Abstract
High versus low novelty exploration predicts a variety of behavioral differences. For example, rats selectively-bred for high novelty exploration (bred High Responders, bHR) exhibit exaggerated aggression, impulsivity, and proclivity to addictive behaviors compared to low novelty-reactive rats (bred Low Responders, bLRs), which are characterized by a high anxiety/depressive-like phenotype. Since bHR/bLR rats exhibit differences in dopaminergic circuitry and differential response to rewarding stimuli (i.e., psychostimulants, food), the present study examined whether they also differ in another key hedonic behavior – sex. Thus, adult bHR/bLR males were given five 30-min opportunities to engage in sexual activity with a receptive female. Sexual behavior and motivation were examined and compared between the groups. The bHR/bLR phenotype affected both sexual motivation and behavior, with bLR males demonstrating reduced motivation for sex compared with bHR males (i.e., fewer animals copulated, longer latency to engage in sex). The bHR males required more intromissions at a faster pace per ejaculation than did bLR males. Thus, neurobiological differences that affect motivation for drugs of abuse, aggression, and impulsivity in rats also affect sexual motivation and performance.
Keywords: bred High Responder (bHR), bred Low Responder (bLR), sexual performance, reward, dopamine
Introduction
Individual differences in temperament and environmental or emotional reactivity predict a variety of behavioral traits and increase vulnerability to certain psychopathologies ranging from mood disorders to drug addiction (Cloninger, 1987; Lukasiewicz et al., 2008; Serretti et al., 2006; Van Laere et al., 2009). In recent years, we developed selectively-bred Sprague-Dawley rats that show extreme differences in novelty exploration (Stead et al., 2006), with bred High Responders (bHRs) vigorously exploring a novel environment and bred Low Responders (bLR) showing limited novelty-induced activity. These high versus low novelty-seeking traits in rats (as in humans) predict distinct behavioral phenotypes, with high novelty-seekers (bHRs) showing high aggression (Kerman et al., 2011), impulsivity (Flagel et al., 2011; Flagel et al., 2010) and proclivity to addictive behaviors (Cummings et al., 2011; Davis, Clinton, Akil, & Becker, 2008; Flagel et al., 2011; Garcia-Fuster, Perez, Clinton, Watson, & Akil, 2010). Low novelty-seekers (bLRs), on the other hand, naturally exhibit exaggerated anxiety (S. M. Clinton, Stead, Miller, Watson, & Akil, 2011; S.M. Clinton, Miller, Watson, & Akil, 2008; Flagel et al., 2010; Kabbaj, Devine, Savage, & Akil, 2000; Perez, Clinton, Turner, Watson, & Akil, 2009; White, Kalinichev, & Holtzman, 2007), depression-like behavior and chronic stress vulnerability (Garcia-Fuster et al., 2012; Stedenfeld et al., 2011).
Numerous studies have examined neurobiological differences that may contribute to the high versus low novelty-seeking traits (e.g., (S. M. Clinton, Stead, et al., 2011; Hooks, Jones, Smith, Neill, & Justice, 1991; Hooks et al., 1994; Hooks & Kalivas, 1994; Kabbaj & Akil, 2001; Kabbaj et al., 2000; Lemaire, Aurousseau, Le Moal, & Abrous, 1999; Perez et al., 2009; Piazza, Maccari, et al., 1991; Rosario & Abercrombie, 1999; Rouge-Pont, Deroche, Le Moal, & Piazza, 1998)), and abundant evidence points to key differences in the dopamine (DA) system. Early studies in commercially available (non-selectively bred) HR/LR rats found that HRs showed increased responsiveness to DAergic drugs (e.g., psychostimulants), but lower D2 mRNA and D2 receptor binding relative to LR rats (Hooks et al., 1994). Our recent work with the bHR/bLR lines augment these findings, showing that bHRs are also more sensitive to the psychomotor activating effects of DAergic agonists (Flagel et al., 2010; Garcia-Fuster et al., 2010), are more inclined to self-administer cocaine (Cummings et al., 2011; Davis et al., 2008), have lower levels of D2 mRNA, and a greater proportion D2-high affinity receptors in the dorsal striatum (Flagel et al., 2010). Interestingly, fast-scan cyclic voltammetry experiments revealed that bHRs also exhibit a greater number of spontaneous DA release events in the nucleus accumbens (NAc) and have higher reward-related DA release compared to bLRs (Flagel et al., 2011). Taken together, much of our research to date points to bLR animals as a useful new rodent model for examining co-morbid depression-like behavior and anxiety.
The display of sexual behavior consists of two separate but complementary components: the appetitive aspects of sex, or sexual motivation, and consummatory components, or sexual behavior/performance. DA in the NAc has been implicated in male sexual behavior and motivation (Everitt, 1990; Hull et al., 1999; Pfaus, 2010; Pfaus et al., 1990; Pfaus & Phillips, 1989, 1991), and the D1/D2 receptor ratio in the medial preoptic area of male rats is critical for sexual motivation and ejaculation (Hull et al., 1989). Given the known bHR/bLR DA system differences and their distinct behavioral responses to a variety of rewarding stimuli (i.e., food and drugs of abuse), the present study determined whether bHR/bLR males also exhibit differences in another key hedonic behavior – sex.
In this experiment, adult bHR/bLR males were subjected to a 5-week sexual behavior testing paradigm where they were exposed to a sexually receptive female 30-min once a week for 5 weeks. Males were allowed to engage in sexual activity, and both motivation for sexual activity as well as sexual performance were examined. Additionally, considering known HR/LR differences in hypothalamic-pituitary-adrenal (HPA) stress axis activity (Kabbaj et al., 2000; Kerman et al., 2012), and possible consequences on the hypothalamic-pituitary-gonadal axis and sexual behavior, we examined seminal vesicles, testes, epididymis, and adrenals in bHR/bLR males as proxy measures for circulating androgens and corticosterone (Akana, Shinsako, & Dallman, 1983; Gonzales, 2001; Swerdloff, Wang, Hines, & Gorski, 1992).
Methods
Animals
bHR and bLR male Sprague Dawley rats (n=20 per phenotype) were acquired from the in-house breeding colony in the Akil laboratory at the University of Michigan. We previously published a description of our breeding strategy and initial behavioral characterization of the bHR/bLR lines (Stead et al., 2006). Animals used in the present experiments were taken from the 28th generation of the colony. Prior to sexual behavior testing, male bHR/bLR rats were screened to assess novelty-induced locomotor activity, and only rats whose scores fell within one standard deviation of their respective group average (from the 28th generation of our bHR/bLR colony) were used for the present studies. Half of the bHR and bLR rats were randomly assigned to the “Sex” group (n=10/phenotype) while the other bHR and bLR animals were placed in the “Naïve” group (n=10/phenotype).
All animals were housed in same-group pairs in standard laboratory cages with free access to food (Harlan 2014) and water. Animals were maintained on a 14:10 light:dark cycle, with lights on at 7:00 am through the locomotor screening tests after which time they were set on a reverse light cycle (lights off at 10:00 am). Males and females were housed in the same room that was maintained at a constant temperature of 22°C. All procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines on laboratory animal use and care, using a protocol approved by the University of Michigan Committee on Use and Care of Animals.
Sexual Behavior Testing
Stimulus females were ovariectomized at approximately 60 days of age and allowed to recover for at least 2 weeks before the start of testing. Sexual receptivity was induced with 17β-estradiol benzoate (10 μg in 0.1 ml peanut oil, s.c.) administered 48 hr prior to testing, followed by progesterone (500 μg in 0.1 ml peanut oil, s.c.) 4-6 hr prior to the encounter. Each female was used only once per test day, and care was taken to ensure that each male received a novel stimulus female for every test. Prior to each test, stimulus females were briefly paired with non-experimental males and verified to display lordosis. The group of sexually-naïve bHR/bHR males served as controls for exposure to the novel environment. Males in this “naïve” group were placed in testing chambers with their cage mate for five 30-minute sessions.
Sexual behavior testing began when males were 90 days of age. Tests were conducted once per week for 5 weeks in a standard glass 10-gallon aquarium between 1:00-4:00 pm. bHR/bLR males were allowed 5 min to acclimate before introduction of the female, and each test lasted 30 min. Behavior was videotaped and analyzed using a computerized program (Noldus Observer 5.0, Leesburg, VA), which recorded the latency, duration, and frequency of events from videotape. The person scoring the tapes was blind to the experimental treatments and trained to observe the following behaviors: mounts (male's front paws on the rump of the female, no penile insertion into vagina), intromissions (a mount with penile insertion), and ejaculations. Due to differences in the percentage of bHR and bLR males engaging in these behaviors across test sessions, sexual behavior during the first three tests with a complete ejaculatory series (i.e. mating to at least one ejaculation) were scored, noting the frequency of each behavior as well as the copulatory efficiency (intromissions divided by the sum of intromissions and mounts). Additional behavioral parameters were also examined, including the number of mounts per ejaculation, the number of intromissions per ejaculation, the post-ejaculatory refractory period (PERP, the amount of time between an ejaculation and the next intromission of the subsequent copulatory series), and inter-intromission interval (III; calculated as average amount of time between intromissions).
Tissue Collection
One week after the final sexual behavior test, naïve and sexually experienced bHR/bLR males were euthanized with an overdose of sodium pentobarbital, followed by transcardiac perfusion with 4% paraformaldehyde. The adrenals, seminal vesicles, testes, and epididymis were dissected out and weighed as proxy measures for HPA axis activity and androgen levels.
Statistical Analysis
Data were analyzed with Statview Statistical Software, version 5 from SAS. Differences in the percentage of bHR/bLR males exhibiting mounts, intromissions, and ejaculations were examined by chi square analyses for each of the five test sessions, and included all experimental animals. Because fewer bLRs engaged in sex through ejaculation during the first two test sessions, an analysis examining sexual behavior across all 5 test sessions included comparisons between groups of animals that were sexually naïve versus those that were active and exhibited one or more ejaculations. As sexual experience alters neurochemical function and, thus, subsequent displays of sexual activity (Pfaus, 2009; Pfaus et al., 2012), we structured our successive analysis such that we could compare bHRs and bLRs with comparable sexual experience by comparing their motivation and behavior by “copulation day”. For example, many bHR males engaged in sex and ejaculated during the first test session, so their “copulation days 1-3” corresponded to test sessions 1-3. However, some bLRs did not engage in sex until the third test session, so their “copulation days 1-3” corresponded to test sessions 3-5. By conducting the analysis in this way, we are comparing the sexual motivation and ability in animals with similar amounts of sexual experience.
Group differences in behavioral parameters (latency to mount/intromit/ejaculate, number of mounts/intromissions, PERP, copulatory efficiency, and III) during the first ejaculatory sequence of each of the three copulation days were examined by repeated measures ANOVA, with copulation day as within subject factor and bHR/bLR phenotype as between subject factor. Mount latency, intromission latency, ejaculation latency, and III data were log transformed prior to analysis due to unequal variance across days. We also examined these parameters within test session to compare behavior during the first ejaculatory sequence (E1) versus second (E2). Accordingly, for each of the first three copulation days, we performed a repeated measures ANOVA with ejaculatory sequence (E1 and E2) as within subject factor and bHR/bLR phenotype as between subject factor.
Organ weights were corrected for body weight and analyzed by 2-way ANOVA, with bHR/bLR phenotype and sexual experience as independent variables. ANOVAs were followed by Fisher's post-hoc comparisons when appropriate. For all tests, α was 0.05.
Results
Male bHR initiate and engage in sexual behavior with less experience than bLR
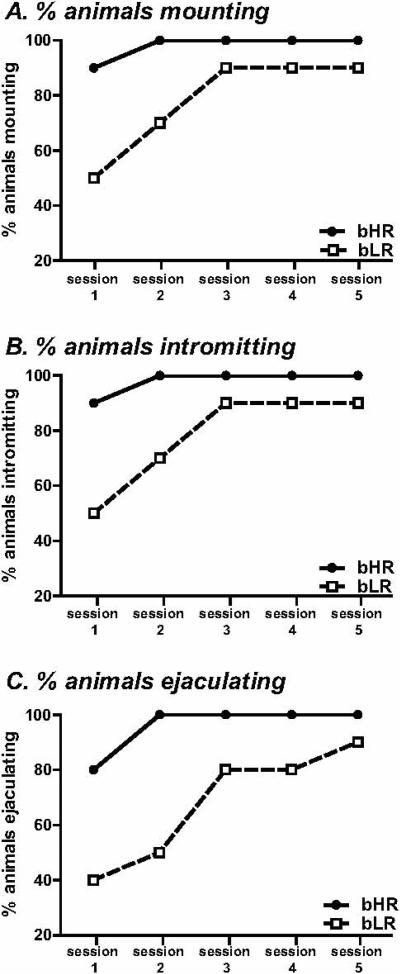
During the first two test sessions, a greater percentage of bHRs mounted the female compared to bLRs (day 1: χ2=5.83, d.f.=1, p<0.05; day 2: χ=4.90, d.f.=1, p<0.05; Fig. 1A). Similarly, a greater percentage of bHRs exhibited intromissions (day 1: χ2=5.83, d.f.=1, p<0.05; day 2: χ2=4.90, d.f.=1, p<0.05; Fig. 1B) and achieved an ejaculation (day 1: χ2=5.10, d.f.=1, p<0.05; day 2: χ2=10.80, d.f.=1, p<0.01; Fig. 1C) during the first two test sessions compared to bLRs. During sessions 3-5, however, there was no group differences in percentage of animals exhibiting mounting behavior, intromissions or ejaculations (Fig. 1A-C).
Figure 1. Percentage of bHR and bLR males engaged in sexual behavior.
During each of the 5 sex test sessions, bHR/bLR males were exposed to a sexually receptive female for 30-min. During the first two test sessions, a greater percentage of bHRs mounted the females compared to bLRs, but by session 3, a similar proportion of bLRs exhibited mounting behavior (A). Similarly, during the first two test sessions, a greater percentage of bHRs exhibited intromissions (B) and ejaculated compared to their bLR counterparts (C). A similar proportion of bLRs and bHRs were engaged in intromissions and ejaculation during test sessions 3-5. * indicates p<0.05
Sexual behavior during the first 3 days of a complete copulatory bout
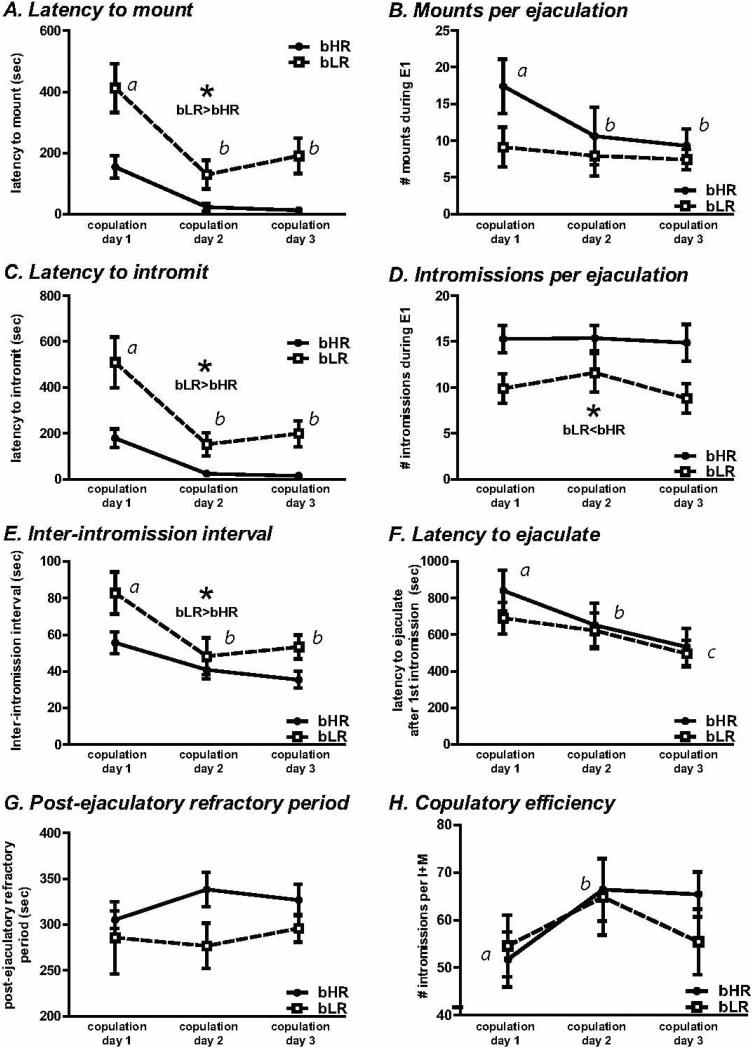
On the first three test days that an animal exhibited an ejaculation (copulation days 1-3), the first ejaculatory bout of each day was analyzed (Figure 2). The latency to mount decreased over time (effect of copulation day, F2, .32 = 37.72, p<0.0001; Fig. 2A) and bLR males took longer to mount than bHRs (F1, 32 = 21.16, p<0.01). There was also a test day × phenotype interaction (F2, 32 = 4.37, p<0.05; Fig. 2A). Post-hoc analysis showed that all animals steadily reduced latency to mount over copulation days 1-3 (p<0.0001 day 1 versus each day 2 and 3), and that bLRs exhibited greater latency to mount compared to bHRs on all copulation days (p<0.001 for each day; Fig. 2A).
Figure 2. bHR and bLR male sexual performance during the first ejaculatory sequence.
For each rat, we designated the first three consecutive test sessions when they successfully engaged in sex (i.e. achieved ejaculation) as their “copulation days 1-3”. Across days, we found that bLRs showed a greater latency to mount the female compared to bHRs (A), and all animals generally showed a reduced latency to mount over the 3 copulation days (difference between days indicated by a versus b). All animals also showed fewer mounts per ejaculation across copulation days (B; indicated by a versus b). bLRs also exhibited increased latency to intromit (C) and fewer intromissions per ejaculation (D) compared to bHR males. All rats showed reduced latency to ejaculate across copulation days (F; indicated by a versus b versus c), although there were no bHR/bLR differences on this measure. Post-ejaculatory refractory period (time between an ejaculation the next mount or intromission) did not change over days, and did not differ between bHR/bLR groups (G). bLR>bHR indicates significant main effect of phenotype; * indicates p<0.05
The number of mounts per ejaculation decreased over copulation days 1-3 (main effect of day F2, 32 = 3.82, p<0.05; Fig. 2B), but there was no significant effect of bHR/bLR phenotype, and no phenotype × day interaction. Post-hoc analysis showed that animals mounted less during copulations days 2 and 3 compared to day 1 (p<0.05).
As shown in Figure 2C, latency to intromit also decreased with experience (F2, 32 = 34.79, p<0.0001) and varied by bHR/bLR phenotype (F1, 32 = 23.95, p<0.01; no significant interaction). All animals steadily reduced the latency to intromit over the 3 copulation days; post-hoc analysis showed that latency to intromit was greater on day 1 versus 2 and 3 (p<0.0001 for each comparison). Post-hoc analysis also showed that bLRs exhibited greater latency to intromit compared to bHRs across copulation days (p<0.0001; Fig. 2C).
The bHR males exhibited more intromissions per ejaculation than bLR males during the first copulatory bout of each copulation day (effect of phenotype, F1, 32 = 7.18, p<0.05; Fig. 2D). There was no effect of copulation day, and no day × phenotype interaction for this measure. In contrast, bLR males exhibited longer inter-intromission intervals than bHR males (F1, 32 = 4.54, p<0.05; Fig 2E), and both phenotypes exhibited decreased intervals with time (F2, 32 = 29.43, p<0.0001). There was no phenotype × day interaction. Post-hoc analysis showed that the inter-intromission interval on test days 2 and 3 were significantly shorter than on day 1 (p<0.05 for each comparison).
Although there were bHR/bLR differences in number of intromissions and inter-intromission interval, the latency to ejaculate decreased over time for both groups (F2, 32 = 9.51, p<0.0001), and there was no effect of bHR/bLR phenotype or day × phenotype interaction (Fig. 2F). Post-hoc analysis showed that animals exhibited steadily shorter latencies to ejaculate across copulation days (p<0.05 for each day compared to one another). Thus, the unique intromission pattern of bHR versus bLR males was equally efficient at achieving ejaculation. For the post-ejaculatory refractory period, there was no effect of bHR/bLR phenotype or copulation day, and no copulation day × phenotype interaction (Fig. 2G). Finally, we found a main effect of copulation day on copulatory efficiency (F2, 32 = 3.94, p<0.05); all animals exhibited greater efficiency on day 2 versus 1 (p<0.05). There was no effect of phenotype, and no day × phenotype interaction (Fig. 2H).
Sexual behavior within test session
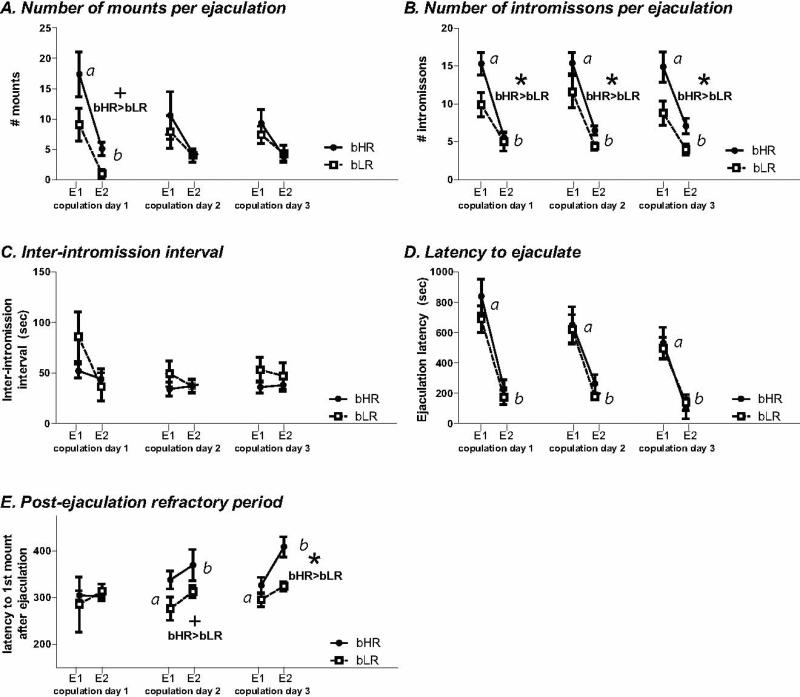
All males exhibited more mounts per ejaculation during the first bout on copulation day 1 than during the second bout (F1, 9 = 6.61, p<0.05; Fig. 3A). For copulation days 2 and 3, there was no main effect of ejaculatory bout or bHR/bLR phenotype, and no bout × phenotype interactions.
Figure 3. bHR versus bLR sexual behavior during the first two ejaculatory sequences of copulation days 1-3.
We examined the number of mounts (A), intromissions (B), inter-intromission interval (C), latency to ejaculate (D), and post-ejaculation refractory period (E) during the first two ejaculatory sequences (E1 and E2) of each rats’ copulation day 1 through 3. This analysis revealed not only how bHR versus bLR males performed across copulation days, but also how their behavior patterns differed during the course of those sessions. bHRs showed a trend for more mounting compared to bLRs on the first copulation day, but no differences on days 2 or 3 (A). All animals showed more mounting during the first ejaculatory bout on copulation day 1 compared to the second ejaculatory bout (indicated by a versus b). bHRs exhibited far more intromissions than bLRs across the three days (B), and all animals showed far more intromissions during the first versus second ejaculatory bout (indicated by a versus b). There were no group differences on inter-intromission interval (C). Similarly, there were no bHR/bLR differences in the latency to ejaculate (D), although all rats showed significantly reduced latency to ejaculate during the second ejaculatory bout compared to the first (indicated by a versus b). Finally, we found that the post-ejaculation refractory period increased between E1 and E2 on copulation days 2 and 3 (indicated by a versus b). There was a trend for bHRs to show increased post-ejaculation refractory period compared to bLR on copulation 2, and this difference was statistically significant on copulation day 3 (E). bHR>bLR indicates significant main effect of phenotype; * indicates p<0.05
The bHR males exhibited more intromissions per ejaculation on each copulation day compared to bLR males (day 1: F1, 9 = 8.66, p<0.05; day 2: F1, 13 = 4.73, p<0.05; day 3: F1, 14 = 6.21, p<0.05; Fig. 3B). On each copulation day there was also a main effect of ejaculatory sequence (day 1: F1, 9 = 22.79, p<0.01; day 2: F1, 13 = 37.37, p<0.0001; day 3: F1, 14 = 27.02, p<0.0001), with all rats showing significantly fewer intromissions during the second compared to the first ejaculatory bout. On the first copulation day, there was a phenotype × ejaculatory bout interaction (F1, 9 = 7.89, p<0.05); post-hoc analysis showed that bHRs exhibited fewer intromissions during the first bout on this day compared to bLRs (p<0.05; Fig. 3B). There were no significant phenotype × ejaculatory sequence interactions for copulation days 2 and 3.
Inter-intromission interval did not vary with session on any of the 3 copulation days (Fig. 3C). Latency to ejaculate decreased over ejaculatory bouts on copulation days 1-3 (day 1: F1, 8 = 38.58, p<0.01; day 2: F1, 8 = 24.42, p<0.01; day 3: F1, 8 = 10.39, p<0.05; Fig. 3D). There was no effect of bHR/bLR phenotype, and no bout × phenotype interaction for copulation days 1-3.
Interestingly, the post-ejaculatory refractory period was greater for bHR than bLR only on copulation day 3 (F1, 11 = 7.50, p<0.05; Fig. 3E). There was no effect of ejaculatory sequence on post-ejaculatory refractory period on copulation day 1, but there was an effect on copulation days 2 (F1, 8 = 21.90, p<0.01) and 3 (F1, 11 = 28.25, p<0.01), with animals showing an increased post-ejaculatory refractory period during E2 versus E1. There was no phenotype × sequence interaction on this measure on copulation days 1-3.
Organ weights
Sexually experienced bHR/bLR males weighed significantly less than sexually-naïve males (F1, 36 = 7.39, p<0.01; Table 1), but there was no effect of phenotype, and no sex × phenotype interaction. For the adrenal glands, there was a main effect of bHR/bLR phenotype (F1, 36 = 10.34, p<0.01), with bHRs having heavier adrenals compared to bLRs regardless of sexual experience. There was no effect of sexual experience, and no phenotype × sex interaction on adrenal size. Sexual experience significantly increased seminal vesicle weight (F1, 36 = 28.93, p<0.0001), and while there was no effect of phenotype, there was a significant phenotype × sex interaction (F1, 36 = 4.58, p<0.05). Sexual experience increased seminal vesicle weight in both bHR and bLR animals, although this effect was more prominent in bLRs (p<0.05 for sexually-experienced versus sexually-naïve bLRs). Finally, there was no effect of phenotype or sexual experience, and no phenotype × sex interaction on weight of the testes or epididymis.
Table 1.
Organ weights in bHR versus bLR males
| bHR sexually experienced | bHR sex naïve | bLR sexually experienced | bLR sex naïve | Statistical effects | |
|---|---|---|---|---|---|
| Body weight (bw; g) | 535 ± 42 | 554 ± 52 | 513 ± 57 | 576 ± 35 | *a |
| Adrenal gland (mg/100g bw) | 10 ± 2 | 10 ± 2 | 9 ± 1 | 8 ± 1 | *b |
| Testes (mg/100g bw) | 622 ± 76 | 590 ± 137 | 680 ± 128 | 625 ± 84 | n.s. |
| Epididymis (mg/100g bw) | 114 ± 16 | 111 ± 18 | 133 ± 30 | 112 ± 20 | n.s. |
| Seminal vessicles (mg/100g bw) | 349 ± 51 | 303 ± 23 | 371 ± 65 | 263 ± 28 | *a, *c |
Organ weights were corrected for body weight. Values represent average corrected organ weights in grams (g) ± standard errors for each of 4 experimental groups: bHR/bLR rats exposed to 5 sex behavior test sessions (sexually experienced), and controls not exposed to females (sex naive). Groups were compared by ANOVA; *a indicates main effect of sexual experience; *b indicates main effect of bHR/bLR phenotype; *c indicates a significant sexual experience × bHR/bLR phenotype interaction; n.s. indicates non-significant p-values >0.05 for statistical comparisons.
Discussion
The present experiments show that male rats selectively-bred for high (bHR) versus low (bLR) reactivity to novelty, known to exhibit drastic differences in behavioral and neurochemical response to reward, also differ in their display of sexual motivation and behavior. During the first two opportunities with sexually receptive females, fewer bLR males engaged in sexual activity compared to bHRs, who readily engaged in sex across all test days. Even after gaining sexual experience, bLRs continued to exhibit reduced sexual motivation when presented with a receptive female, evident by increased mount and intromission latencies on each of the three copulation days. There were also significant differences in sexual behavior, with bHRs requiring more intromissions per ejaculation and demonstrating shorter intervals between intromissions than bLRs. Interestingly, bHR/bLR males were similar in several other sex behavioral measures, including latency to ejaculate, post-ejaculatory refractory period, and copulatory efficiency. Additionally, both bHRs and bLRs demonstrated experience-induced behavioral plasticity, since both groups improved their sexual performance with increased experience. Several of the known bHR/bLR neurobiological differences occur in neurochemical and/or hormonal systems important for sexual motivation and performance; thus, these selectively-bred lines offer a unique tool for studying neural circuits relevant for male sexual function and dysfunction.
As mentioned in the Introduction, successful sexual activity requires the proper functioning of both motivation and ability. Numerous studies have delineated specific brain regions, hormones, and neurotransmitters that mediate the disparate components of sexual activity (for example, see (Conrad & Pfaff, 1976; Hull et al., 1999; Meisel & Sachs, 1994; Pfaff, 1970; Pfaus & Heeb, 1997). While there is some overlap, the control of sexual ability and motivation appear to be largely independent of one another. Indeed, elegant experiments by Everitt (Everitt, 1990; Everitt & Stacey, 1987) have shown that motivation and ability are dissociable; that is, either performance or motivation can be altered while the other remains intact.
Sexual motivation in bHR and bLR males
Sexual motivation in male rats is traditionally evaluated by investigating how long it takes a male to express sexual interest in a female (latency to mount or intromit with a female), as well as examining the number of males within a given group that engage in sexual behaviors (Meisel & Sachs, 1994). The present experiments revealed that significantly fewer bLR males engage the stimulus female in sex during the first two encounters, and continue to exhibit longer latencies to copulation even with sexual experience. Together, these data indicate that bLR males exhibit reduced sexual motivation in comparison to bHR males. Novel techniques have since been designed that directly examine differences in sexual motivation (e.g. via operant responding to gain access to a sexually active conspecific (Cummings & Becker, 2012)). Future studies utilizing these techniques would allow for a more detailed examination of the possible differences in sexual motivation in the bHR/bLR line.
It is also important to consider that the number of bLR males engaging in sexual activity during the first two test sessions may be affected by their tendency to show reduced novelty-induced activity and exploration. bLRs are more inhibited in a novel situation as compared to bHRs, which results in reduced general activity and exploration. In a recent experiment, we found that bHRs approached a novel male or female stimulus rat more quickly than bLRs in a social interaction test (Clinton, unpublished observations). In the present study, most bLRs begin to engage in sexual activity by the third test, while many bHRs copulate in the first session. It is possible that by the third exposure to the test environment, bLRs have grown more familiar with the situation, reducing their inhibition of engaging the females in sex. Nonetheless, while this may contribute to the fewer number of bLR males engaging in sexual activity during the initial tests, the significant differences in sexual motivation that remain even after the animals have gained sexual experience cannot be explained by a novelty-induced reduction in activity.
Sexual motivation in bHR/bLR males: A potential role for neurochemical systems
Several studies have identified a series of neurobiological factors that likely contribute to marked behavioral differences in commercially-available HR/LR (Ballaz, Akil, & Watson, 2007; Cecchi, Capriles, Watson, & Akil, 2007; Hooks et al., 1994; Kabbaj, 2004; Kabbaj et al., 2000; Piazza, Rouge-Pont, et al., 1991; Rosario & Abercrombie, 1999) and selectively-bred bHR/bLR (S. M. Clinton, Bedrosian, Abraham, Watson, & Akil, 2010; S. M. Clinton, Stead, et al., 2011; Flagel et al., 2011; Flagel et al., 2010; Kerman et al., 2011; Kerman et al., 2012) rats. Many of these same neurotransmitter and neuropeptide systems play critical roles in regulating sexual motivation. While future experiments will be required to investigate possible roles of each of these neurotransmitter systems in shaping bHR/bLR sexual motivation, here we outline a few major systems that it will be important to consider.
DA is a key neurotransmitter involved in the neural processing of natural and artificial rewards, including sex and drugs of abuse (Dominguez & Hull, 2005; Kelley, 2004; Koob & Le Moal, 2001). DA influences human male sexual motivation, with attenuated sexual desire being associated with reduced levels of DA (Giargiari, Mahaffey, Craighead, & Hutchison, 2005; Pfaus, 2009; Stahl, 2010). Rodent studies support these findings, demonstrating that DA is released in the medial preoptic area (MPOA) and nucleus accumbens (NAc) both upon the presentation of a sexually receptive female as well as during copulation (Damsma, Pfaus, Wenkstern, Phillips, & Fibiger, 1992; Hull et al., 1999; Hull, Muschamp, & Sato, 2004; Pfaus et al., 1990; Robinson, Heien, & Wightman, 2002; Robinson et al., 2001). Furthermore, pharmacologically reducing NAc DA levels delays the onset of sexual activity (Hull et al., 1999). DA facilitates sexual activity, in part, by removing tonic inhibition of GABAergic neurons in the MPOA; this permits increased sensory processing and the display of appropriate motoric behaviors (Chevalier & Deniau, 1990; Hull, Du, Lorrain, & Matuszewich, 1997; Hull et al., 1999). Thus, DA release increases the probability that sexually relevant stimuli will elicit the appropriate sexual behavioral response (i.e., initiation of sex). Prior studies in our bHR/bLR rats revealed reduced DAergic responsivity in the NAc of bLR versus bHR males, including lower tonic DA levels and less reward-induced DA release (Flagel et al., 2011). bLRs’ reduced sexual motivation may therefore be due to reduced DA release in response to the sexually receptive female. If bLR males release less DA in response to sexual cues (i.e., placement into the testing chamber and introduction of the female), they may require longer cue exposure to stimulate sufficient DA release and prime the circuitry to respond with sexual activity.
Serotonin is another key neurotransmitter system that impacts sexual motivation and behavior. Serotonin inhibits the molecular mechanisms required for increased sexual excitation, and elevated serotonin levels have been shown to reduce sexual motivation (Giargiari et al., 2005; Pfaus, 2009). Indeed, it is widely reported that depressed men and women taking selective serotonin reuptake inhibitors (SSRIs)—which increases brain levels of serotonin—experience reduced sexual motivation (Stahl, 2010). Dysregulation of serotonin neurotransmission has also been shown to contribute toward highly aggressive and depressive phenotypes in animals, and importantly, bLR males demonstrate increased activation of specific serotonergic brainstem cell groups (S. M. Clinton, Kerman, et al., 2011; Kerman et al., 2011). This increased activation could be a mechanism by which bLR males manifest alterations in sexual motivation, similar to that reported in humans experiencing sexual dysfunction.
Another neurotransmitter system known to stimulate sexual motivation is oxytocin (Pfaus, 2009). Oxytocin is a neuropeptide found in the hypothalamus that stimulates sexual arousal and induces response to sexual cues. Sexual excitation can be primed by activating the oxytocin system or blunted by inhibiting oxytocin action. For example, sexual incentives and cues previously paired with sexual activity stimulate oxytocin release in the brain of male rats (Hillegaart, Alster, Uvnas-Moberg, & Ahlenius, 1998), which facilitates erection (Kita, Yoshida, & Nishino, 2006). Previous experiments in bHR/bLR females found reduced oxytocin mRNA levels in the hypothalamus of bLRs versus bHRs (S. M. Clinton et al., 2010). If male bLRs also demonstrate a less responsive oxytocinergic system, this could contribute to their reduced sexual motivation.
Sexual Behavior in bHR and bLR males
In addition to apparent bHR/bLR differences in sexual motivation, bHR and bLR males also differ in certain consummatory aspects of copulation – that is, the act of engaging in sexual activity. The most pronounced bHR/bLR difference in sexual performance is the number and timing of intromissions, with bHR males demonstrating more intromissions at a faster pace compared to bLR males. Commercially-available Long Evans rats have been shown to exhibit intromission numbers (per ejaculation) that vary widely based on an animal's type of sexual experience (Ismail, Gelez, Lachapelle, & Pfaus, 2009; Ismail, Zhao, & Pfaus, 2008). Early reports examining male sexual behavior note that the number of intromissions per ejaculation for outbred male rats would be closer to our bLR males than bHR males (Erskine, 1989), indicating that it is the bHR group demonstrating perturbed intromission timing.
bHR males exhibit more intromissions at a faster rate than bLRs. In many rodent species, the identification and processing of appropriate environmental and sensory cues are imperative for successful copulation (Been & Petrulis, 2012; Liu, Salamone, & Sachs, 1997; Meisel & Sachs, 1994). Brain regions that are crucial to this processing include the bed nucleus of the stria terminalis (BNST) and the medial amygdala (MeA). Lesions to these areas increase the number of intromissions preceding ejaculation, and lesions to lateral nuclei in the amygdala increase the rate of copulation (Meisel & Sachs, 1994). It is possible, therefore, that perturbed signaling or responsiveness in the amygdala, BNST, or interconnections between these areas may play a role shaping bHRs’ pattern of sex behavior.
Both bHR and bLR males demonstrate experience-induced plasticity in their sexual behavior. Indeed, all animals show shorter ejaculation latencies both between and within sessions—a pattern of behavior that is typical of laboratory rats (Sachs, Barfield, Jay S. Rosenblatt, & Colin, 1976). Sexual experience produces morphological and functional changes in the related neural circuitry (McEwen, 2010; Pfaus et al., 2012; Pitchers et al., 2010), leading to enhanced reproductive success for the animal as it increases performance. Therefore, while the brains of bHR and bLR males are surely different prior to (and perhaps upon) engaging in sexual activity, it appears that both are capable of learning and improving sexual performance in subsequent copulatory sessions, leading to increased reproductive success.
While the post-ejaculatory refractory period (PERP) analysis did not find an overall phenotypic difference across all copulation days, we did observe an effect of phenotype on copulation day 3 (with a trend on day 2), where bLR males demonstrated shorter refractory periods than bHRs. This difference could be related to altered glutamatergic signaling in the MPOA of bLRs or bHRs during or immediately following sexual activity. The MPOA is imperative in the display of male sexual behavior, and levels of glutamate in particular have been linked to PERP duration (Dominguez, Gil, & Hull, 2006; Dominguez & Hull, 2005). Specifically, glutamate increases in the male MPOA during sexual activity and drops during the refractory period; the magnitude of this drop correlates with the PERP duration. Thus, bLRs’ shortened duration to reinitiate sex after ejaculation may result from an attenuated drop in glutamate, stemming from either reduced glutamate release during ejaculation or increased levels of glutamate in the MPOA during the refractory period (i.e., enhanced released or attenuated reuptake of glutamate). As increased MPOA glutamate levels have also been associated with enhanced sexual performance (Dominguez et al., 2006), and here the bLR males exhibit reduced sexual performance on some of the measures, it is more likely that their reduced PERP is caused by a lower glutamate peak during ejaculation than enhanced glutamate release during the PERP.
Serotonin has also been implicated in sexual satiety in humans and animals (Pfaus, 2009; Stahl, 2010). As mentioned above, an earlier study found altered activation of certain serotonergic cell groups in bLR versus bHR males, as well as altered expression of key molecules involved in serotonin neurotransmission (tryptophan hydroxylase-2, the key synthetic enzyme for serotonin, and the serotonin transporter, which removes serotonin from the synaptic cleft) (Kerman et al., 2011). These serotonin system differences may contribute to a variety of bLRs’ behavioral abnormalities (relative to bHRs), including high anxiety- and depression-like behavior, diminished aggression, as well as their diminished sexual performance. Perhaps bLRs exhibit increased serotonin reuptake or turnover in brain areas relevant for sexual behavior and satiety, which may reduce the refractive period after ejaculation by an early clearing of serotonin.
Sex behavior in bHR/bLR: A potential role for hormones
The observed bHR/bLR differences in sexual behavior may also be driven indirectly by differences in hypothalamic-pituitary adrenal (HPA) stress axis responsivity. Previous experiments demonstrated that non-selectively-bred HR males compared to LR males display lower glucocorticoid receptor mRNA expression in the hippocampus and lower CRH mRNA in the amygdala (Kabbaj et al., 2000), which may contribute to their exaggerated stress-induced corticosterone release and reduced anxiety (Kabbaj et al., 2000; Marquez, Nadal, & Armario, 2006; Piazza et al., 1993; Piazza, Maccari, et al., 1991). Similar differences have been observed in bHR/bLR rats (S.M. Clinton et al., 2008; Kerman et al., 2012). Given the well-known reciprocal interactions between the stress and gonadal axes (Dallman et al., 2002; Handa et al., 1994; Rivier & Rivest, 1991; Viau & Meaney, 1996; Young, 1996), and the importance of androgen action for reproductive function (Arnold & Breedlove, 1985; Hull, Du, Lorrain, & Matuszewich, 1995; McGinnis & Dreifuss, 1989), it is possible that bHR/bLR HPA stress axis differences contribute to differences in gonadal steroid production or sensitivity and, thus, differences in sexual activity. Indeed, testosterone is imperative for the proper display of both sexual behavior and motivation in men (Pfaus, 2009). We report here that bLR males have significantly lighter adrenal glands compared to bHRs, which parallels previous findings and indicates reduced activity of the HPA axis (Kabbaj et al., 2000; Kerman et al., 2012). Since the HPA axis typically opposes hypothalamic-pituitary-gonadal function, it would stand to reason that bLR males, with their lighter adrenals (and reduced HPA activity), would demonstrate enhanced reproductive function. However, we do not find a general enhancement in reproductive function in bLRs, as they take longer to engage in sexual activity upon introduction of the female, and require more exposures to the female before reproducing. Additional studies will be required to further examine how the unique tuning of the bHR vs. bLR HPA axis impacts their sexual performance and motivation.
To determine if differences in sexual activity may be the result of differential androgenic activity between bHR and bLR males, we also harvested and weighed the seminal vesicles as seminal vesicle weight can be used as a marker for androgenic activity (Gonzales, 2001; Swerdloff et al., 1992). While we found that sexual experience increased seminal vesicle weight, we did not find any effect of phenotype on seminal vesicle weight. Thus, there are not likely any differences in circulating androgens between bHR/bLR males. We have not examined the central pattern of androgen receptors however, and a difference in receptor number would make one set of animals more sensitive to androgenic action, which could alter sexual activity.
Finally, it is important to consider the potential impact that the differences in bHR/bLR sexual behavior may have on mating success. Indeed, that bHR males engage in sex more quickly than bLRs following exposure to a female may be an advantage; female rats are only sexually receptive for a short time, and therefore, delayed onset of male sexual activity could result in fewer females inseminated. On the other hand, timing of intromissions for a successful mating is also important. Female rats prefer a longer period of time between intromissions in order to induce the progestational reflex, which enhances implantation of the fertilized embryo (Erskine, 1989). In this case, bLR males may be considered to have a reproductive advantage since their timing between sexual contacts is closer to the rate preferred by females. However, care must be taken when generalizing the exhibition of sexual activity in the laboratory to mating success in an evolutionary context, as rats in the wild mate in groups and the timing of their interactions (as well as the display of their behavior) can be different to what is observed in the laboratory (McClintock & Anisko, 1982; McClintock, Anisko, & Adler, 1982).
Sexual Behavior in bHR and bLR males: Contribution of early-life experiences
Finally, some of the observed bHR/bLR differences in sexual activity may be related to early-life experiences of the animals. bHR/bLR mothers exhibit distinct maternal styles when caring for their pups, with bHR mothers spending less time licking and grooming their offspring compared to bLR dams (S. M. Clinton et al., 2007). Reduced licking and grooming of rat pups can elicit a variety of physiological and behavioral effects in adult offspring (Meaney, 2001), including lasting effects on sexual behavior (Cameron et al., 2005; Moore, 1984). For example, decreased licking and grooming has been shown to lead to increased ejaculation latencies and inter-intromission intervals in males. While these changes are not an exact parallel to what is seen in bHR males, maternal care is an important way in which sexual behavior can be altered and should be considered as a potential mechanism when considering perturbations in sexual behavior.
Conclusions
Sexual dysfunction is a common symptom of major depression and anxiety, with patients frequently experiencing a range of sexual problems including reduced libido, difficulty with arousal, and/or difficulty achieving orgasm (Kennedy & Rizvi, 2009; Laurent & Simons, 2009). These effects can result not only from the condition, but also from the medications prescribed to treat such a condition (Stahl 2010). Results from animal studies parallel these findings, showing that there is evolutionary conservation of the neural mechanisms controlling sexual perturbations across species. This presents a unique opportunity for clinical and pre-clinical researchers, as it offers a mechanism by which scientists can attempt to further unravel the neurological and behavioral mysteries that present with sexual function and dysfunction. We posit that the bLR rat represents a useful new rodent model of co-morbid anxiety- and depression given their high levels of anxiety- and depressive-like behavior, chronic stress vulnerability, diminished aggression, and reduced responsivity to reward (S. M. Clinton, Stead, et al., 2011; Garcia-Fuster et al., 2012; Kerman et al., 2011; Stead et al., 2006; Stedenfeld et al., 2011). We now show that bLRs also exhibit attenuated sexual behavior compared to bHRs, suggesting that they may be a particularly useful animal model to study the neurobiology of sexual dysfunction in depression.
In summary, a substantial body of work demonstrates how innate differences in novelty reactivity and exploration predicts a variety of behaviors, including response to natural and drug reward (Cummings et al., 2011; Davis et al., 2008; Flagel et al., 2011; Garcia-Fuster et al., 2010; Kabbaj, 2004; Piazza, Deminiere, Le Moal, & Simon, 1989), impulsivity (Flagel et al., 2011; Flagel et al., 2010), aggression (Kerman et al., 2011), anxiety (S. M. Clinton, Stead, et al., 2011; S.M. Clinton et al., 2008; Flagel et al., 2010; Kabbaj et al., 2000; Perez et al., 2009; White et al., 2007), and depressive-like behavior (Stedenfeld et al., 2011). The present study extends these findings, showing that high (bHR) versus low (bLR) novelty-seeking male rats also exhibit differences in sexual behavior and motivation, with bLR males demonstrating reduced sexual motivation and bHR males showing alterations in some measures of sexual behavior. These differences demonstrate that neurobiological differences in rats that affect motivation for drugs of abuse, aggression, and impulsivity also affect sexual motivation and performance.
Acknowledgements
This study was supported by NIMH R00 MH085859-03 (SMC), Office of Naval Research N00014-02-1-0879 (HA and SJW), NIDA PPG 5P01DA021633-02 (HA and SJW), NIH 5R21-DA27924-2 (JAC and JBB) and NIH R01-DA012677 (JBB).
References
- Akana SF, Shinsako J, Dallman MF. Relationships among adrenal weight, corticosterone, and stimulated adrenocorticotropin levels in rats. Endocrinology. 1983;113(6):2226–2231. doi: 10.1210/endo-113-6-2226. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior - a reanalysis. Hormones and Behavior. 1985;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience. 2007;147(2):428–438. doi: 10.1016/j.neuroscience.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Dissociated functional pathways for appetitive and consummatory reproductive behaviors in male Syrian hamsters. Hormones and behavior. 2012;61(2):204–211. doi: 10.1016/j.yhbeh.2011.12.007. doi: 10.1016/j.yhbeh.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32(3):589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends in Neurosciences. 1990;13(7):277–280. doi: 10.1016/0166-2236(90)90109-n. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Bedrosian TA, Abraham AD, Watson SJ, Akil H. Neural and environmental factors impacting maternal behavior differences in high-versus low-novelty-seeking rats. Hormones and Behavior. 2010;57(4-5):463–473. doi: 10.1016/j.yhbeh.2010.02.004. doi: 10.1016/j.yhbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Kerman IA, Orr HR, Bedrosian TA, Abraham AD, Simpson DN, Akil H. Pattern of forebrain activation in high novelty-seeking rats following aggressive encounter. Brain research. 2011;1422:20–31. doi: 10.1016/j.brainres.2011.08.033. doi: 10.1016/j.brainres.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JD, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. The European journal of neuroscience. 2011;34(6):994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Hormones and Behavior. 2007;51(5):655–664. doi: 10.1016/j.yhbeh.2007.03.009. doi:10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33(2):162–177. doi: 10.1016/j.psyneuen.2007.10.012. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of General Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Conrad LCA, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in rat - autoradiographic study of anterior hypothalamus. Journal of Comparative Neurology. 1976;169(2):221–261. doi: 10.1002/cne.901690206. doi: 10.1002/cne.901690206. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Becker JB. Quantitative assessment of female sexual motivation in the rat: Hormonal control of motivation. Journal of neuroscience methods. 2012;204(2):227–233. doi: 10.1016/j.jneumeth.2011.11.017. doi: 10.1016/j.jneumeth.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biology of sex differences. 2011;2:3. doi: 10.1186/2042-6410-2-3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Viau V, Bhatnagar S, Laugero K, Gomez F, Bell ME. Corticotropin-releasing factor (CRF), corticosteroids and stress, energy balance, the brain and behavior. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; New York: 2002. [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacology, biochemistry, and behavior. 2008;90(3):331–338. doi: 10.1016/j.pbb.2008.03.008. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26(6):1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. doi: 26/6/1699 [pii] 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiology & behavior. 2005;86(3):356–368. doi: 10.1016/j.physbeh.2005.08.006. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Hormones and behavior. 1989;23(4):473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14(2):217–232. doi: 10.1016/s0149-7634(05)80222-2. doi: S0149-7634(05)80222-2 [pii] [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (rattus norvegicus) - Effects of preoptic area lesions, castration and testosterone. Journal of Comparative Psychology. 1987;101(4):407–419. doi: 10.1037/0735-7036.101.4.407. [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(2):388–400. doi: 10.1038/npp.2009.142. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Parks GS, Clinton SM, Watson SJ, Akil H, Civelli O. The melanin-concentrating hormone (MCH) system in an animal model of depression-like behavior. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22(8):607–613. doi: 10.1016/j.euroneuro.2011.12.001. doi: 10.1016/j.euroneuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. The European journal of neuroscience. 2010;31(1):79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giargiari TD, Mahaffey AL, Craighead WE, Hutchison KE. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Archives of sexual behavior. 2005;34(5):547–556. doi: 10.1007/s10508-005-6280-y. doi: 10.1007/s10508-005-6280-y. [DOI] [PubMed] [Google Scholar]
- Gonzales GF. Function of seminal vesicles and their role on male fertility. Asian Journal of Andrology. 2001;3:251–258. [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology & behavior. 1994;55(1):117–124. doi: 10.1016/0031-9384(94)90018-3. doi: 0031-9384(94)90018-3 [pii] [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Alster P, Uvnas-Moberg K, Ahlenius S. Sexual motivation promotes oxytocin secretion in male rats. Peptides. 1998;19(1):39–45. doi: 10.1016/s0196-9781(97)00250-7. doi: 10.1016/s0196-9781(97)00250-7. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9(2):121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr., Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. Journal of Neuroscience. 1994;14(10):6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. Involvement of dopamine and excitatory amino acid transmission in novelty-induced motor activity. Journal of Pharmacological and Experimental Theraputics. 1994;269(3):976–988. [PubMed] [Google Scholar]
- Hull EM, Du JF, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area - implications for sexual motivation and hormonal control of copulation. Journal of Neuroscience. 1995;15(11):7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du JF, Lorrain DS, Matuszewich L. Testosterone, preoptic dopamine, and copulation in male rats. Brain Research Bulletin. 1997;44(4):327–333. doi: 10.1016/s0361-9230(97)00211-6. doi: 10.1016/s0361-9230(97)00211-6. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105(1):105–116. doi: 10.1016/s0166-4328(99)00086-8. doi: S0166-4328(99)00086-8 [pii. [DOI] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiology & behavior. 2004;83(2):291–307. doi: 10.1016/j.physbeh.2004.08.018. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hull EM, Warner RK, Bazzett TJ, Eaton RC, Thompson JT, Scaletta LL. D2/D1 ratio in the medial preoptic area affects copulation of male rats. J Pharmacol Exp Ther. 1989;251(2):422–427. [PubMed] [Google Scholar]
- Ismail N, Gelez H, Lachapelle I, Pfaus JG. Pacing conditions contribute to the conditioned ejaculatory preference for a familiar female in the male rat. Physiology & behavior. 2009;96(2):201–208. doi: 10.1016/j.physbeh.2008.09.013. doi: 10.1016/j.physbeh.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Ismail N, Zhao Y, Pfaus JG. Context-dependent acquisition of copulatory behavior in the male rat: role of female availability. Behavioral Neuroscience. 2008;122(5):991–997. doi: 10.1037/a0012831. doi: 10.1037/a0012831. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Archives of Neurology. 2004;61(7):1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Akil H. Individual differences in novelty-seeking behavior in rats: a c-fos study. Neuroscience. 2001;106(3):535–545. doi: 10.1016/s0306-4522(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience. 2000;20(18):6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. Journal of clinical psychopharmacology. 2009;29(2):157–164. doi: 10.1097/JCP.0b013e31819c76e9. doi: 10.1097/JCP.0b013e31819c76e9. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Bedrosian TA, Abraham AD, Rosenthal DT, Akil H, Watson SJ. High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain research. 2011 doi: 10.1016/j.brainres.2011.08.038. doi: 10.1016/j.brainres.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology. 2012;37(2):256–269. doi: 10.1016/j.psyneuen.2011.06.010. doi: 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita I, Yoshida Y, Nishino S. An activation of parvocellular oxytocinergic neurons in the paraventricular nucleus in oxytocin-induced yawning and penile erection. Neuroscience Research. 2006;54(4):269–275. doi: 10.1016/j.neures.2005.12.005. doi: 10.1016/j.neures.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. doi: 10.1016/s0893-133x(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laurent SM, Simons AD. Sexual dysfunction in depression and anxiety: conceptualizing sexual dysfunction as part of an internalizing dimension. Clinical psychology review. 2009;29(7):573–585. doi: 10.1016/j.cpr.2009.06.007. doi: 10.1016/j.cpr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci. 1999;11(11):4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behavioral Neuroscience. 1997;111(6):1361–1367. doi: 10.1037//0735-7044.111.6.1361. doi: 10.1037//0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz M, Neveu X, Blecha L, Falissard B, Reynaud M, Gasquet I. Pathways to substance-related disorder: a structural model approach exploring the influence of temperament, character, and childhood adversity in a national cohort of prisoners. Alcohol Alcohol. 2008;43(3):287–295. doi: 10.1093/alcalc/agm183. doi: agm183 [pii] 10.1093/alcalc/agm183. [DOI] [PubMed] [Google Scholar]
- Marquez C, Nadal R, Armario A. Influence of reactivity to novelty and anxiety on hypothalamic-pituitary-adrenal and prolactin responses to two different novel environments in adult male rats. Behav Brain Res. 2006;168(1):13–22. doi: 10.1016/j.bbr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- McClintock Martha K., Anisko Joseph J. Group mating among Norway rats I. Sex differences in the pattern and neuroendocrine consequences of copulation. Animal Behaviour. 1982;30(2):398–409. doi: http://dx.doi.org/10.1016/S0003-3472(82)80051-1. [Google Scholar]
- McClintock Martha K., Anisko Joseph J., Adler Norman T. Group mating among Norway rats II. The social dynamics of copulation: Competition, cooperation, and mate choice. Animal Behaviour. 1982;30(2):410–425. doi: http://dx.doi.org/10.1016/S0003-3472(82)80052-3. [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. In: Mehler MF, Malaspina D, editors. Epigenetics and Neuropsychiatric Diseases: Mechanisms Mediating Nature and Nurture. Vol. 1204. Wiley-Blackwell; Malden: 2010. pp. E38–E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Dreifuss RM. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinology. 1989;124(2):618–626. doi: 10.1210/endo-124-2-618. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meisel Robert L., Sachs Benjamin D. The physiology of male sexual behavior. Raven Press {a}; 1185 Avenue of the Americas, New York, New York 10036-2806, USA: 1994. [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Developmental Psychobiology. 1984;17(4):347–356. doi: 10.1002/dev.420170403. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29(19):6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. doi: 29/19/6379 [pii] 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D. Nature of sex hormone effects on rat sex behavior - specificity of effects and individual patterns of response. Journal of Comparative and Physiological Psychology. 1970;73(3):349–&. doi: 10.1037/h0030242. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. doi: JSM1309 [pii] 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG. Dopamine: helping males copulate for at least 200 million years: theoretical comment on Kleitz-Nelson et al. (2010). Behav Neurosci. 2010;124(6):877–880. doi: 10.1037/a0021823. discussion 881-873. doi: 2010-24688-009 [pii] 10.1037/a0021823. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain research. 1990;530(2):345–348. doi: 10.1016/0006-8993(90)91309-5. doi: 0006-8993(90)91309-5 [pii] [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Research Bulletin. 1997;44(4):397–407. doi: 10.1016/s0361-9230(97)00219-0. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, Parada M. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Archives of sexual behavior. 2012;41(1):31–62. doi: 10.1007/s10508-012-9935-5. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Differential effects of dopamine receptor antagonists on the sexual behavior of male rats. Psychopharmacology (Berl) 1989;98(3):363–368. doi: 10.1007/BF00451688. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105(5):727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proceedings of the National Academy of Science of the USA. 1993;90(24):11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences of the USA. 1991;88(6):2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991;567(1):169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biological psychiatry. 2010;67(9):872–879. doi: 10.1016/j.biopsych.2009.09.036. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45(4):523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien MLAV, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. Journal of Neuroscience. 2002;22(23):10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Phillips PEM, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001;12(11):2549–2552. doi: 10.1097/00001756-200108080-00051. doi: 10.1097/00001756-200108080-00051. [DOI] [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Research Bulletin. 1999;48(6):595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10(12):3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Sachs Benjamin D., Barfield Ronald J., Jay S, Rosenblatt Robert A., Hinde Evelyn Shaw, Colin Beer. Functional analysis of masculine copulatory behavior in the rat Advances in the Study of Behavior. Vol. 7. Academic Press; 1976. pp. 91–154. [Google Scholar]
- Serretti A, Mandelli L, Lorenzi C, Landoni S, Calati R, Insacco C, Cloninger CR. Temperament and character in mood disorders: influence of DRD4, SERTPR, TPH and MAO-A polymorphisms. Neuropsychobiology. 2006;53(1):9–16. doi: 10.1159/000089916. doi: 89916 [pii] 10.1159/000089916. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Circuits of sexual desire in hypoactive sexual desire disorder. The Journal of clinical psychiatry. 2010;71(5):518–519. doi: 10.4088/JCP.10bs06115whi. doi: 10.4088/JCP.10bs06115whi. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36(5):697–712. doi: 10.1007/s10519-006-9058-7. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & behavior. 2011;103(2):210–216. doi: 10.1016/j.physbeh.2011.02.001. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdloff Ronald S., Wang Christina, Hines Melissa, Gorski Roger. Effect of androgens on the brain and other organs during development and aging. Psychoneuroendocrinology. 1992;17(4):375–383. doi: 10.1016/0306-4530(92)90042-6. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Bormans G, Casteels C, Mortelmans L, de Hoon J, Pieters G. Relationship of type 1 cannabinoid receptor availability in the human brain to novelty-seeking temperament. Arch Gen Psychiatry. 2009;66(2):196–204. doi: 10.1001/archgenpsychiatry.2008.530. doi: 66/2/196 [pii] 10.1001/archgenpsychiatry.2008.530. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16(5):1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Research. 2007;1149:141–148. doi: 10.1016/j.brainres.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA. Sex differences in response to exogenous corticosterone: a rat model of hypercortisolemia. Mol Psychiatry. 1996;1(4):313–319. [PubMed] [Google Scholar]