Abstract
One of the factors affecting the pharmacokinetics (PK) of a drug during pregnancy is the activity of hepatic and placental metabolizing enzymes. Recently, we reported on the biotransformation of glyburide by human hepatic and placental microsomes to six metabolites that are structurally identical between the two tissues. Two of the metabolites, 4-trans- (M1) and 3-cis-hydroxycyclohexyl glyburide (M2b), were previously identified in plasma and urine of patients treated with glyburide and are pharmacologically active. The aim of this investigation was to identify the major human hepatic and placental CYP450 isozymes responsible for the formation of each metabolite of glyburide. This was achieved by the use of chemical inhibitors selective for individual CYP isozymes and antibodies raised against them. The identification was confirmed by the kinetic constants for the biotransformation of glyburide by cDNA-expressed enzymes. The data revealed that the major hepatic isozymes responsible for the formation of each metabolite are as follows: CYP3A4 (ethylene-hydroxylated glyburide (M5), 3-trans-(M3) and 2-trans-(M4) cyclohexyl glyburide); CYP2C9 (M1, M2a( 4-cis-) and M2b); CYP2C8 (M1 and M2b); and CYP2C19 (M2a). Human placental microsomal CYP19/aromatase was the major isozyme responsible for the biotransformation of glyburide to predominantly M5. The formation of significant amounts of M5 by CYP19 in the placenta could render this metabolite more accessible to the fetal circulation. The multiplicity of enzymes biotransforming glyburide and the metabolites formed underscores the potential for its drug interactions in vivo.
1. INTRODUCTION
Glyburide (glibenclamide) is a second generation sulfonylurea drug used for treatment of type 2 diabetes. Clinical trials and retrospective chart reviews demonstrated the efficacy of glyburide in treatment of pregnant patients inflicted with gestational diabetes as well as the absence of adverse effects on maternal and neonatal outcome [1,2]. On the other hand, the maternal physiological changes associated with the onset of pregnancy, fetal organogenesis and development, as well as placental disposition of drugs, contributes to changes in the pharmacokinetics (PK) of administered medications. Therefore, a multi-center clinical trial conducted by the Obstetrics-Pharmacology Research Units network of NICHD to investigate the PK of glyburide administered for treatment of gestational diabetics was initiated four years ago and was recently completed [3]. During this clinical trial, our center also investigated human placental transfer of glyburide utilizing the ex vivo model system of dual perfusion of placental lobule [4, 5] as well as the biotransformation of glyburide by human hepatic and placental microsomes [6,7].
Hepatic enzymes extensively metabolized glyburide to 4-trans- (M1) and 3-cis- (M2b) hydroxycyclohexyl derivatives that were identified in plasma, urine, and feces of patients and volunteers following administration of the hypoglycemic agent [8–11]. These two metabolites were as potent as glyburide in lowering the concentration of glucose [12, 13].
Recent investigations of the biotransformation of glyburide by human placental and hepatic microsomes revealed the formation of six major metabolites [6, 7, 14]. Five metabolites are formed by the hydroxylation of the cyclohexyl ring of glyburide and include previously described 4-trans- (M1) and 3-cis- (M2b) [8,9] and recently identified 4-cis- (M2a),3- trans- (M3), and 2-trans- (M4) hydroxycyclohexyl glyburide [6,7]. The sixth metabolite of glyburide (M5) was formed by hydroxylation of the ethylene bridge linking the benzenesulfonyl-N′-cyclohexyl urea group to the remainder of the molecule [6, 7, 14]. The metabolites formed by the two organs were structurally identical but their rates of formation and the ratios of each to the total amount formed were substantially different. For example, the major metabolites formed by human hepatic microsomes were M1 and M5, whereas in the placenta M5 was the predominant metabolite [6, 7]. The apparent Km values for the biotransformation of glyburide by hepatic and placental microsomes as well as the Vmax values for each metabolite formed suggested that several hepatic and placental microsomal cytochrome P450 (CYP) isozyme were responsible for the reaction [7].
Current reports on the role of hepatic CYP isozymes in the biotransformation of glyburide are not consistent. The metabolism of glyburide in vivo was affected by polymorphism in the CYP2C9 gene [15–17]. However, the in vitro activity of CYP2C9, either recombinant or in human hepatic microsomes, was meager [18] or not detectable [19]. Moreover, CYP3A4 was the predominant metabolizing enzyme in vitro [18–20]. The in vitro activity of recombinant CYP2C19 was demonstrated [18, 19] but polymorphism(s) in its gene did not affect the PK of glyburide [17]. The discrepancy between the in vivo and in vitro results suggests that multiple CYP isozymes could be involved in hepatic biotransformation of glyburide. However, the role of each isozyme in the metabolism of the drug and the formation of each individual metabolite remains unclear. Moreover, to the best of our knowledge, there are no reports, other than from our laboratory, on the biotransformation of glyburide by human placenta.
Therefore, the aim of this investigation is to identify the CYP isozyme(s) responsible for the formation of each metabolite formed by human hepatic and placental microsomes.
2. MATERIAL AND METHODS
2.1. Chemicals and other supplies
Acetonitrile, dichloromethane, hexane, acetic and trichloroacetic acid were purchased from Fisher Scientific (Fair Lawn, NJ). Glyburide (glibenclamide), or N-4-[β-(5-chloro-2-methoxybenzamido-ethyl) benzenesulfonyl]-N′-cyclohexyl urea), chemicals, and reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Metabolites of glyburide—4-trans-, 3-trans-, 2-trans-, 3-cis-, and 4-cis-hydroxycyclohexyl glyburide—were synthesized as previously reported [21]. Polyclonal (CYP1A1) and monoclonal antibodies raised against CYP 1A2, 2A6, 2B6, 2C8, 2C9, and 2E1 were purchased from XenoTech, LLC (Lenexa, KS). Polyclonal antibodies against CYP 3A4, 2C19, and 2D6 were purchased from MorphoSys UK Ltd, (Oxford, UK). Rabbit antiserum to human placental aromatase was purchased from Hauptman-Woodward Institute (Buffalo, NY); human cDNA-expressed CYP450 (Supersomes): 1A1, 1A2, 2A6, 2B6, 2C8, 2C9*1 (Arg144), 2C19, 2D6*1 (Val374), 2E1, 3A4, and 19 from Gentest BD Biosciences (Woburn, MA).
2.2. Human placental and hepatic microsomes
Human term placentas were obtained from healthy pregnancies immediately after delivery according to a protocol approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston. Homogenates of placental trophoblast tissue were used to prepare crude microsomal fractions by differential centrifugation as previously described [22]. The microsomes were stored in 0.1 M potassium phosphate buffer (pH 7.4) at −70°C until used. Protein content of the microsomes was determined by a commercially available kit (Bio-Rad Laboratories, Hercules, CA) using bovine serum albumin as a standard. A pool of microsomal preparations from 18 placentas and a pool of 15 human liver microsomes purchased from CellzDirect (Austin, TX) were used in all experiments.
2.3. Identification of human placental and hepatic CYP isozymes responsible for the biotransformation of glyburide
The effects of selective chemical inhibitors of CYP isoforms and antibodies raised against them on the formation of each metaboliteby the pools of placental or hepatic microsomes were investigated. The total amount of metabolites formed in the control reactions (absence of inhibitors) was set as 100%. Stock solutions of glyburide, its metabolites, and the HPLC internal standard (estrone) were prepared as previously described [7].
2.3.1. The effect of the chemical inhibitors on the formation of metabolites
Known selective inhibitors of CYP 450 isozymes were used at concentrations between 5 and 10 fold their reported Ki or IC50 values [23–28]. These concentrations were sufficiently higher to ensure an inhibition of ≥ 80% which is critical when multiple CYP isozymes are involved in the biotransformation of one drug [25]. The following are the inhibitors selective for each CYP isozyme and their concentrations used: CYP1A, α-naphthoflavone (0.05 μM); CYP2C, sulfaphenazole (10 and 40 μM); CYP2C19, lansoprazole (10 and 20 μM); CYP2D6, quinidine (5 μM); CYP2E1, 4-methylpyrazole (25 μM); CYP3A4, ketoconazole (0.5 uM) and troleandomycin (10, 50, 200 μM); CYP19, 4-hydroxyandrostenedione (10 μM). The total volume of the reaction solution was 1 ml of 0.1 M potassium phosphate buffer (pH 7.4) containing either 0.125 mg protein of hepatic or 1 mg protein of placental microsomes. The final concentration of glyburide in the reaction solution was approximately its reported apparent Km value [7]. Glyburide and each inhibitor were added simultaneously to the reaction solution and pre-incubated with the microsomes for 5 min at 37°C. The reaction was initiated by the addition of NADPH-regenerating system (0.4 mM NADP, 4 mM glucose-6-phosphate, 1 U/ml glucose-6-phosphate dehydrogenase, and 2 mM MgCl2), incubated for 30 min, then terminated by adding 15μl of 10% (w/v) trichloroacetic acid and placing on ice. Estrone, 100 ng/ml, was added as an internal standard. The reaction components were centrifuged at 9500 × g for 12 min and 15 μL of acetic acid was then added to the supernatant. The metabolites formed were extracted from the supernatant in 3 ml of dichloromethane-hexane (1:1 v/v). The organic layer was evaporated to dryness, and the residue was reconstituted in 150 μl of the HPLC mobile phase. An aliquot of 100 μl was injected into the column. The amounts of each metabolite formed were calculated (see Section 2.4) and the data reported as the mean of tree experiments.
2.3.2. The Effect of antibodies against CYP isozymes on the formation of metabolites
Monoclonal antibodies raised against human liver CYP isoforms 1A2, 2A6, 2B6, 2C8, 2C9, and 2E1; and rabbit antiserum to human placental aromatase (CYP19) were added in the amount that causes 80%–90% inhibition of their activity as stated by the manufacturer. Polyclonal antibodies against CYP 1A1, 2C19, 2D6, and 3A4 were used at three concentrations in the range recommended. Each antibody was pre-incubated with the microsomes for 15 min at room temperature followed by the addition of glyburide. The remainder of the procedure was as described for the chemical inhibitors (section 2.3.1), except for the reaction volume being 250 μl and the incubation time which was 45 min for hepatic and 60 min for placental microsomes. The relationship between metabolite formation and incubation time was linear up to 80 min.
2.4. Biotransformation of glyburide by cDNA-expressed CYP isozymes (Supersomes)
The biotransformation of glyburide by the following commercially available human cDNA-expressed CYP isozymes (supersomes) was investigated: 1A1, 1A2, 2A6, 2B6, 2C8, 2C9*1 (Arg144), 2C19, 2D6*1 (Val374), 2E1 (+ b5), 3A4, and 19.
2.4.1. Kinetics of glyburide biotransformation by cDNA-expressed CYP isozymes
The biotransformation of glyburide at concentrations between 0 and 80 μM by each supersome was determined in a reaction volume of 250μl. The incubation time was 45 min at 37°C and other reaction conditions were identical to those described above (section 2.3.1). The data obtained for the saturation curves for each cDNA expressed enzyme were used to calculate the kinetic parameters of the reaction (apparent Km and Vmax). The rates of metabolites formation are expressed as pmole of metabolite / pmole of CYP isozyme.
2.4.2. The effect of antibodies against CYP isozymes on the activity of cDNA-expressed CYP isozymes
The potency and cross activity of antibodies raised against individual CYP isozymes was investigated as follows: the effect of CYP3A4 antibodies on the activity of expressed CYP19; the effect of CYP 1A1 and 2C19 antibodies on the activity of CYP 1A1, 2C8, 2C9, 2C19, 3A4, and 19. Two concentrations for each antibody were investigated: the first, which causes 80–90% inhibition of activity, and the second, one half of it, i.e. 45–50%. Each concentration was added to the reaction catalyzed by the investigated supersome preparation as mentioned above. All other experimental conditions were as described in 2.3.2.
The cross reactivity of an antibody was determined by its inhibition of the cDNA-expressed CYPs and presented as % of its effect on the isozyme it was raised against.
2.4.3. Contribution of hepatic CYP isozymes to the formation of glyburide metabolites
The relative contribution of hepatic CYP isozymes in the biotransformation of glyburide was determined according to Rodrigeus et al. [25]by utilizing the kinetics parameters obtained for cDNA-expressed enzymes. The concentrations of glyburide used in calculations corresponded to its minimal effective [29] and mean peak serum concentration, Cmax, following the administration of a therapeutic dose of the drug [9, 10, 12, 13, 18]. For each CYP isozyme the Vmax for metabolite formation was related to its reported content (pmol CYP/mg) in native human liver microsomes [25]. The rate (v) for the formation of the metabolite/s at the given concentration of glyburide [S] was calculated for each CYP using the equation: , and expressed per mg of liver protein (pmol/min·mg). The total rate (vtot) was calculated as follows: ; and the contribution of each CYP was expressed as a percent, i.e. % contribution = v/vtot · 100.
2.5. Identification and quantitative determination of glyburide metabolites
The metabolites formed in each reaction solution were identified by their retention time and mass spectra using LC-MS. The HPLC column was a reverse phase C18 (Symmetry @ 3.5 μM, 4.6 × 75 mm, Waters, Milford, MA). The mobile phase was made of acetonitrile: water (33:67 v/v) at pH 3.5. The volume of the sample injected was 100 μl. The effluent flow rate was 1.3 ml/min, and the UV absorbance was monitored at wavelength of 203 nm. One third of the effluent was directed to the mass spectrometer (Waters EMD 1000 single-quadrupole; Milford, MA).
Quantities of the metabolites formed were determined by selected ion monitoring at m/z 510 [M+H]+ and for the internal standard (IS) at m/z 272. The ratios of the peak areas of the metabolites to that of the internal standard (M/IS) were used to calculate the amount of each metabolite, using standard curves obtained with synthesized standards as previously reported [7].
2.6. Data analysis
All data are represented as mean ± SE. The effect of the antibodies on the formation of glyburide metabolites was evaluated by analysis of variance (ANOVA) with multiple comparisons.
The apparent Km and Vmax values for each cDNA-expressed CYP enzyme were calculated from the saturation curves for glyburide metabolites using the Michaelis-Menten equation and nonlinear regression analysis (SPSS 13.0 for Windows, SPSS Inc., Chicago, IL).
3. RESULTS
3.1. Biotransformation of glyburide by human hepatic microsomes
The biotransformation of glyburide by a pool of 15 human hepatic microsmes was determined. The metabolites formed in the reaction solution were separated by HPLC and identified by their retention times versus those of synthetic standards and confirmed by LC-MS, ion monitoring and fragmentation patterns, as previously described [6,7].
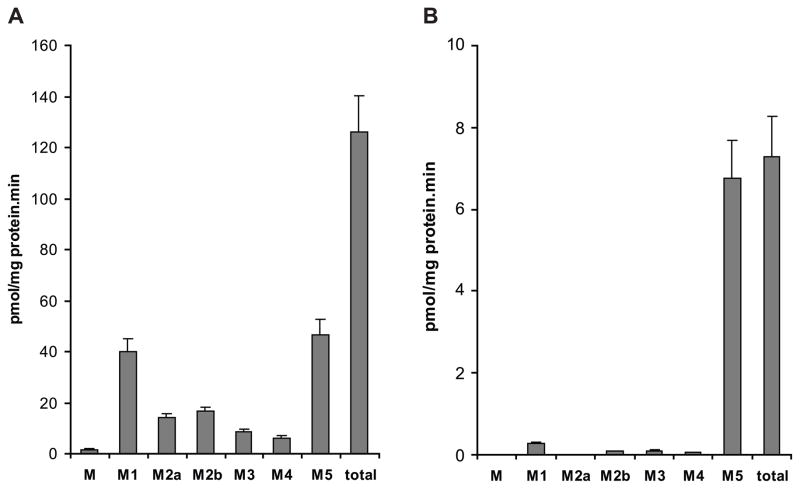
The major metabolites formed were M1 and M5 (Fig. 1A) which represented 31 and 34% of the total, respectively. The metabolites M2a and M2b accounted for 11% and 12%, respectively, while M3 contributed 6% and M4 4% to the total. The amount of metabolite M, an unidentified cyclohexyl hydroxylated derivative of glyburide [7], was <1%, and hence, its inhibition was not considered in analysis of the data.
Figure 1.
Histograms representing the rates of formation of glyburide metabolites by pools of (A) human hepatic microsomes and (B) human placental microsomes. Each histogram represents the mean of data obtained from 18 experiments ± standard error of the mean (S.E.M.).
3.1.1. Effect of selective chemical inhibitors of CYP isozymes on the biotransformation of glyburide by human hepatic microsomes
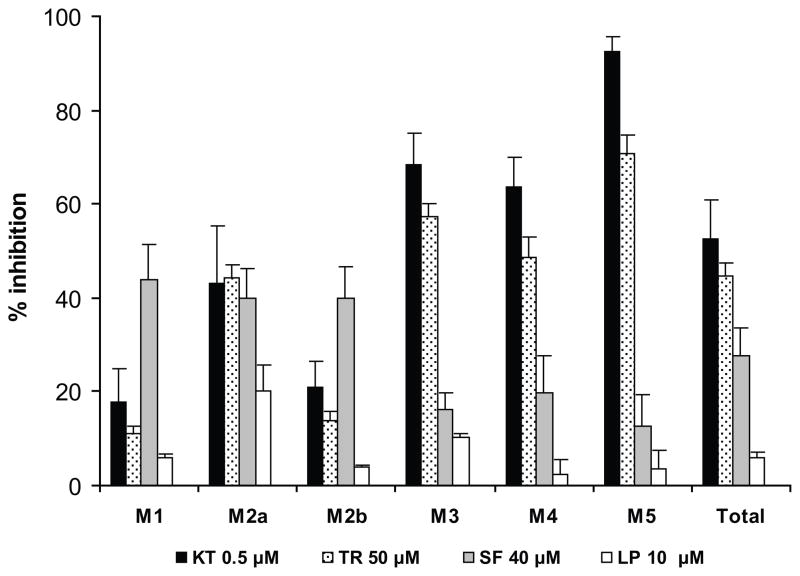
The most pronounced decrease in the aggregate amount of metabolites formed was caused by the inhibitors selective for CYP3A4 (Fig. 2), namely, ketoconazole (0.5 μM, 53%) and troleandomycin (50 μM, 45%). Both compounds inhibited the formation of the following metabolites: M2a by approximately 40%; M3 and M4 by ≥ 50%, and M5 — ketoconazole by 93% and troleandomycin by 71%; the formation of M1 and M2b was decreased by ≤ 20%.
Figure 2.
Histograms representing the effect of inhibitors selective for specific CYP isozymes on the metabolism of glyburide by the pool of human hepatic microsomes. Each inhibitor is followed by the corresponding CYP isoform: ketoconazole, KT, and troleandomycin, TR (CYP3A4); sulfaphenazole, SF (CYP2C9); lansoprazole, LP (CYP2C19). The amount of each metabolite formed in a presence of the inhibitor is expressed as a percent of that in the control (absence of inhibitor). Each histogram represents the mean ± S.E.M. of two experiments.
The inhibitor of CYP2C, sulfaphenazole (40 μM), caused a 28% decrease in the total amount of metabolites formed. The formation of each of the three metabolites M1, M2a, and M2b was decreased by approximately 40%, whereas the formation of M3, M4, and M5 by ≤ 20%.
Lansoprazole (selective for CYP2C19) at its concentration of 10 μM caused only a slight decrease in M2a formation (≈ 20%).α-Naphthaflavone (0.05 μM), selective for CYP1A, did not have any significant effect on the amounts of metabolites formed.
3.1.2. Effect of antibodies on the biotransformation of glyburide by human hepatic microsomes
The identification of CYP isozymes responsible for the formation of individual metabolites of glyburide was confirmed by the effect of the mono- and polyclonal antibodies raised against the isozymes.
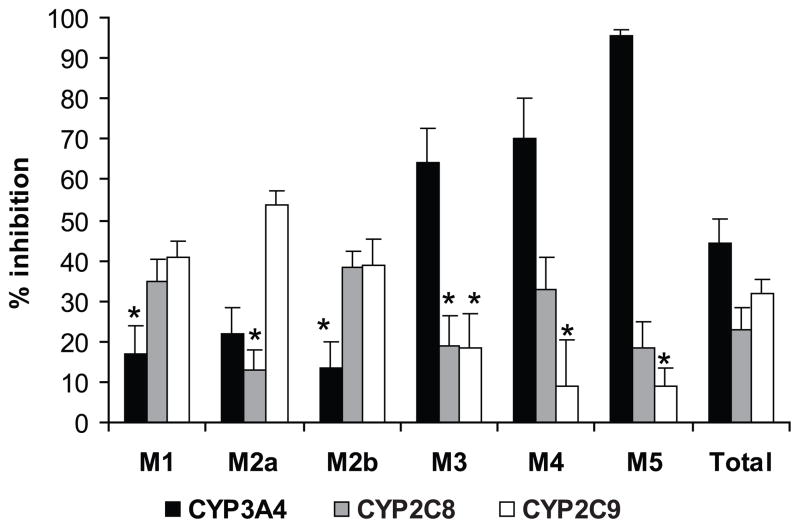
Antibodies against CYP3A4 inhibited the formation of M5by 95%; M4 by 70%; M3by 64%; and M2a by 22%. Antibodies against CYP2C9 inhibited the formation of M1 by 41%, M2a by 54%, and M2b by 39%. Antibodies against CYP2C8 decreased M1 and M2b formation by 35% 40% and M4 by 33% (Fig. 3).
Figure 3.
The effect of antibodies against human CYP 3A4, 2C8, and 2C9 on the formation of each metabolite and their total by the pool of human hepatic microsomes. The amounts of metabolites are represented as described in Fig. 2. Each histogram is the mean of the data from three experiments ± S.E.M. * Not statistically significant (P>0.05).
The inhibition of metabolites formation by antibodies raised against CYP 1A1 and 2C19 (by ≤ 50%) was due to their cross reactivity with other isozymes which was determined as described in section 2.4.2. Namely, antibodies against CYP1A1 cross reacted with 2C8 (as it was reported by the manufacturer); antibodies against CYP2C19 cross reacted with 2C8, and both antibodies cross reacted to a lesser extent with CYP3A4 (Table 1).
Table 1.
Cross reactivity (%) of antibodies raised against CYP 1A1, 2C19 and 3A4 with cDNA-expressed CYP450 (Supersomes).
| Antibodies raised against CYP isozymes | Cross reactivity (%) with cDNA-expressed CYP isozymes
|
||||
|---|---|---|---|---|---|
| 2C8 | 2C9 | 3A4 | 19 | ||
| 1A1 | 1 μl | 63 ± 15 | 10 ± 17 | 29 ± 8 | 35 ± 3 |
| 2 μl | 83 ± 6 | 18 ± 21 | 46 ± 10 | 51 ± 10 | |
| 2C19 | 1 μl | 69 ± 11 | 4 ± 1 | 30 ± 16 | |
| 2 μl | 77 ± 22 | 50 ± 10 | 56 ± 1 | ||
| 3A4 | 1 μl | 28 ± 7 | |||
| 2 μl | 51 ± 12 | ||||
All values are expressed as% of the inhibitory effect of antibodies on the corresponding CYP isozyme (set as 100%) as described in section 2.4.2. Data are mean ± standard error of the mean (S.E.M.) of 3 experiments. Two concentrations of antibodies were used to confirm the cross reactivity (lesser than 20% is considered not significant).
Antibodies raised against CYP 1A2, 2A6, 2B6, 2D6, and 2E1 had no effect on the biotransformation of glyburide.
3.2. Biotransformation of glyburide by human placental CYP microsomes
A pool of 18 term placental microsomal preparations was used for identification of the CYP isozymes catalyzing the biotransformation of glyburide. The major metabolite formed by the pool of placental microsomes is M5 which amounted to 92% of the total (Fig. 1B). The amounts of the other, minor, metabolites were as follows: M1 4%; M2b, M3, and M4 1% each; and the contribution of M and M2a was negligible.
3.2.1. Effects of selective chemical inhibitors of CYP isozymes on the biotransformation of glyburide by human placental CYP microsomes
The effects of the selective inhibitors for CYP 19, 1A, 2C, 2C19, 2D6, 2E1, and 3A4 were investigated. The effect of each inhibitor on the aggregate amount of metabolites formed by placental microsomes was in proportion to that on M5 formation (Fig. 4). This data confirms that M5 is the major metabolite formed by placental microsomes.
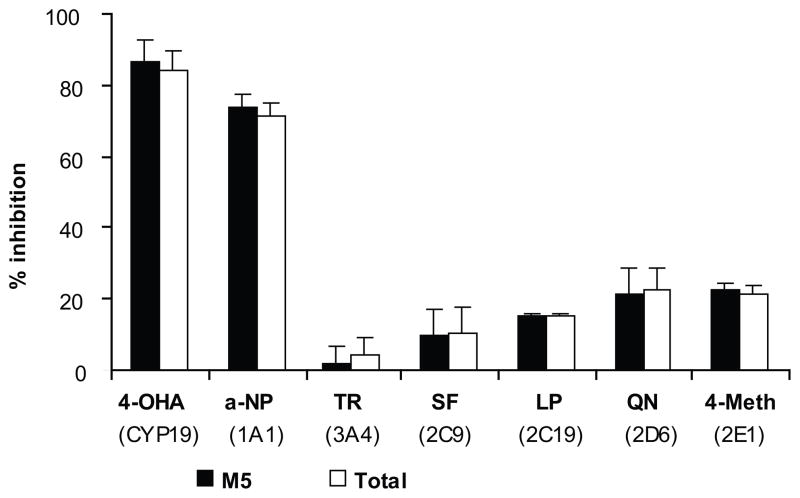
Figure 4.
The effect of inhibitors selective for specific CYP isozymes on the metabolism of glyburide by pooled term human placental microsomes. The inhibitors and their concentrations (in parentheses) are as follows: 4-hydroxy-androstendione, 4-OHA (10 μM); α-naphthoflavone, α-NP (0.05 μM); troleandomycin, TR (50 μM); sulfaphenazole, SF (10 μM); lansoprazole, LP (10 μM); quinidine, QN (5 μM); and 4-methylpyrazole, 4-MP (25 μM). Data presented are the mean of two experiments ± S.E.M.
The two most potent inhibitors of glyburide metabolism were 4-hydroxyandrostenedione (10 μM) selective for CYP19/aromatase and α-naphthaflavone (0.05 μM) selective for CYP1A that decreased M5 formation by 87% and 74%, respectively.
The inhibitors selective for CYP2C family, sulfaphenazole (10 μM for CYP2C) and lansoprazole (10 μM for CYP2C19), as well as troleandomycin (50 and 200 μM) selective for CYP3A4), quinidine (5 μM) selective for 2D6, and 4-methylpyrazole (25 μM) selective for 2E1, had no effect on M5 formation. Consequently, their effects on the aggregate amount of metabolites were negligible (Fig. 4).
3.2.2. Effects of antibodies against individual CYP isozymes on the biotransformation of glyburide by human placental microsomes
Rabbit antiserum to human placental aromatase (CYP19) inhibited the formation of M5 by 94% and, to a lesser extent, M4by 80%; M3 by 70%; M1 by 65%; and M2b by 60%. Antibodies against CYP 1A1 and 3A4 caused approximately 40% reduction in the amounts of M5 and consequently the aggregate metabolites. However, the same antibodies showed cross reactivity (≈ 40%) with cDNA-expressed CYP19 (Table 1).
The antibodies against CYP 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, and 2D6 caused negligible inhibition (≤ 20%) or no effect on the biotransformation of glyburide.
3.3. Biotransformation of glyburide by cDNA-expressed CYP isozymes (Supersomes)
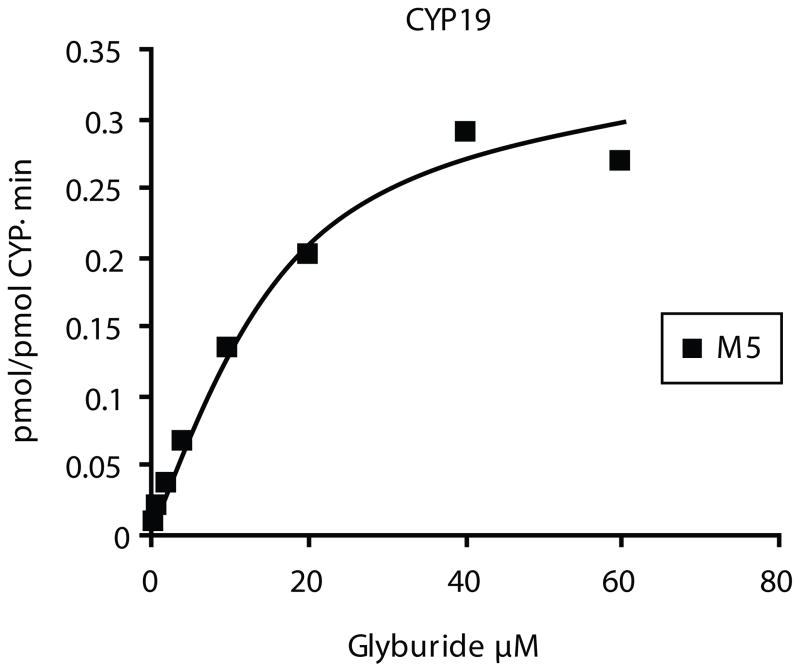
Human cDNA-expressed CYP 1A2, 2A6, 2B6 and 2E1 did not biotransform glyburide. The biotransformation of glyburide by cDNA-expressed CYP 1A1, 2C8, 2C9*1, 2C19, 2D6*1, 3A4, and 19 exhibited typical saturation kinetics (Fig. 5) and apparent Km and Vmax values sited in Table 2. The affinity of glyburide was the highest to CYP2C9. The highest rate of metabolite formation (Vmax) was that achieved by CYP2C19 supersomes followed by the other isozymes in the following descending order: 1A1 > 3A4 > 2C8 > 2D6 > 19 > 2C9. The highest metabolic activity (Clint) was achieved by CYP 2C19, 3A4, and 2C9, and the lowest by CYP 2D6 and 19 (Table 2).
Figure 5.
Biotransformation of glyburide by cDNA-expressed CYP19. The rate of the M5 formation exhibits monophasic saturation kinetics of a typical experiment.
Table 2.
Kinetics parameters for metabolism of glyburide by DNA-expressed CYP isozymes.
| CYP isozymes | Clint Vmax/Km | Km (μM) | Vmax total (pmol /pmol CYP·min) |
|---|---|---|---|
| 1A1 | 0.05 | 40.2 ± 10 | 2 ± 0.2 |
| 2C8 | 0.09 | 10.2 ± 4.7 | 0.9 ± 0.15 |
| 2C9 | 0.25 | 0.8 ± 0.3 | 0.2 ± 0.02 |
| 2C19 | 0.33 | 15.3 ± 6.4 | 4.6 ± 0.7 |
| 2D6 | 0.01 | 44.2 ± 7.6 | 0.5 ± 0.05 |
| 3A4 | 0.34 | 5.1 ± 2.5 | 1.5 ± 0.2 |
| 19 | 0.02 | 16.5 ± 3.3 | 0.4 ± 0.03 |
Data are mean ± standard error of the mean (S.E.M.) of 2 experiments.
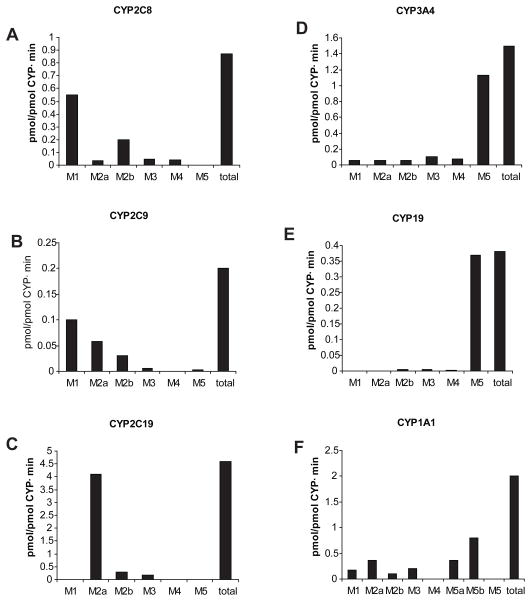
The maximum rates (Vmax) for the formation of each metabolite by the supersomes are shown in Fig. 6. The major metabolites formed by each CYP isoform were as follows: M1 by 2C9 and 2C8; M2a by 2C19, 2D6, and 2C9; M2b by 2C8 and 2C9, and M5 by 3A4 and 19. It is apparent that only CYP3A4 catalyzed the formation of all previously identified metabolites of glyburide [7]. On the other hand, CYP1A1 formed 2 additional metabolites, M5a and M5b, but not M5. These two metabolites exhibited mass spectra identical to that of M5, ethylene-hydroxylated metabolite [6], but had shorter retention times.
Figure 6.
Histograms illustrating the rates (Vmax ) for the formation of the glyburide metabolites by cDNA-expressed CYP isozymes. Each histogram represents the mean of three experiments.
3.4. Contribution of hepatic CYP isozymes to the formation of glyburide metabolites
The relative contribution of six hepatic microsomal CYP isozymes (1A1, 2C8, 2C9, 2C19, 2D6, and 3A4) in the biotransformation of glyburide was determined using two concentrations as described in section 2.4.3. The contribution of CYP 1A1 and 2D6 to the formation of each metabolite and the total formed was negligible (< 1%). The involvement of other four CYP isozymes in the formation of the aggregate was as follows: 3A4 > 2C9 > 2C19 ≥ 2C8 (Table 3). Their contribution to the formation of each metabolite was as follows: M1, 2C9 > 2C8; M2a, 2C9 > 2C19 > 3A4; M2b, 2C9 > 3A4 > 2C8; M3, 3A4 > 2C9; M4, 3A4 > 2C8; and M5, 3A4 only.
Table 3.
Relative contribution of the hepatic microsomal CYP 2C8, 2C9, 2C19, and 3A4 in the biotransformation of glyburide.
| CYP enzymes | Contribution of CYPs (%) in the formation of metabolites
|
||||||
|---|---|---|---|---|---|---|---|
| M1 | M2a | M2b | M3 | M4 | M5 | Total | |
| 2C8 | 20 (26) | 1 (2) | 18 (21) | 7 (7.5) | 10 (11) | 0* | 7 (8) |
| 2C9 | 70 (62) | 47 (39) | 52 (44) | 18 (14) | 0* | 1* | 30 (24) |
| 2C19 | 0.1* | 39 (46) | 6 (7) | 6 (7) | 0* | 0* | 8 (9) |
| 3A4 | 10 (12) | 11 (13) | 24 (28) | 69 (72) | 89 (90) | 99 | 54 (58) |
The values are calculated for the reported minimal effective plasma concentration of glyburide, 10 ng/ml, and for its Cmax, 200 ng/ml (in parentheses), as described in section 2.4.3.
Values are equal for 2 concentrations.
4. DISCUSSION
The aim of this investigation was to identify the major human hepatic and placental microsomal CYP isozymes responsible for the formation of each of the six metabolites of glyburide.
Investigations of glyburide PK provided partial information on the enzymes involved in its biotransformation and suggested that it is markedly influenced by polymorphism(s) in the gene coding for CYP2C9 [15–17]. Investigations of the effects of rifampin [30] and bosentan [18] administration revealed that they lowered plasma levels of glyburide suggesting the involvement of CYP2C9 [30] or 3A4 [18], respectively. However, each of these two drugs has a potential to induce both CYPs [31, 32]. Therefore, the involvement of CYP3A4 in the in vivo biotransformation of glyburide was not conclusive. On the other hand, previous reports on the biotransformation of glyburide by human hepatic microsomes and recombinant enzymes suggested a major role for CYP3A4 [18–20] a meager involvement of CYP2C9 [19] or its lack of contribution [20].
In this investigation, human hepatic and placental CYP isozyme(s) responsible for the formation of each metabolite of glyburide were identified. The data revealed that CYP3A4 is responsible for the formation of three metabolites, namely, M3 (3-trans-), M4 (2-trans-cyclohexyl glyburide), and M5 (ethylene-hydroxylated glyburide), while its contribution to the formation of the previously known metabolites M1 (4-trans-) and M2b (3-cis-) [8–11], as well as M2a (4-cis-), was relatively small. Therefore, human hepatic CYP3A4 introduces a hydroxyl group into all positions of the cyclohexyl ring [6,7,20] as well as in one of the two positions [6, 14] in the ethylene bridge of glyburide (M5). The amount of M5 represents approximately 45% of the aggregate metabolites formed by hepatic microsomes and is followed by M1 at 40% (Fig. 1A). On the other hand, M5 has not been previously determined in plasma or urine of patients under treatment with glyburide. Three possible explanations are offered: M5 is not formed in vivo; it is formed in vivo but rapidly metabolized; or it is formed but was not detected. Our preliminary data (not shown) indicate that M5 is excreted in small amounts in urine of pregnant patients treated with glyburide. However, at this time, there are no data to support the in vivo formation of M5 either in smaller amounts or in larger amounts that are further metabolized rapidly. Moreover, our data on the major role of CYP3A4 in the biotransformation of glyburide by hepatic microsomes are consistent with previous reports [18, 19]. However, data sited here indicate that the contribution of CYP3A4 to the metabolism of glyburide accounted for approximately 55% which is lower than previously reported (96.4%) [19]. This discrepancy is most likely due to the detection limits of the analytical methods used. In our case, the detection of the metabolites formed was achieved by LC-MS. In the previous report, the decrease in the concentration of glyburide was determined by an HPLC detector i.e. spectrophotometrically [19].
The data cited here indicate that CYP 2C9 and 2C8 are the major contributors to the biotransformation of glyburide and are responsible for the formation of 4-trans- (M1) and 3-cis- (M2b) cyclohexyl glyburides (Table 3), the two pharmacologically active metabolites [12,13] that accounted for 43% of the aggregate amount of metabolites formed by hepatic microsomes.
It should be also emphasized that the affinity of glyburide was highest to CYP2C9 as determined for the cDNA-expressed CYPs (Table 2) and as reported for hepatic microsmal CYP enzymes when glyburide was used as an inhibitor of their activities [33]. Similarly, the biotransformation of glyburide by hepatic microsomes to the metabolites M1, M2a, and M2b revealed the apparent Km values ranging between 3.2 – 3.8 μM whereas the apparent Km values for the metabolites M3, M4, and M5 ranged between 7.4 – 8 μM. Accordingly, the concentrations of glyburide in vivo are lower than those used in vitro and consequently CYP2C9 will have an advantage over the other isozymes in its biotransformation. The contribution of CYP2C9 to the in vivo metabolism of glyburide, at its concentration in blood, was approximately 30% i.e. lower than for CYP3A4 but higher than other CYP2C isozymes (Table 3). Therefore, the formation of the pharmacologically active metabolites (M1 and M2b) should also contribute to glycemic control of patient as previously reported [12–13].
To the best of our knowledge, the involvement of CYP2C8 in the in vivo and in vitro hepatic biotransformation of glyburide has not been reported. The expression of CYP2C8 at the mRNA and protein levels, 10%–12% [25, 34, 35], is close to that for CYP2C9 (18%) [34, 25,3–5]. However, the expression of CYP2C8 protein has high variability between individuals (60 fold) that could reflect on its activity as opposed to the variability in CYP2C9 expression which is much less (3-fold between individuals) [37].
Hepatic CYP2C19 more commonly serves as a secondary contributor in the metabolism of drugs [37]. Previous reports indicated that recombinant CYP2C19 biotransformed glyburide [18, 19] at a rate higher than that for CYP2C9 [18] and are in agreement with our data on the cDNA-expressed enzymes (Table 3). However, our data indicate that CYP2C19 biotransforms glyburide to one metabolite, namely, M2a (Fig. 6). The pharmacological activity of this metabolite has not been yet determined. The enzyme contribution to hepatic metabolism of the drug accounts to <10% (Table 3). This could be due to the low affinity of glyburide to CYP2C19 and its low expression (4%) in hepatic tissue [25] as opposed to the expression of CYP 3A4 (20–30%) or 2C9 (18%) [25, 36]. The above mentioned data could explain the lack of effect of polymorphism(s) in the gene coding for CYP2C19 on the in vivo metabolism of glyburide despite its demonstrated high in vitro metabolic activity (CLint = 0.3).
Term human placental microsomes biotransform glyburide to M5 which contributes to 90 ± 3% of the aggregate amount of metabolites formed [6, 7]. In this investigation, neither the inhibitors selective for the CYP isozymes: 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, and 2E1, nor antibodies raised against them, caused a significant decrease in the formation of any of the metabolites. Therefore, it can be concluded that these CYP isozymes are not involved in the metabolism of glyburide by term placental microsomes.
The formation of the major placental metabolite M5, and other metabolites, was affected by chemical inhibitors selective for CYP19 (4-hydroxyandrostedione) and CYP1A (α-naphtoflavone) (Fig. 4) as well as by antibodies raised against CYP 19. Interestingly, the biotransformation of glyburide by placental microsomes was also inhibited, but to a lesser extent, by antibodies against CYP 1A1 and 3A4. However, this inhibition does not indicate the involvement of these two enzymes for the following reasons: first, the major placental metabolite (M5) was not among the products of the biotransformation of glyburide by cDNA expressed CYP1A1 (Fig. 6); second, antibodies against CYP1A1 and CYP3A4 cross reacted with CYP19 supersomes (Table 1); third, α-naphtoflavone is also an inhibitor of CYP19 [27]. Moreover, CYP19 supersomes and human placental microsomes biotransformed glyburide to the same metabolites with a similar ratio of each to the total amount (Fig. 6 and 1B) and similar Km values (Table 2) [7]. Finally, CYP19/aromatase is a key enzyme in the biosynthesis of estrogens in the placenta and is the most abundant CYP isoform in this tissue [38]. Therefore, the high expression and activity of CYP19/aromatase and in absence of CYP3A4 activity in human placenta [38–40] indicates that CYP19 is the major enzyme responsible for glyburide biotransformation in the tissue. This conclusion is in agreement with the role of CYP19 in the biotransformation of other drugs that are metabolized by hepatic CYP3A4 [22, 41–43].
In conclusion, the data presented indicate that more than one CYP isozyme is involved in the biotransformation of glyburide. In the liver, glyburide is biotransformed by CYP 3A4, 2C9, 2C8, and 2C19. The major enzymes contributing to the formation of the previously reported and pharmacologically active [12, 13] metabolites, 4-trans- (M1) and 3-cis- hydroxycyclohexyl (M2b) glyburides, are CYP 2C9 and 2C8 while CYP2C9 and CYP2C19 are responsible for the formation of 4-cis-hydroxycyclohexyl glyburide (M2a). CYP3A4 is the major enzyme responsible for the formation of the ethylene-hydroxylated glyburide (M5), 3-trans- (M3) and 2-trans-hydroxycyclohexyl (M4) metabolites.
In the placenta, glyburide is biotransformed by CYP19 to predominantly M5. The formation of M5 by the placental enzyme, if true in vivo, suggests that this metabolite could be more accessible to the fetal circulation. Accordingly, the pharmacological effects of this metabolite, if any, could have a significant effect on fetal euglycemia.
The multiplicity of glyburide biotransforming enzymes, the number of metabolites formed, and lack of information on their pharmacological activity underscores the need for further investigations.
Acknowledgments
This investigation was supported by the Obstetric-Fetal Pharmacology Research Units Network (OPRU, U10-HD0478, NICHD). The authors appreciate the assistance of the medical staff of the Labor and Delivery Ward of the John Sealy Hospital, the Perinatal Research Group, and the Publication, Grant, & Media Support Office of the Department of Obstetrics & Gynecology, University of Texas Medical Branch, Galveston, Texas.
Abbreviations
- M
metabolite
- IS
internal standard
- CYP
cytochrome P450
- HPLC
high performance liquid chromatography
- MS
mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer O, Conway DL, Bercus MD, Xenakis EM-J, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–8. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field R. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol. 2005;193:118–24. doi: 10.1016/j.ajog.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans J, Hankins GDV, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607–14. doi: 10.1038/clpt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanovskaya TN, Nekhayeva I, Hankins GDV, Ahmed MS. Effect of human serum albumin on transplacental transfer of glyburide. Biochem Pharmacol. 2006;72:632–9. doi: 10.1016/j.bcp.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Nanovskaya TN, Patrikeeva S, Hemauer S, Fokina V, Mattison D, Hankins GD, et al. Effect of albumin on transplacental transfer and distribution of rosiglitazone and glyburide. J Matern Fetal Neonatal Med. 2008;21:197–207. doi: 10.1080/14767050801929901. [DOI] [PubMed] [Google Scholar]
- 6.Ravindran S, Zharikova OL, Hill RA, Nanovskaya TN, Hankins GD, Ahmed MS. Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol. 2006;72:1730–7. doi: 10.1016/j.bcp.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Zharikova OL, Ravindran S, Nanovskaya TN, Hill RA, Hankins GDV, Ahmed MS. Kinetics of glyburide metabolites by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol. 2007;73:2012–9. doi: 10.1016/j.bcp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Rupp W, Christ O, Heptner W. Resorption, excretion and metabolism after intravenous and oral administration of HB 419–14C in man. Arzneimittelforschung. 1969;19:1428–34. German. [PubMed] [Google Scholar]
- 9.Fuccella LM, Tamassia V, Valzelli G. Metabolism and kinetics of the hypoglycemic agent glipizide in man – comparison with glibenclamide. J Clin Pharmacol. 1973;13:68–75. doi: 10.1002/j.1552-4604.1973.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 10.Balant L, Fabre J, Zahnd GR. Comparison of the pharmacokinetics of glipizide and glibenclamide in man. Europ J Clin Phamacol. 1975;8:63–9. doi: 10.1007/BF00616416. [DOI] [PubMed] [Google Scholar]
- 11.Rydberg T, Whlin-Boll E, Melander A. Determination of glibenclamide and its two major metabolites in human serum and urine by column liquid chromatography. J Chromatgr. 1991;564:223–33. doi: 10.1016/0378-4347(91)80084-p. [DOI] [PubMed] [Google Scholar]
- 12.Rydberg T, Jönsson A, Karisson MO, Melander A. Concentration-effect relations of glibenclamide and its active metabolites in man: modelling of pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol. 1997;43:373–81. doi: 10.1046/j.1365-2125.1997.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jönsson A, Hallengren B, Rydberg T, Melander A. Effects and serum levels of glibenclamide and its active metabolites in patients with type 2 diabetes. Diabetes Obes Metab. 2001;3:403–9. doi: 10.1046/j.1463-1326.2001.00152.x. [DOI] [PubMed] [Google Scholar]
- 14.Tiller PR, Land AP, Jardine I, Murphy DM, Sozio R, Ayrton A, et al. Application of liquid chromatography-mass spectrometryn analyses to the characterization of novel glyburide metabolites formed in vitro. J Chromatogr A. 1998;794:15–25. doi: 10.1016/s0021-9673(97)00881-9. [DOI] [PubMed] [Google Scholar]
- 15.Kirchheiner J, Brockmöller J, Meineke I, Bauer S, Rohde W, Meisel C, et al. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther. 2002;71:286–96. doi: 10.1067/mcp.2002.122476. [DOI] [PubMed] [Google Scholar]
- 16.Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivistö KT. Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther. 2002;72:326–32. doi: 10.1067/mcp.2002.127495. [DOI] [PubMed] [Google Scholar]
- 17.Yin OQP, Tomlinson B, Chow MSS. CYP2C9, but not CYP2C19, polymorphisms affect the pharmacokinetics and pharmacodynamics of glyburide in Chinese subjects. Clin Pharmacol Ther. 2005;78:370–7. doi: 10.1016/j.clpt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Van Giersbergen PL, Treiber A, Clozel M, Bodin F, Dingemanse J. In vivo and in vitro studies exploring the pharmacokinetic interaction between bosentan, a dual endothelin receptor antagonist, and glyburide. Clin Pharmacol Ther. 2002;71:253–62. doi: 10.1067/mcp.2002.122473. [DOI] [PubMed] [Google Scholar]
- 19.Naritomi Y, Terashita S, Kagayama A. Identification and relative contributions of human cytochrome P450 isoforms involved in the metabolism of glibenclamide and lansoprazole: evaluation of an approach based on the in vitro substrate disappearance rate. Xenobiotica. 2004;34:415–27. doi: 10.1080/00498250410001685728. [DOI] [PubMed] [Google Scholar]
- 20.Fischer V, Rodríguez-Gascón A, Heitz F, Tynes R, Hauck C, Cohen D, et al. The multidrug resistance modulator valspodar (PSC 833) is metabolized by human cytochrome P450 3A. Implications for drug-drug interactions and pharmacological activity of the main metabolite. Drug Metab Dispos. 1998;26:802–11. [PubMed] [Google Scholar]
- 21.Hill RA, Rudra S, Peng B, Roane DS, Bounds JK, Zhang Y, et al. Hydroxyl-substituted sulfonylureas as potent inhibitors of specific [3H] glyburide binding to rat brain synaptosomes. Bioorg Med Chem. 2003;11:2099–113. doi: 10.1016/s0968-0896(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 22.Nanovskaya TN, Deshmukh SV, Nekhayeva IA, Zharikova OL, Hankins GD, Ahmed MS. Methadone metabolism by human placenta. Biochem Pharmacol. 2004;68:583–91. doi: 10.1016/j.bcp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Newton DJ, Wang RW, Lu AYH. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsoms. Drug Metab Dispos. 1995;23:154–8. [PubMed] [Google Scholar]
- 24.Bourrie M, Meunier V, Berger Y, Fabre G. Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human microsomes. J Pharmacol Exp Ther. 1996;277:321–32. [PubMed] [Google Scholar]
- 25.Rodrigues AD. Integrated cytochrome P450 reaction phenotyping. Attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57:465–80. doi: 10.1016/s0006-2952(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 26.Sai Y, Dai R, Yang TJ, Krausz KW, Gonzalez FJ, Gelboin HVM, et al. Assesment of specificity of eight chemical inhibitors using cDNA-expressed cytochromes P450. Xenobiotica. 2000;30:327–343. doi: 10.1080/004982500237541. [DOI] [PubMed] [Google Scholar]
- 27.Stresser DM, Turner SD, McNamara J, Stocker P, Miller VP, Crespi CL, et al. A high-throughput screen to identify inhibitors of aromatase (CYP19) Anal Biochem. 2000;284:427–30. doi: 10.1006/abio.2000.4729. it is added for a-napht. [DOI] [PubMed] [Google Scholar]
- 28.Li XQ, Andersson TB, Ahlström, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–7. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 29.Groop LC, Barzilai N, Ratheiser K, Luzi L, Wåhlin-Boll E, Melander A, Defronzo RA. Dose-dependent effects of glyburide on insulin secretion and glucose uptake in humans. Diabetes Care. 1991;14:724–7. doi: 10.2337/diacare.14.8.724. [DOI] [PubMed] [Google Scholar]
- 30.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69:400–6. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 31.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–57. [PubMed] [Google Scholar]
- 32.Dingemanse J, van Giersbergen PL. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet. 2004;43:1089–115. doi: 10.2165/00003088-200443150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Kim KA, Park JY. Inhibitory effect of glyburide on human cytochrome P450 isoforms in human liver microsomes. Drug Metab Dispo. 2003;31:1090–2. doi: 10.1124/dmd.31.9.1090. [DOI] [PubMed] [Google Scholar]
- 34.Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17(9):731–42. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 35.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23. [PubMed] [Google Scholar]
- 36.Forrester LM, Henderson CJ, Glancey MJ, Back DJ, Park BK, Ball SE, Kitteringham NR, McLaren AW, Miles JS, Skett P. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281:359–68. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedlund PJ. The CYP2C19 enzyme polymorphism. Pharmacology. 2000;61:174–83. doi: 10.1159/000028398. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123:369–75. doi: 10.1248/yakushi.123.369. [DOI] [PubMed] [Google Scholar]
- 39.Hakkola J, Pasanen M, Hukkanen J, Pelkonen O, Mäenpää J, Edwards RJ, et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human full-term placenta. Biochem Pharmacol. 1996;51:403–411. doi: 10.1016/0006-2952(95)02184-1. [DOI] [PubMed] [Google Scholar]
- 40.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 41.Deshmukh SV, Nanovskaya TN, Ahmed MS. Aromatase is the major enzyme metabolizing buprenorphine in human placenta. J Pharmacol Exp Ther. 2003;306:1099–105. doi: 10.1124/jpet.103.053199. [DOI] [PubMed] [Google Scholar]
- 42.Deshmukh SV, Nanovskaya TN, Hankins GD, Ahmed MS. N-demethylation of levo-alpha-acetylmethadol by human placental aromatase. Biochem Pharmacol. 2004;67:885–92. doi: 10.1016/j.bcp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Nanovskaya TN, Deshmukh SV, Nekhayeva IA, Zharikova OL, Hankins GD, Ahmed MS. Methadone metabolism by human placenta. Biochem Pharmacol. 2004;68:583–91. doi: 10.1016/j.bcp.2004.04.011. [DOI] [PubMed] [Google Scholar]