Abstract
Purpose
Our objective was to evaluate the pharmacokinetics (PK) of doxorubicin during pregnancy compared to previously published data from non-pregnant subjects.
Methods
During mid- to late-pregnancy, serial blood and urine samples were collected over 72 hours from 7 women treated with doxorubicin for malignancies. PK parameters were estimated using noncompartmental techniques. Pregnancy parameters were compared to those previously reported non-pregnant subjects.
Results
During pregnancy, mean (± SD) doxorubicin PK parameters utilizing 72 hour sampling were: clearance (CL), 412 ± 80 mL/min/m2; steady-state volume of distribution (Vss), 1132 ± 476 L/m2; and terminal half-life (T1/2), 40.3 ± 8.9 hr. The BSA-adjusted CL was significantly decreased (p < 0.01) and T1/2 was not different compared to non-pregnant women. Truncating our data to 48 hours, PK parameters were: CL, 499 ± 116 ml/min/m2; Vss, 843 ± 391 L/m2; and T1/2, 24.8 ± 5.9 hr. The BSA-adjusted CL in pregnancy compared to non-pregnant data was significantly decreased in 2 of 3 non-pregnant studies (p < 0.05, < 0.05, NS). Vss and T1/2 were not significantly different.
Conclusions
In pregnant subjects, we observed significantly lower doxorubicin CL in our 72 hour and most of our 48 hour sampling comparisons with previously reported non-pregnant subjects. However, the parameters were within the range previously reported in smaller studies. At this time, we cannot recommend alternate dosage strategies for pregnant women. Further research is needed to understand the mechanism of doxorubicin pharmacokinetic changes during pregnancy and optimize care for pregnant women.
Keywords: doxorubicin, doxorubicinol, Adriamycin, pregnancy, pharmacokinetics
Introduction
Cancer complicates approximately 1 in 1,000 pregnancies. Chemotherapy for the mother poses risks for the fetus, but treatment cannot always be delayed until after delivery [1]. Reports of safe use of chemotherapeutic agents such as cyclophosphamide and vinca alkaloids in mid- and late-pregnancy exist, but in practice, management is mostly based on case reports and retrospective studies [1]. Body surface area (BSA)-adjusted doses in the same range as utilized in non-pregnant population have been used in pregnant subjects, despite changes in drug pharmacokinetics that may occur during pregnancy. Pharmacokinetic studies have rarely been performed on pregnant women receiving chemotherapy. Only two reports estimating the pharmacokinetic parameters of chemotherapeutic drugs during pregnancy have been published. A case report on a single subject described enhanced clearance (CL) of paclitaxel, compared to literature values, during late pregnancy [2]. These findings were supported by a recent study that found both increased CL and increased volume of distribution (Vd) of paclitaxel and carboplatin in mid- to late-pregnancy [3]. Doxorubicin CL and Vd were reported to not be significantly altered by pregnancy following 48 hours of sampling post-dose [3]. However, the pharmacokinetic analysis did not account for gestational increases in body weight or for active metabolites. More data are required to understand the mechanisms for changes in chemotherapeutic drug disposition during pregnancy and improve dosage guidelines.
Doxorubicin, an anthracycline antibiotic, has been used during pregnancy for the treatment of malignant tumors such as breast cancer, lymphoma, and leukemia [4,5]. In non-pregnant subjects treated with short-term intravenous infusions, doxorubicin’s disposition is usually tri-phasic, with half-lives of less than 5 to 10 minutes, 0.5 to 3 hours, and 24 to 36 hours [6]. Doxorubicin is bound to plasma proteins (74-76%), primarily albumin [7-9]. Previously reported volumes of distribution (mean ± SD) for doxorubicin in non-pregnant subjects with 48 hour sampling duration are 572 ± 215 to 682 ± 433 L/m2 and plasma clearance 492 ± 155 to 677 ± 229 mL/min/m2 [10-12]. In a single study with 72 hour sampling duration, plasma clearance was 598 ± 142 mL/min/m2 [13]. Doxorubicin is eliminated primarily by hepatic metabolism and biliary excretion [6]. The only metabolite regularly found in subjects’ plasma is doxorubicinol, an active metabolite formed primarily by carbonyl reductases and aldoketoreductases [14, 15]. Other inactive doxorubicin metabolites in humans include aglycones, glucuronides, and sulphates. Additionally, one-electron redox cycling of doxorubicin, catalyzed by a number of oxidoreductases, results in the formation of free radicals, which have been associated with the drug’s cardiotoxicity [3, 16, 17].
Doxorubicin accumulates in the placenta, and the parent drugs as well as its metabolites have been detected in fetal tissues [18, 19]. Adverse pregnancy outcomes, including preeclampsia at 28 weeks gestation [20], intrauterine growth restriction [21], and fetal demise [19] have been reported when doxorubicin was administered during mid- or late-pregnancy. However, doxorubicin appears to have a relatively better safety profile for the post-first trimester fetus when compared with other anthracyclines [1]. The lack of optimization of doxorubicin dosage regimen in pregnancy potentially impacts the efficacy and safety for these patients. Given the limited information available, this study was designed to evaluate doxorubicin pharmacokinetics in mid- and late-pregnancy as compared to previously reported data in non-pregnant subjects.
Methods
The study was conducted at the University of Washington and Swedish Medical Centers, Seattle, and the Georgetown University Medical Center, Washington DC, between 2006 and 2010. The protocol was approved by the institutional review boards at each site and conducted in accordance with their guidelines. All subjects gave written informed consent.
Patients
We studied the plasma pharmacokinetics of intravenous doxorubicin in seven pregnant subjects. Subjects were eligible to participate if they were pregnant, at least 18 years of age, had a hematocrit of at least 28%, and received doxorubicin for therapeutic purposes. Blood and urine samples were collected during mid-(19, 22 and 26 weeks gestation) or late-pregnancy (29, 32, 32 and 34 weeks) as well as at 2.6 weeks postpartum (n=1). Subjects received cycles of intravenous doxorubicin as part of combination chemotherapy, according to standard clinical protocols (Table 1). Six subjects received short-term infusions over 3 to 30 minutes (23.4-60.4 mg/m2). One subject received a 48-hour infusion (74.0 mg/m2).
Table 1.
Concomitant medications
| Patient | Chemotherapeutic protocol |
Other medications |
|---|---|---|
| 1 (Late- pregnancy) |
ABVD (every 2 weeks) |
dexamethasone, ondansetron, prenatal vitamins, ferrous sulfate, folic acid |
| 1 (Postpartum) | AVD (every 2 weeks) |
dexamethasone, ondansetron, oxycodone, acetaminophen |
| 2 | CAF (every 3 weeks) |
dexamethasone, ondansetron, ranitidine, lorazepam, acetaminophen, prenatal vitamins, vitamin D, calcium |
| 3 | PAD (every 4 weeks) |
dexamethasone, ondansetron, metoclopramide, prochlorperazine, promethazine, diphenhydramine, midazolam, lorazepam, bupivacaine, fentanyl, acetaminophen, oxycodone, cefpodoxime, mannitol, docusate, caffeine, potassium phosphate, sodium phosphate, calcium carbonate, prenatal multivitamin |
| 4 | AC (every 3 weeks) |
dolasetron, metoclopramide, promethazine, aprepitant, doxylamine, acetaminophen, hydrocodone, polyethylene glycol, multivitamin |
| 5 | ABVD (every 2 weeks) |
dexamethasone, palonosetron, ondansetron, diphenhydramine, prenatal vitamins, vitamin D, ferrous sulfate |
| 6 | AC (every 3 weeks) |
Vitamin D, ranitidine, calcium, magnesium, docusate, amoxicillin/clavulante, probiotics, multivitamin, docusate, palonosetron, dexamethasone, pegfligrastim, digestive enzyme |
|
| ||
| 7 | AC (every 2 weeks) |
dexamethasone, palonosetron, diphenhydramine, famotidine |
A, doxorubicin; B, bleomycin; V, vinblastine; D, dacarbazine; C, cyclophosphamide; F, 5-fluorouracil; P, cisplatin
Sample collection
Serial venous blood samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 48 and 72 hours following the initiation of the doxorubicin infusion. Urine was collected over the 72-hour period following the initiation of doxorubicin administration for estimation of creatinine clearance as well as renal excretion of doxorubicin and doxorubicinol. None of the women had a urinary catheter.
Plasma and urine sample analysis
Plasma and urine samples were stored at −80° C until analysis. Doxorubicin and doxorubicinol concentrations were measured as previously described [22]. The recovery of doxorubicin and doxorubicinol was approximately 94%. The limit of detection of doxorubicin and doxorubicinol was 2 nM; the limit of quantification was 5 nM. The standard curve was linear between 5 and 10,000 nM. The intra-day coefficient of variation was < 5% and the inter-day variation was < 10% based on 5 replicates each.
Pharmacokinetic analysis
The pharmacokinetic parameters of doxorubicin were determined utilizing standard non-compartmental techniques as previously described [23, 24] with WinNonLin, version 5.2.1 (Pharsight Corporation, Mountain View, CA). Compartmental modeling of the data was not performed.
Statistical analysis
Unpaired Student’s T-test with the Welch-Satterthwaite correction for unequal variances was used to compare our pharmacokinetic parameters to previously reported data in non-pregnant subjects [10-13]. Subject 3 received a prolonged doxorubicin infusion and was included only in comparisons of clearance. Subject 1 pregnancy data was included in the statistical comparison to previously published data in non-pregnant individuals, but her postpartum data was not used in statistical comparisons. Results are reported as mean ± standard deviation, with P < 0.05 considered significant.
Comparator studies
Doxorubicin pharmacokinetic studies published from 1978 to 2012 involving 20 or more non-pregnant adult subjects (both adult men and women) with normal liver function were selected as the non-pregnant control group. Sampling durations were either 48 or 72 hours (Table 2). One comparator study with 72 hour sampling duration was compared to our 72 hour sampling results. Three comparator studies with 48 hour sampling durations were compared to our values truncated to 48 hours. Subjects who received doxorubicin as bolus administrations, short-term infusions (3 minutes to 15 minutes), or long-term infusions (45 minutes to 16 hours) were included.
Table 2.
Doxorubicin pharmacokinetics in pregnant women, a single pregnancy study, and in non-pregnant subjects
| Author & Ref | N (n) |
Sample duration (hours) |
Alb (g/L) |
CL (ml/min) | CL (mL/min/m2) |
Vss (L)a | Vss (L/m2)a | T1/2 (hr)a | AUCdoxorubicinol/ AUCdoxorubicina |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Current Study |
Pregnancy | 7 (7) |
72 | 29 ± 4 | 751 ± 184 (577 - 1053) |
412 ± 80 (310 - 536) |
2089 ± 909 (916 - 3097) |
1132 ± 476 (493 - 1778) |
40.3 ± 8.9 (31.4 - 56.8) |
0.45 ± 0.10 (0.33 - 0.61) |
| Hochster et al. [13] |
Non- pregnant |
20 (17) |
72 | ND | ND | 598 ± 142** | ND | ND | 39.5 ± 18.3 | ND |
| Current Study |
Pregnancy | 6 (6) |
48 (truncated) |
29 ± 4 | 918 ± 258 (624 – 1297) |
499 ± 116 (336 – 628) |
1560 ± 730 (662 - 2359) |
843 ± 391 (385 - 1354) |
24.8 ± 5.9 (18.2 - 34.9) |
0.43 ± 0.1 (0.31 – 0.57) |
| Van Calsteren et al. [3] |
Pregnancy | 7 (7) |
48 | ND | 1143 ± 156 | ND | 2486 ± 657 | ND | 25.6 ± 7.7 | ND |
| Robert et al. [10] |
Non- pregnant |
26 (8) |
48 | ND | ND | 492 ± 155 (192 - 918) |
ND | ND | 34.7 ± 16.6 (12.8 - 72.0) |
0.33 ± 0.10 |
| Piscitelli et al. [11] |
Non- pregnant |
31 (11) |
48 | 39 ± 4 | ND | 666 ± 339* | ND | 682 ± 433 | 25.6 ± 16.9 | 1.43 ± 1.44** |
| Rodvold et al. [12] |
Non- pregnant |
21 (13) |
48 | ND | 1229 ± 385 | 677 ± 229* | 1049 ± 432 | 572 ± 215 | 16.2 ± 5.8** | 0.49 ± 0.24 |
Results are reported as mean ± SD (range) when available.
P < 0.05;
P < 0.01
Subject 3 was omitted from the analysis due to interruption of her infusion and limited sampling after termination of infusion.
N (n): number of subjects (number of females); Alb: Albumin; AUC: area under the curve; CL: total body clearance; T1/2: terminal half-life; ND: not determined; Vss: volume of distribution at steady state
Results
Subject population
A total of 7 subjects participated in the study, whose demographics are described in Table 3. All the pregnant women were treated with doxorubicin as a component of their cancer chemotherapeutic regimen, along with antiemetic drugs (n=7) and prenatal vitamins (n=6) (Table 1). As expected, serum albumin concentrations were lower than reference values in all the pregnant subjects (29 ± 4 g/L, compared to reference mean values for non-pregnant adults of 39-44 g/L), and total protein was below normal in 6 of the subjects. Alkaline phosphatase concentration was mildly elevated in Subject 4, as can occur in normal pregnancy (2.5 μkat/L; normal range, 0.6-2.0 μkat/L). Otherwise, hepatic and renal function values were within the normal range.
Table 3.
Patient characteristics
| Pregnant | Pregnant Mean ± SD |
Postpartum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | |
| Age (years) | 31 | 45 | 20 | 34 | 34 | 37 | 34 | 34 ± 7 | 31 |
| Gestational age (weeks) | 29 | 32 | 19 | 26 | 32 | 34 | 22 | 28 ± 6 | 2.6 |
| Body weight (kg) | 75.0 | 100.9 | 62.3 | 69.0 | 81.3 | 72.7 | 52.8 | 73.4 ± 15.2 | 69.0 |
| Body surface area (m2) | 1.80 | 2.19 | 1.73 | 1.74 | 1.91 | 1.87 | 1.51 | 1.82 ± 0.21 | 1.76 |
| Doxorubicin dose (mg) | 42 | 108 | 128 | 100 | 46 | 113 | 88 | 42 | |
| Indication for doxorubicin | Hodgkin’s lymphoma |
Breast cancer |
Osteo- sarcoma |
Breast cancer |
Hodgkin’s lymphoma |
Breast cancer | Breast cancer |
Hodgkin’s lymphoma |
|
Pharmacokinetics
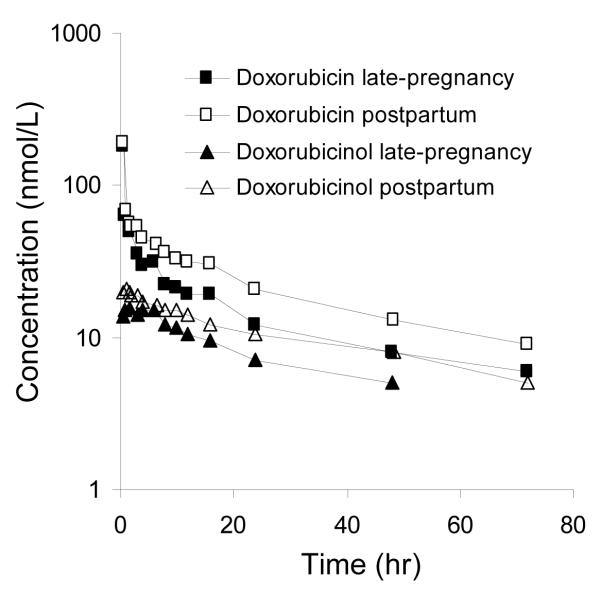
Fig.1 depicts the plasma concentration versus time curves for doxorubicin and doxorubicinol in Subject 1 during late pregnancy and 2.6 weeks postpartum. On both study days, the initial rapid decline in doxorubicin concentration was followed by a slower decline, which became log-linear beyond 24 hours. Doxorubicinol appeared rapidly in plasma and its concentrations decreased in parallel with those of doxorubicin. Doxorubicinol concentrations at 72 hours on the late-pregnancy study day were below the limit of quantification (< 5 nM). The concentration-time profiles of the other subjects who received short intravenous injections were similar. Slow infusion of doxorubicin to Subject 3 resulted in a slow increase in the doxorubicin plasma concentrations and doxorubicinol was not detectable in plasma until 8 hours after the initiation of the infusion. For all subjects, the pre-dose concentrations of doxorubicin were below the limit of detection (2 nM).
Fig.1.
Doxorubicin and doxorubicinol plasma concentration time profiles in Subject 1 during late pregnancy (29 weeks gestation) and 2.6 weeks postpartum. On both study days, the subject received 42 mg of doxorubicin intravenously over 3 or 6 minutes. On the late-pregnancy study day doxorubicinol concentration at 72 was below the limit of quantification
The subjects’ pharmacokinetic parameters are reported in Table 2. The duration of sample collection affected doxorubicin pharmacokinetic parameter estimates. Therefore for statistical comparisons, data was truncated to the duration of sampling in the previously published comparator studies. Utilizing our full 72 hour sample collection, doxorubicin CL was 412 ± 80 mL/min/m2 (range, 310-536 mL/min/m2). The fraction of total AUC that was extrapolated from 72 hours to infinite time was 21.1 ± 8.4% (data not shown). The estimated clearance for Subject 3 (374 mL/min/m2), who was treated with a prolonged doxorubicin intravenous infusion, was within this range. Doxorubicin pharmacokinetic parameters other than CL were not calculated for this subject given the limited sampling following the end of drug administration. Doxorubicin Vss and T 2 1/2 were 1132 ± 476 L/m (range, 493-1778 L/m2) and 40.3 ± 8.9 hr (range, 31.4-56.8 hr), respectively. The AUCdoxorubicinol/AUCdoxorubicin ratio was 0.45 ± 0.10 (range, 0.33-0.61). When comparing the pharmacokinetics of our pregnant subjects to non-pregnant subjects in the literature with 72 hour sampling duration, the BSA-adjusted CL was significantly decreased (p < 0.01) and the terminal half-life was not significantly different. Previously published Vss and AUCdoxorubicinol/AUCdoxorubicin ratio estimates that utilized 72 hour sampling are not available for statistical comparison. When Subject 1 was studied again 2.6 weeks after delivery, her doxorubicin BSA-adjusted clearance and volume of distribution at steady state were 36% and 28% lower than her late-pregnancy values, respectively, and doxorubicin AUC was 57% greater. Half-life remained unchanged.
Utilizing our data truncated to 48 hours, doxorubicin clearance was 499 ± 116 mL/min/m2 (range, 336-628 mL/min/m2). The fraction of total AUC that was extrapolated from 48 hours to infinite time was 19.9 ± 8.3% (data not shown). Doxorubicin Vss and T1/2 were 843 ± 390 L/m2 (range, 385-1354 L/m2) and 24.8 ± 5.9 hr (range, 18.2-34.9 hr), respectively. The AUCdoxorubicinol/AUCdoxorubicin ratio was 0.43 ± 0.1 (range, 0.31-0.57). When comparing our truncated 48 hour sampling pharmacokinetics of pregnant women to the non-pregnant subjects with 48 hour sample collection, our BSA-adjusted CL was significantly decreased in 2 of 3 comparator studies. Our Vss was not significantly different compared to all three studies. Our half-life and AUCdoxorubicinol/AUCdoxorubicin ratio did not show differences compared to the majority of the previously reported studies.
The mean renal clearance of doxorubicin (n=4) was 178 ± 101 mL/min. The cumulative urinary excretion of doxorubicin and doxorubicinol over the first 72 hours accounted for 19.1 ± 10.2% and 3.6 ± 3.0% of doxorubicin dose, respectively (data not shown). The fraction of the dose collected in the urine (truncated to 48 hours following drug administration) as doxorubicin was 18.9 ± 8.5% (n=5) and doxorubicinol was 3.2 ± 2.2%. In comparison, previous studies reported 3.4 and 9.6% of doxorubicin dose recovered in the urine as the parent compound and 0.8 and 3.3% as the alcohol metabolite over 5 to 7 days following dosing [25, 26].
Patient outcome
All seven women and infants survived pregnancy. Median gestational age at delivery was 37 (range, 27-39) weeks and median birth weight was 2911 (range, 724-3856) g. The infant birth weights ranged between the 5th and 90th percentile of age matched infant girls, with the exception of one infant, born preterm, who was at less than the 2nd percentile of age matched infant girls [27]. Subjects 3 and 6 prematurely delivered infants at 27 5/7 and 36 6/7 weeks, respectively. Subject 4 developed congestive heart failure that was diagnosed 4 months after delivery, following 10 weeks of treatment with trastuzumab for lung metastases. She later developed brain metastases and died 20 months after delivery. Subject 5 briefly experienced hypotension (BP range 77-85/47-49 mmHg) and tachycardia (HR range 117-129 beat per minute) in response to drug administration. The subject was successfully treated with intravenous hydration. Her vital signs were within normal limits 24 hours post-dose. None of the subjects had unusual drops in blood counts and we did not observe any exaggeration of clinical side effects. Temporary bruising was noted at the blood sample collection site for Subject 1, 3, 4, and 6. Subjects 2 and 7 completed the study without any short-term adverse events. Six subjects responded well to their therapy, and the mothers and their infants are alive.
Discussion
Given the rarity of cancer during pregnancy, limited research has been conducted evaluating doxorubicin pharmacokinetics during gestation. Since efficacy and safety are of major concern when administering chemotherapy, understanding the effects of pregnancy on doxorubicin concentrations is important to optimize care. Although we had a small sample size, the findings from this study contribute to the literature by providing doxorubicin pharmacokinetic data on seven additional pregnant subjects. This is the first study to report the concentrations of doxorubicinol, an active metabolite of doxorubicin, in pregnant women. This study also provides doxorubicin pharmacokinetic parameter estimates utilizing a longer sampling duration than previously reported in pregnancy, which allows for more accurate parameter determination [3]. Three previously published studies in non-pregnant subjects (n ≥ 20) collected samples over 48 hours. Only one previously published study in non-pregnant subjects with n ≥ 20 continued sampling for 72 hours. Since duration of administration does not profoundly influence doxorubicin pharmacokinetics, we included studies with IV bolus and prolonged infusions in our comparison studies [16]. Studies including subjects with liver disease were excluded from comparison, as CL decreases significantly with liver impairment [11].
The CL of doxorubicin in our study was significantly lower than reported in the non-pregnant comparator study utilizing 72 hour sampling [13], as well as in 2 out of 3 studies with 48 hour sampling [11, 12]. This was in contrast to Van Calsteren et al., who used a 48 hour sample collection protocol and found no significant differences in CL between 7 women in mid- to late-pregnancy and 5 age-matched, non-pregnant women [3]. The mechanism for the decrease in doxorubicin CL is unknown. Several possible factors might contribute to changes observed during pregnancy. The effects of age and sex on doxorubicin CL have been reported with conflicting results [8, 16]. Although doxorubicin CL has been reported to be slower in women than in men [28], a more recent study by Rudek et al. did not find a significant difference when CL was normalized to BSA [29]. Therefore, we included comparator studies that include adults of either sex. The assay method that we used was similar to the previous studies and is unlikely to have caused the observed differences. Another possible explanation could be drug interactions. It has been suggested that cyclophosphamide slows elimination of doxorubicin through 7-deoxyaglycone formations in rats [30]. However, the 4 subjects who received concurrent cyclophosphamide in our study did not show differences in CL as compared to those who did not receive the drug. We did not find any other significant drug interactions that have been reported to affect doxorubicin CL.
Doxorubicin appears to be an intermediate to high extraction ratio drug; therefore, the decreased CL could potentially be due to alterations in hepatic blood flow, protein binding, and/or enzyme activity. Potential changes in hepatic blood flow are unlikely to explain the decreased CL, because liver blood flow is typically unchanged or increased rather than decreased during pregnancy [31]. In addition, given that decreased protein binding would be expected to cause an increase in CL, our findings are not explained by changes in protein binding. Interestingly, Carbonyl Reductase 1 (CBR1), a major contributor to doxorubicin metabolism [32], has decreased activity in the rat ovary throughout pregnancy [33]. More recently, 17β-estradiol has been shown to decrease CBR1 protein expression in porcine endometrium [34].
However, CBR activity differs between species [16] and whether those changes occur in other tissues (such as the liver) or in humans has not been reported. Of note, if all studies (small and large) reporting adult doxorubicin pharmacokinetics are included, CL values for our subjects fall within the range previously reported in non-pregnant subjects. This is likely explained by the high inter-individual variability reported for doxorubicin pharmacokinetics in the smaller studies [35].
For Subject 1, the only subject for whom we have postpartum data (at 2.6 weeks), we observed that changes in BSA-adjusted CL were in the opposite direction of the population effect. Doxorubicin CL was 56% higher during pregnancy than postpartum. Similarly, Van Calsteren et al. found two patients with 60-98% higher doxorubicin CL during pregnancy compared to postpartum at 6.5 - 7 weeks [3]. In the only available baboon study (n=3), CL was 77% higher during pregnancy compared to postpartum [3]. These findings differ from our pregnancy comparisons with previously published data in non-pregnant subjects. Our study is limited by the fact that we did not have postpartum controls for all our subjects. However, the difference that we have observed at 2.6 weeks postpartum may reflect intra-individual variability in doxorubicin pharmacokinetics or possibly 2.6 weeks is not long enough postpartum for CBR1 activity to return to baseline.
Renal elimination appears to play a larger role in doxorubicin elimination in pregnant subjects than in non-pregnant subjects. In pregnancy, renal plasma flow, glomerular filtration rate, and P-glycoprotein (P-gp) mediated net renal tubular secretion increases [36, 37]. Consistent with this, the fraction of the dose collected in the urine over the first 48 hours following doxorubicin administration was 18.9 ± 8.5% (n=5) as compared to previous reports of 3.4 and 9.6%. In addition, the fraction of the dose collected in the urine over 48 hours as doxorubicinol was 3.2 ± 2.2% as compared to 0.8 and 3.3% over 5 to 7 days from dosing [25, 26]. The fraction of the dose collected in the urine over the first 72 hours was 19.1 ± 10.2% as doxorubicin and 3.6 ± 3.0% as doxorubicinol (n=4). Although doxorubicin is predominantly metabolized by the liver and excreted in the bile, the increased renal elimination during pregnancy to some extent counteracts the decreased extra-renal clearance.
Elis et al. recently reported on the association between doxorubicin AUC and clinical response in 19 non-pregnant adult patients with Hodgkin’s lymphoma [38]. Using the same method to estimate the AUC in our subjects who were treated with short-term infusion, the values were within or greater than the range previously reported (274-742 ng•hr/mL). Our mean doxorubicinol/doxorubicin AUC ratio (0.45 ± 0.1) was not significantly different than that reported for non-pregnant subjects in most previous studies [6, 10-13]. Breast cancer patients who receive chemotherapy during pregnancy appear to have a similar outcome, stage for stage, as non-pregnant patients. Notably, recent results from the Cancer and Pregnancy Registry (n=130) demonstrated that survival rates at 3 years for pregnant women diagnosed with stages I to III breast cancer appear comparable with non-pregnant women [39]. Doxorubicin is also a substrate of the multidrug resistance transporter, P-gp. Pregnancy appears to increase renal P-gp activity [36]. Although P-gp activity in the intestine and liver have not been tested, if they respond in a similar manner as in the kidneys, then plasma concentrations for P-gp substrates are likely to be reduced. The impact of pregnancy on P-gp activity in tissues involved in doxorubicin elimination as well as in tumor cells remains to be investigated.
Subject 4, who received doxorubicin as a short-term infusion with concurrent cyclophosphamide and trastuzumab during her pregnancy, developed cardiotoxicity after her delivery. Cardiotoxicity is the main adverse effect of doxorubicin. Increasing the duration of doxorubicin administration significantly decreased the risk of congestive heart failure, likely due to decreased peak plasma concentration [40]. In other studies, CBR-mediated intracellular conversion of doxorubicin to doxorubicinol has been suggested as an important factor in the development of doxorubicin cardiotoxicity. Although human studies are lacking, mice with CBR1 overexpression showed severe myocardial damage with doxorubicin administration. Inhibition of CBR1 decreased circulating levels of doxorubicinol, decreasing cardiotoxicity [41].
The volume of distribution at steady state was not reported with the comparator study utilizing 72 hour sample collection. However, the comparator studies that collected samples for 48 hours did report Vss values, which were not significantly different from our data. This was in agreement with the findings by Van Calsteren et al. in both baboons and humans [3]. Doxorubicin is a hydrophilic drug. In a normal pregnancy, the volume of distribution of hydrophilic drugs is expected to increase due to the expanded intravascular and extravascular water. The estimated gestational increase in total body water ranges from 6.3 – 8.5 L, which accounts for ~60% of gestational weight gain [42]. This would increase the total body water by 15-20% over normal in non-pregnant subjects. Although this is a substantial increase in total body water, it is a small increase relative to doxorubicin volume of distribution > 1000 L. Therefore, pregnancy does not appear to alter the Vss of doxorubicin.
When examining the full 72 hours of sample collection, doxorubicin T1/2 was not significantly different from values previously reported in non-pregnant subjects. In addition, there was no change in half-life when our data were compared to the majority of previously published studies utilizing 48 hours of sample collection. The duration of sample collection impacts estimation of half-life, with longer sampling time increasing accuracy. Therefore, the comparison with studies utilizing prolonged sample collections should be the most valid.
In summary, these findings are an important contribution to the literature, providing additional data for doxorubicin pharmacokinetics in pregnancy with a longer sampling duration. In addition, this is the first study to report doxorubicinol concentrations in pregnancy; although further metabolite research is recommended. At this time, there is insufficient data to draw generalizable conclusions regarding optimum doxorubicin dosing for pregnant women. Our data from the only subject for whom we have both pregnancy and postpartum data (at 2.6 weeks), the individual change in BSA-adjusted CL are in the opposite direction of the population effect. Doxorubicin pharmacokinetics are highly variable in adults regardless of pregnancy state. Although studying chemotherapy during pregnancy is extremely challenging, further pharmacokinetic and pharmacodynamic data are required to optimize doxorubicin therapy for pregnant women.
Acknowledgements
S. Eyal, T.R. Easterling, S.L. Berg, K.A. Scorsone and M.F. Hebert: grant number U10HD047892 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health and National Center for Research Resources grant numbers M01RR00037 and RR023256. J.G. Umans and M. Miodovnik: grant number U10HD047891 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Abbreviations
- Terminal half-life
T1/2
- AUC
Area under the concentration-time curve
- BSA
Body Surface Area
- P-gp
P-glycoprotein
- Vd
Volume of distribution
- Vss
Volume of distribution at steady state
- CL
Clearance
- SD
standard deviation
- CBR1
Carbonyl Reductase 1
Footnotes
Disclosure: None of the authors have a conflict of interest.
References
- 1.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–91. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 2.Lycette JL, Dul CL, Munar M, et al. Effect of pregnancy on the pharmacokinetics of paclitaxel: a case report. Clin Breast Cancer. 2006;7:322–4. doi: 10.3816/CBC.2006.n.049. [DOI] [PubMed] [Google Scholar]
- 3.Van Calsteren K, Verbesselt R, Ottevanger N, et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: a preclinical and clinical study. Acta Obstet Gynecol Scand. 2010;89:1338–45. doi: 10.3109/00016349.2010.512070. [DOI] [PubMed] [Google Scholar]
- 4.Doll DC, Ringenberg QS, Yarbro JW. Management of cancer during pregnancy. Arch Intern Med. 1988;148:2058–64. [PubMed] [Google Scholar]
- 5.Donegan WL. Cancer and pregnancy. CA Cancer J Clin. 1983;33:194–214. doi: 10.3322/canjclin.33.4.194. [DOI] [PubMed] [Google Scholar]
- 6.Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet. 1988;15:15–31. doi: 10.2165/00003088-198815010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chassany O, Urien S, Claudepierre P, Bastian G, Tillement JP. Comparative serum protein binding of anthracycline derivatives. Cancer Chemother Pharmacol. 1996;38:571–3. doi: 10.1007/s002800050529. [DOI] [PubMed] [Google Scholar]
- 8.Eksborg S, Ehrsson H, Ekqvist B. Protein binding of anthraquinone glycosides, with special reference to adriamycin. Cancer Chemother Pharmacol. 1982;10:7–10. doi: 10.1007/BF00257228. [DOI] [PubMed] [Google Scholar]
- 9.Greene RF, Collins JM, Jenkins JF, Speyer JL, Myers CE. Plasma pharmacokinetics of adriamycin and adriamycinol: implications for the design of in vitro experiments and treatment protocols. Cancer Res. 1983;43:3417–21. [PubMed] [Google Scholar]
- 10.Robert J, Bui NB, Vrignaud P. Pharmacokinetics of doxorubicin in Sarcoma Patients. Eur J Clin Pharmacol. 1987;31:695–699. doi: 10.1007/BF00541297. [DOI] [PubMed] [Google Scholar]
- 11.Piscitelli SC, Rodvold KA, Rushing DA, Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther. 1993;53:555–61. doi: 10.1038/clpt.1993.69. [DOI] [PubMed] [Google Scholar]
- 12.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6:1321–7. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 13.Hochster H, Liebes L, Wadler S, et al. Pharmacokinetics of the cardioprotector ADR-529 (ICRF-187) in escalating doses combined with fixed-dose doxorubicin. J Natl Cancer Inst. 1992;84:1725. doi: 10.1093/jnci/84.22.1725. [DOI] [PubMed] [Google Scholar]
- 14.Kassner N, Huse K, Martin HJ, et al. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab Dispos. 2008;36:2113–20. doi: 10.1124/dmd.108.022251. [DOI] [PubMed] [Google Scholar]
- 15.Robert J. Anthracyclines. In: Grochow LB, Ames MM, editors. A clinician’s guide to chemotherapy pharmacokinetics and pharmacodynamics. Williams & Wilkins; Baltimore: 1998. pp. 93–173. [Google Scholar]
- 16.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 17.Takanashi S, Bachur NR. Adriamycin metabolism in man. Evidence from urinary metabolites. Drug Metab Dispos. 1976;4:79–87. [PubMed] [Google Scholar]
- 18.d’Incalci M, Broggini M, Buscaglia M, Pardi G. Transplacental passage of doxorubicin. Lancet. 1983;1:75. doi: 10.1016/s0140-6736(83)91614-8. [DOI] [PubMed] [Google Scholar]
- 19.Karp GI, von Oeyen P, Valone F, et al. Doxorubicin in pregnancy: possible transplacental passage. Cancer Treat Rep. 1983;67:773–7. [PubMed] [Google Scholar]
- 20.Lambert J, Wijermans PW, Dekker GA, Ossenkoppele GJ. Chemotherapy in non-Hodgkin’s lymphoma during pregnancy. Neth J Med. 1991;38:80–5. [PubMed] [Google Scholar]
- 21.García L, Valcárcel M, Santiago-Borrero PJ. Chemotherapy during pregnancy and its effects on the fetus--neonatal myelosuppression: two case reports. J Perinatol. 1999;19:230–3. doi: 10.1038/sj.jp.7200138. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PA, Rosner GL, Matthay KK, et al. Impact of body composition on pharmacokinetics of doxorubicin in children: A glaser pediatric research network study. Cancer Chemother Pharmacol. 2009;64:243–51. doi: 10.1007/s00280-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 23.Hebert MF, Roberts JP, Prueksaritanont T, Benet LZ. Bioavailability of cyclosporine with concomitant rifampin administraiton is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther. 1992;52:453–7. doi: 10.1038/clpt.1992.171. [DOI] [PubMed] [Google Scholar]
- 24.Hebert MF, Carr DB, Anderson GD, Blough D, Green GE, Brateng DA, Kantor E, Benedetti TJ, Easterling TR. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45:25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin RS, Riggs CE, Bachur NR. Pharmacokinetics and metabolism of adriamycin in man. Clin Pharmacol Ther. 1973;14:592–600. doi: 10.1002/cpt1973144part1592. [DOI] [PubMed] [Google Scholar]
- 26.Riggs CE, Benjamin RS, Serpick AA, Bachur NR. Bilary disposition of adriamycin. Clin Pharmacol Ther. 1977;22:234–41. doi: 10.1002/cpt1977222234. [DOI] [PubMed] [Google Scholar]
- 27.WHO [Accessed 05 May 2013];Weight for Age Chart- Birth to 6 months girl’s percentiles. 2013 http://www.who.int/childgrowth/standards/cht_wfa_girls_p_0_6.pdf.
- 28.Dobbs NA, Twelves CJ, Gillies H, James CA, Harper PG, Rubens RD. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother Pharmacol. 1995;36:473–6. doi: 10.1007/BF00685796. [DOI] [PubMed] [Google Scholar]
- 29.Rudek MA, Sparreboom A, Garrett-Mayer ES, Armstrong DK, Wolff AC, Verweij J, Baker SD. Factors affecting pharmacokinetic variability following doxorubicin and docetaxel-based therapy. Eur J Cancer. 2004 May;40(8):1170–8. doi: 10.1016/j.ejca.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Dodion P, Riggs CE, Akman SR, et al. Interactions between cyclophosphamide and adriamycin metabolism in rats. J Pharmacol Exp Ther. 1984;229:51. [PubMed] [Google Scholar]
- 31.Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet. 2002;266:25–9. doi: 10.1007/pl00007495. [DOI] [PubMed] [Google Scholar]
- 32.Oppermann U. Carbonyl reductases: the complex relationships of mammalian carbonyl- and quinone-reducing enzymes and their role in physiology. Annu Rev Pharmacol Toxicol. 2007;47:293–322. doi: 10.1146/annurev.pharmtox.47.120505.105316. [DOI] [PubMed] [Google Scholar]
- 33.Iwata N, Inazu N, Satoh T. Changes and localization of ovarian carbonyl reductase during pseudopregnancy and pregnancy in rats. Biol Reprod. 1990;43:397–403. doi: 10.1095/biolreprod43.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Waclawik A, Jabbour HN, Blitek A, Ziecik AJ. Estradiol-17beta, prostaglandin E2 (PGE2), and the PGE2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium. Endocrinology. 2009;150:3823–32. doi: 10.1210/en.2008-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquet JM, Bressolle F, Galtier M, et al. Doxorubicin and doxorubicinol: intra and inter-individual variations of pharmacokinetic parameters. Cancer Chemother Pharmacol. 1990;39:507. doi: 10.1007/BF00685716. [DOI] [PubMed] [Google Scholar]
- 36.Hebert MF, Easterling TR, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84:248–53. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 37.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–61. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 38.Elis A, Lishner M, Walker S, Atias D, Korenberg A, Koren G. Doxorubicin in lymphoma: association between pharmacokinetic variability and clinical response. Ther Drug Monit. 2010;32:50–2. doi: 10.1097/FTD.0b013e3181c3a16d. [DOI] [PubMed] [Google Scholar]
- 39.Cardonick E, Dougherty R, Grana G, Gilmandyar D, Ghaffar S, Usmani A. Breast cancer during pregnancy: maternal and fetal outcomes. Cancer J. 2010;16:76–82. doi: 10.1097/PPO.0b013e3181ce46f9. [DOI] [PubMed] [Google Scholar]
- 40.Legha SJ, Benjamin RS, McKay B. Reduction of doxorubicin cardiotoxicity by prolonged continuous infusion. Ann Intern Med. 1982;96:133. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 41.Olson L, Bedja D, Alvey S, Cardounel A, Gabrielson K, Reeves R. Protection from Doxorubicin-Induced Cardiac Toxicity in Mice with a Null Allele of Carbonyl Reductase 1. Cancer Research. 2003;63:6602–6606. [PubMed] [Google Scholar]
- 42.Lukaski H, Skiers W, Nielsen E, Hall C. Total body water in pregnancy: assessment by using bioelectrical impedance. Am J Clin Nutr. 1994;59:578–85. doi: 10.1093/ajcn/59.3.578. [DOI] [PubMed] [Google Scholar]