibroblast-generated extracellular Ang II (14). Consistent with this hypothesis, the latter agents have been beneficial in improving several physiological parameters in human diabetic nephropathy, retinopathy, and cardiomyopathy; however, long-term morbidity and mortality in diabetic patients remain high compared to non-diabetics (15, 16). In our study, all three inhibitors of the RAS were effective at preventing alterations in left ventricular function associated with diabetic cardiomyopathy to a similar extent. However, there were significant differences in protection provided against oxidative stress, inflammatory status, and prorenin receptor expression. Renin inhibition was completely effective in all three parameters, while the other two agents were partially effective in one or two parameters studied. Though these differences did not impact the heart function at 10 wks of hyperglycemia, their long-term effect is not clear.
Oxidative stress is a major mechanism which contributes to impaired heart function in diabetes and the RAS has a significant role in the generation of oxidative stress (22, 24, 34). Consistently, a renin inhibitor, which inhibits both intra- and extracellular Ang II formation, completely protected the diabetic heart from oxidative stress. Treatment with an ARB, which did not block intracellular Ang II actions in cardiac myocytes and in diabetic hearts (4, 5), was only partially effective at preventing oxidative stress. The incomplete protection was likely due to the ARB only inhibiting extracellular Ang II dependent ROS generation and limited cellular permeability to inhibit intracellular AT1 receptor. These observations corroborated our previously reported observations in rat heart after one wk of diabetes and suggest involvement of the intracellular RAS in cardiac oxidative stress (5). We also observed complete inhibition of oxidative stress by an ACE inhibitor, which was inconsistent with high glucose-induced, chymase-mediated, intracellular Ang II synthesis in cardiac myocytes. Intracellular Ang II would generate ROS likely through intracellular AT1-dependent and non-AT1-dependent mechanisms. In support of the former mechanism, Ang II was shown to bind to nuclear AT1 receptors and increase ROS generation in kidney cells and cardiac myocytes (35–37). Recently, both AT1 and AT2 receptors were described in the inner mitochondrial membrane and were associated with the age-related increase in mitochondrial oxidative stress (38). In support of an AT1-independent mechanism, increased oxidative stress was observed in kidneys of transgenic mice expressing intracellular Ang II through direct interaction of Ang II with proteins of the mitochondrial respiratory chain (39, 40). These studies supported, and provided the likely mechanism of, intracellular Ang II-mediated oxidative stress in diabetes.
Elevated levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, IFN-γ, and IL-6 have been reported in diabetic animals, as well as in patients (41). In the present study, we observed that TNF-α, IL-1β, IFN-γ, and MCP-1 levels were significantly increased in diabetic mice, compared to controls. Renin and ACE inhibitors completely prevented the increase in all four cytokines; but, the ARB was only partially effective. Similar to our findings, an ARB and ACE inhibitor were ineffective at preventing the increased TNF-α or IL-1β in diabetic rats in another study (42). In addition to Ang II, PRR-mediated renal and retinal inflammation has been described in diabetes (43, 44). In another study, the glucose-induced increase in IL-1β expression in mesangial cells was blocked by PRR siRNA, but only partially by valsartan (45). We observed increased PRR expression in diabetic hearts (Fig. 8); however, whether PRR contributed to inflammation and aliskiren had a role in preventing such PRR-mediated inflammation is not clear from our studies.
Despite our previous reports of the inability of ACE inhibitors and ARBs to inhibit the intracellular RAS (4, 5, 46), a complete preservation of cardiac function by these agents was observed in this study. One possibility for the observed difference was that even partial effects of these drugs were sufficient for the heart to maintain function in the short-term. Another explanation was that the non-RAS related effects of ARBs and ACE inhibitors, such as those on PPARγ and bradykinin, might have contributed to the cardioprotective effects of these drugs (47, 48). Additionally, it was reported that valsartan suppressed LPS-induced macrophage activation and improved insulin resistance, independent of AT1 receptor or PPARγ, through an unknown mechanism (49). ACE inhibitors enhanced not only kinin levels, but kinin B1 and B2 receptor function, via allosteric mechanisms that involved ACE and B2 receptor heterodimerization and direct binding to B1 receptor (50). Blockade of B2 receptor reduced the renal protective effects of ramipril, an ACE inhibitor, in diabetic nephropathy (51). Whereas ARBs and ACE inhibitors have pleiotropic mechanisms of cardiac protection, aliskiren is known as a specific inhibitor of renin. The latter observation and an overall better protection profile of aliskiren have suggested, but not confirmed, a role of intracellular Ang II in diabetic cardiomyopathy. Studies utilizing genetically modified animal models, such as AT1a knockout mice in which extracellular Ang II effects will be prevented without a need for pharmacological agents, are needed to provide more definite information about the role of intracellular Ang II in diabetes.
It may be argued that the differential effects of the three drugs on some parameters in this study were due to a different degree of RAS blockade related to differences in dosing. There is no standard measure of equipotent RAS inhibition by different agents (15, 52), especially in diabetes wherein a significant shift occurs from ACE- to chymase-mediated Ang II synthesis and ARBs are ineffective in preventing intracellular Ang II actions in some cells (11). Therefore, we selected the doses of the tested drugs based on their effects on Ang II production in the target cell relevant to this study- the diabetic cardiac myocyte (Supplement). Therefore, the observed differences among the drugs in this study were more likely related to their inability to inhibit the RAS to the same extent in diabetes, as was our hypothesis, than due to dose related differences.
In this study, we describe a novel mechanism of PRR-induced PLZF activation in diabetic heart. Upregulation and detrimental effects of PRR in diabetes have been described (44, 53–55). In the heart, PRR, which was primarily co-localized with the cardiac ryanodine receptor (RyR2) in the Z-disc, was significantly elevated in diabetic TGR(mRen2)-27 rats (27). Recently, direct intracellular interaction of PRR with the transcription factor PLZF was demonstrated in several cell types in vitro (28). This interaction caused nuclear translocation of PLZF resulting in negative regulation on PRR and positive regulation of PI3K-p85 expression (56). Studies in the heart reported direct interaction of PLZF with the AT2 receptor, which resulted in Ang II-induced nuclear localization of PLZF and enhanced expression of PI3K-p85 and GATA4 (57, 58). In other systems, PLZF targets included smooth muscle α-actin in chicken embryonic fibroblasts, Redd1 in spermatogonial progenitor cells, and c-myc in several cell lines (59, 60). If the latter targets of PLZF are validated in cardiac myocytes, these would imply a significant role of this transcription factor in heart contraction and growth. We observed that the hyperglycemia increased prorenin (5) and PRR gene expression in cardiac myocytes, which was associated with increased nuclear translocation of PLZF (Fig. 8). Aliskiren, benazeprilat, and valsartan reduced cardiac myocyte PRR density and nuclear translocation of PLZF in diabetic mice. The effect of aliskiren on PRR expression corroborates earlier findings in TGR(mRen2)-27 rats (27). The mechanism by which these three agents regulate the PRR-PLZF pathway and a precise role of PLZF in diabetic cardiomyopathy remain to be determined. Association of PRR with PLZF in diabetic cardiomyopathy and prevention of nuclear co-localization of the latter by all three drugs, represent significant novel findings.
Supplementary Material
CLINICAL PERSPECTIVE.
Both ARBs and ACE inhibitors have been shown to be cardioprotective in various pathological states. A renin inhibitor is expected to have a similar profile. However, no single agent among ARBs and ACE inhibitors has been sufficient to reduce morbidity and mortality to the desired levels in patients with cardiovascular diseases. The latter observations have suggested the existence of residual risk and the potential benefits of dual therapy (17). Unexpectedly, a combination of ACE inhibitors and ARBs produced a worse outcome than any single RAS blocker, the reasons for which are not clear (61). Similarly, addition of aliskiren to an ARB and ACE inhibitor has not provided a favorable outcome (16). Interestingly, head-to-head comparison of the three classes of drugs has not been performed in the context of diabetes. The intracellular RAS, which is strongly activated by hyperglycemia, might be the residual risk that is not mitigated by ARBs and ACE inhibitors. The intracellular RAS is inhibited completely by a renin inhibitor, suggesting that a renin inhibitor might have an advantage in diabetes. In this study, direct renin inhibition provided similar protection from cardiac dysfunction in the short term; but, better protection from oxidative stress and inflammation, as compared to an ARB in diabetic cardiomyopathy. However, whether the latter superior effects of renin inhibition would translate into a better outcome in long-term treatment in diabetes, remains to be investigated.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas.
FUNDING
This work was supported by funding from Novartis and NIH grant 5R01HL090817.
This study was partly funded by a research grant from Novartis Pharmaceuticals Corporation to R.K. and K.M.B. In addition, Novartis provided aliskiren, valsartan, and benazeprilat for use in this study. D.L.F. is a full time employee of Novartis Pharmaceuticals Corporation.
Footnotes
Abbreviations: ACE, angiotensin-converting enzyme; AGT, angiotensinogen; Alsk, aliskiren; Ang, angiotensin; AT1, angiotensin type 1; AT2, angiotensin type 2; AT1a, angiotensin type 1a receptor; ARB, angiotensin receptor blocker; Benz, benazeprilat; CO, cardiac output; DHE, dihydroethidium; EDTA, ethylenediaminetetraacetic acid; EF, ejection fraction; FS, fractional shortening; HR, heart rate; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; IVRT, isovolumic relaxation time; LPS, lipopolysaccharide; LVIDd, left ventricular internal dimension in diastole; LVIDs, left ventricular internal dimension in systole; MAP, mean arterial pressure; MCP-1, monocyte chemotactic protein-1; PBS, phosphate buffered saline; PI3K, phosphotidyl inositol 3-kinase; PLZF, promyelocytic zinc finger protein; PPAR, peroxisome proliferator-activated receptor; PRR, pro renin receptor; RAS, renin-angiotensin system; ROS, reactive oxygen species; RyR, ryanodine receptor; SHR, spontaneously hypertensive rats; STZ, streptozotocin; TNF-α, tumor necrosis factor-α; Vals, valsartan; Veh, vehicle; WT, wild type
AUTHOR CONTRIBUTION
C.M.T. researched data and wrote the manuscript, Q.C.Y. researched data and reviewed the manuscript, R.S. researched data, N.C. researched data, D.L.F. assisted in research design, provided drugs and reviewed the manuscript, K.M.B. reviewed/edited the manuscript and contributed to discussion, R.K. designed and supervised research and wrote the manuscript. K.M.B. and R.K. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential Conflicts of Interest
C.M.T., Q.C.Y., R.S. and N.C. do not have any conflicts of interest.
This work was published in abstract form in Hypertension 2011, 58(5):e128.
References
- 1.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci. 2012;123:273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker KM, Kumar R. Intracellular Angiotensin II Induces Cell Proliferation Independent of AT1 Receptor. Am J Physiol Cell Physiol. 2006;291:C995–1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 4.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clausmeyer S, Reinecke A, Farrenkopf R, Unger T, Peters J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology. 2000;141:2963–2970. doi: 10.1210/endo.141.8.7623. [DOI] [PubMed] [Google Scholar]
- 7.Grobe JL, Xu D, Sigmund CD. An intracellular Renin-Angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system in the heart. Curr Hypertens Rep. 2009;11:104–110. doi: 10.1007/s11906-009-0020-y. [DOI] [PubMed] [Google Scholar]
- 9.Re R. Intracellular renin-angiotensin system: the tip of the intracrine physiology iceberg. Am J Physiol Heart Circ Physiol. 2007;293:H905–906. doi: 10.1152/ajpheart.00552.2007. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32:1551–1565. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R, Yong QC, Thomas CM, Baker KM. Review: Intracardiac intracellular angiotensin system in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R510–517. doi: 10.1152/ajpregu.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 13.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H939–948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 14.Singh VP, Baker KM, Kumar R. Activation of the Intracellular Renin-Angiotensin System in Cardiac Fibroblasts by High Glucose: Role in Extracellular Matrix Production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 15.Hollenberg NK, Fisher ND, Nussberger J, Moukarbel GV, Barkoudah E, Danser AH. Renal responses to three types of renin-angiotensin system blockers in patients with diabetes mellitus on a high-salt diet: a need for higher doses in diabetic patients? J Hypertens. 2011;29:2454–2461. doi: 10.1097/HJH.0b013e32834c627a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azizi M, Menard J. Renin Inhibitors and Cardiovascular and Renal Protection: An Endless Quest? Cardiovasc. Drugs Ther. 2012 doi: 10.1007/s10557-012-6380-6. In press. [DOI] [PubMed] [Google Scholar]
- 17.Volpe M, Danser AH, Menard J, Waeber B, Mueller DN, Maggioni AP, Ruilope LM. Inhibition of the renin-angiotensin-aldosterone system: is there room for dual blockade in the cardiorenal continuum? J Hypertens. 2012;30:647–654. doi: 10.1097/HJH.0b013e32834f6e00. [DOI] [PubMed] [Google Scholar]
- 18.Shah AM, Shin SH, Takeuchi M, Skali H, Desai AS, Kober L, Maggioni AP, Rouleau JL, Kelly RY, Hester A, Keefe D, McMurray JJ, Pfeffer MA, Solomon SD. Left ventricular systolic and diastolic function, remodelling, and clinical outcomes among patients with diabetes following myocardial infarction and the influence of direct renin inhibition with aliskiren. Eur J Heart Fail. 2012;14:185–192. doi: 10.1093/eurjhf/hfr125. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-De Leon A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 21.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977;60:884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acar E, Ural D, Bildirici U, Sahin T, Yilmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg. 2011;11:732–737. doi: 10.5152/akd.2011.196. [DOI] [PubMed] [Google Scholar]
- 23.Aksakal E, Akaras N, Kurt M, Tanboga IH, Halici Z, Odabasoglu F, Bakirci EM, Unal B. The role of oxidative stress in diabetic cardiomyopathy: an experimental study. Eur Rev Med Pharmacol Sci. 2011;15:1241–1246. [PubMed] [Google Scholar]
- 24.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 25.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin system. Kidney Int. 1997;51:1553–1567. doi: 10.1038/ki.1997.214. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- 27.Connelly KA, Advani A, Kim S, Advani SL, Zhang M, White KE, Kim YM, Parker C, Thai K, Krum H, Kelly DJ, Gilbert RE. The cardiac (pro)renin receptor is primarily expressed in myocyte transverse tubules and is increased in experimental diabetic cardiomyopathy. J Hypertens. 2011;29:1175–1184. doi: 10.1097/HJH.0b013e3283462674. [DOI] [PubMed] [Google Scholar]
- 28.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A Novel Signal Transduction Cascade Involving Direct Physical Interaction of the Renin/Prorenin Receptor With the Transcription Factor Promyelocytic Zinc Finger Protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 29.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–39. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 30.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. American journal of physiology Renal physiology. 2006;290:F214–222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 31.van Esch JH, Moltzer E, van Veghel R, Garrelds IM, Leijten F, Bouhuizen AM, Danser AH. Beneficial cardiac effects of the renin inhibitor aliskiren in spontaneously hypertensive rats. J Hypertens. 2010;28:2145–2155. doi: 10.1097/HJH.0b013e32833d01ae. [DOI] [PubMed] [Google Scholar]
- 32.Boschmann M, Nussberger J, Engeli S, Danser AH, Yeh CM, Prescott MF, Dahlke M, Jordan J. Aliskiren penetrates adipose and skeletal muscle tissue and reduces renin-angiotensin system activity in obese hypertensive patients. J Hypertens. 2012;30:561–566. doi: 10.1097/HJH.0b013e32834f6b43. [DOI] [PubMed] [Google Scholar]
- 33.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of Aliskiren on Blood Pressure, Albuminuria, and (Pro)Renin Receptor Expression in Diabetic TG(mREN-2)27 Rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 34.Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360–365. doi: 10.1007/s11906-012-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Review: Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302:R518–530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2010;298:H1807–1818. doi: 10.1152/ajpheart.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook JL, Singh A, Aguiluz RN, Re RN, Alam J. Intracellular Angiotensin II Binds to Mitochondrial Proteins of the NADH Dehydrogenase Complex and Modifies Oxidative Phosphorylation. Hypertension. 2010;56:E135–E135. [Google Scholar]
- 41.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1 beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Luyckx VA, Ots M, Lee KW, Ziai F, Troy JL, Brenner BM, MacKenzie HS. Renin-angiotensin blockade lowers MCP-1 expression in diabetic rats. Kidney Int. 1999;56:1037–1048. doi: 10.1046/j.1523-1755.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- 43.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J, Siragy HM. Glucose promotes the production of interleukine-1 beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–5565. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High Glucose Induced Regulation of Intracellular Angiotensin II Synthesis and Nuclear Redistribution in Cardiac Myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 47.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. The Journal of clinical investigation. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 49.Iwashita M, Sakoda H, Kushiyama A, Fujishiro M, Ohno H, Nakatsu Y, Fukushima T, Kumamoto S, Tsuchiya Y, Kikuchi T, Kurihara H, Akazawa H, Komuro I, Kamata H, Nishimura F, Asano T. Valsartan, independently of AT1 receptor or PPARgamma, suppresses LPS-induced macrophage activation and improves insulin resistance in cocultured adipocytes. Am J Physiol Endocrinol Metab. 2012;302:E286–296. doi: 10.1152/ajpendo.00324.2011. [DOI] [PubMed] [Google Scholar]
- 50.Erdos EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension. 2010;55:214–220. doi: 10.1161/HYPERTENSIONAHA.109.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buleon M, Allard J, Jaafar A, Praddaude F, Dickson Z, Ranera MT, Pecher C, Girolami JP, Tack I. Pharmacological blockade of B2-kinin receptor reduces renal protective effect of angiotensin-converting enzyme inhibition in db/db mice model. Am J Physiol Renal Physiol. 2008;294:F1249–1256. doi: 10.1152/ajprenal.00501.2007. [DOI] [PubMed] [Google Scholar]
- 52.Fraune C, Lange S, Krebs C, Holzel A, Baucke J, Divac N, Schwedhelm E, Streichert T, Velden J, Garrelds I, Danser AH, Frenay AR, van Goor H, Jankowski V, Stahl RA, Nguyen G, Wenzel UO. AT1 antagonism and renin inhibition in mice: pivotal role of targeting angiotensin II in chronic kidney disease. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00672.2011. In Press. [DOI] [PubMed] [Google Scholar]
- 53.Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 54.Huang J, Matavelli LC, Siragy HM. Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-beta1-connective tissue growth factor signalling cascade. Clin Exp Pharmacol Physiol. 2011;38:215–221. doi: 10.1111/j.1440-1681.2011.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng H, Fan X, Moeckel GW, Harris RC. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol. 2011;22:1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 57.Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E, Jr, Roberts RL, Imboden H, Fitzgerald TG, Gaffney FA, Inagami T. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. The EMBO journal. 2003;22:6471–6482. doi: 10.1093/emboj/cdg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang N, Frank GD, Ding R, Tan Z, Rachakonda A, Pandolfi PP, Senbonmatsu T, Landon EJ, Inagami T. Promyelocytic leukemia zinc finger protein activates GATA4 transcription and mediates cardiac hypertrophic signaling from angiotensin II receptor 2. PLoS One. 2012;7:e35632. doi: 10.1371/journal.pone.0035632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. 2010;142:468–479. doi: 10.1016/j.cell.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Sun M, Vogt PK. Smooth muscle alpha-actin is a direct target of PLZF: effects on the cytoskeleton and on susceptibility to oncogenic transformation. Oncotarget. 2010;1:9–21. doi: 10.18632/oncotarget.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.