Summary
Sox9 expression defines cell progenitors in a variety of tissues during mouse embryogenesis. To establish a genetic tool for cell-lineage tracing and genefunction analysis, we generated mice in which the CreERT2 gene was targeted to the endogenous mouse Sox9 locus. In Sox9CreERT2/+;R26R embryos, tamoxifen activated Cre recombinase exclusively in Sox9-expressing tissues. To determine the suitability of this mouse line for developmental stage-specific gene recombination, we investigated the cellular origins of the cruciate ligaments of the knee joint and the limb tendons, in which precursor cells have not been defined. The cells in these tissues were labeled after tamoxifen treatment before or at the stage of chondrogenic mesenchymal condensation, indicating that ligament and tendon cells originated from Sox9-expressing cells and that cell fate determination occurred at mesenchymal condensation. This mouse line is a valuable tool for the temporal genetic tracing of the progeny of, and inducible gene modification in Sox9-expressing cells. genesis 48:635–644, 2010.
Keywords: Sox9, CreERT2, cell fate, cruciate ligament, limb tendon
INTRODUCTION
The Cre/loxP site-specific DNA recombination system is a powerful tool in the field of mouse genetics. The conditional ablation or induction of specific genes allows the control of gene activity in a tissue- and time-dependent manner, thus permitting analysis of gene-function and cell-lineage tracing in vivo (Zou et al., 1994). To achieve a more precise control of Cre activity both spatially and temporally, ligand-dependent chimeric Cre recombinases have been developed (Feil et al., 1996). A fusion polypeptide of Cre recombinase and a mutant ligand-binding domain of the estrogen receptor, CreERT2, are inserted into the mouse genome, and the synthetic estrogen receptor ligand, tamoxifen, activates Cre recombinase, resulting in the excision of loxP-containing alleles at specific times during embryogenesis and after birth (Indra et al., 1999).
Sox9 is a member of the SOX family of transcription factors that contains a high-mobility group DNA binding domain and was identified as a causative gene of Campomelic dysplasia, a rare skeletal dysplasia associated with XY sex reversal. Campomelic dysplasia is an autosomal-dominant condition caused by haploinsufficiency of the gene via heterozygous mutation in and around SOX9 gene. The distinct clinical features of this disease include a disproportionately short stature, bowing of the limbs, low ears, a depressed nasal bridge, talipes equinovarus, elongated philtrum, and micrognathia (Foster et al., 1994). Our recent cell-lineage tracing analysis using Sox9-Cre knock-in mice indicate that cells in a variety of tissues are derived from Sox9-expressing precursors (Akiyama et al., 2005). Furthermore, the conditional gene ablation of Sox9 using the Cre/loxP-mediated recombination system reveals that Sox9 plays crucial roles in cell-lineage determination at an initial stage of organogenesis, as well as in cell proliferation and differentiation and in the maintenance of cellular phenotypes in a stage-specific manner (Akiyama et al., 2002; Cha-bossier et al., 2004; Mori-Akiyama et al., 2003; Seymour et al., 2007; Stolt et al., 2003). Thus, the control of DNA recombination at different stages during organogenesis using tamoxifen-inducible CreERT2 under the control of the endogenous Sox9 promoter should open new avenues for the study of gene function and cell-lineage tracing.
In this study, we used homologous recombination to introduce a CreERT2 construct, encoding a tamoxifen-inducible Cre, into the 3′ untranslated region (UTR) of the endogenous mouse Sox9 gene and created a Sox9CreERT2 mouse line. We report here that tamoxifen administration to offspring of crosses between Sox9CreERT2 and Rosa26 reporter (R26R) mice (Soriano, 1999) efficiently induced expression of the Cre reporter in Sox9-expressing tissues.
In our previous report, Sox9-expressing mesenchymal cells contribute to the formation of the ligaments, tendons and synovium in Sox9Cre/+;R26R embryos (Akiyama et al., 2005), but the cellular origins of these tissues have not been well defined. Using this mouse line, we found that cells in the cruciate ligament of the knee joint and limb tendons, the Achilles tendon and the patellar tendon, originated from Sox9-expressing precursors.
RESULTS
Generation of a Sox9-CreERT2 Mouse Line
We generated a knock-in allele in mouse ES cells in which a CreERT2 was integrated into the 3′ UTR of the endogenous mouse Sox9 gene (Fig. 1). The construct consisted of a 5′ internal ribosome entry sequence (IRES) followed immediately by the CreERT2 open reading frame with a SV40 polyadenylation sequence. Consequently, the Sox9 mRNA transcribed from the targeted allele incorporated the nucleotide sequence of IRES-CreERT2, thus forming a bicistronic mRNA. Heterozygous Sox9CreERT2/+ mice were viable and fertile, developed normally, and were not smaller than their wild-type littermates. Homozygotes Sox9CreERT2/+ mice were also viable and fertile, indicating that the knock-in gene did not disrupt Sox9 expression, which is essential for normal growth (Bi et al., 2001). Sox9CreERT2/+ mice were maintained by crossing with wild-type C57BL/6 mice and were backcrossed eight times. All heterozygous Sox9CreERT2/+ mice in this study were analyzed on a C57BL/6 genetic background.
FIG. 1.
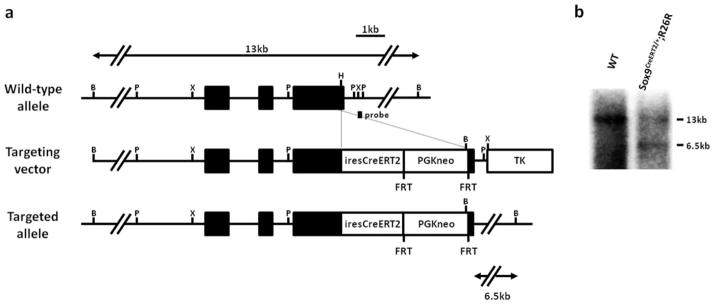
Targeting strategy for the insertion of the tamoxifen-inducible CreERT2 construct into the 3′ UTR of the Sox9 gene. (a) Structure of the genomic Sox9 gene, targeting vector, and targeted allele. Exons are depicted as filled boxes, and intronic sequences are shown as solid lines. IRES-CreERT2-pA and the FRT-PGK-neo-bpA cassettes are depicted as open boxes. The FRT-PGK-neo-bpA cassette was not removed in the final targeted allele. DNA fragments identified in the Southern blot analysis are indicated by double arrows, with the restriction enzymes and the probe. iresCreERT2, IRES-CreERT2-pA; PGKneo, PGK-neo-bpA; B, BamHI; P, PstI; X, XbaI; H, HpaI. (b) Southern blot analysis of genomic DNA isolated from the tails of mice carrying the wild-type and targeted allele. The DNA is digested with BamHI and then hybridized with the 3′ probe. Southern blotting reveales that the wild-type and targeted alleles are detected as 13 kb and 6.5 kb fragments, respectively.
Sox9-specific Cre Induction by Tamoxifen Treatment
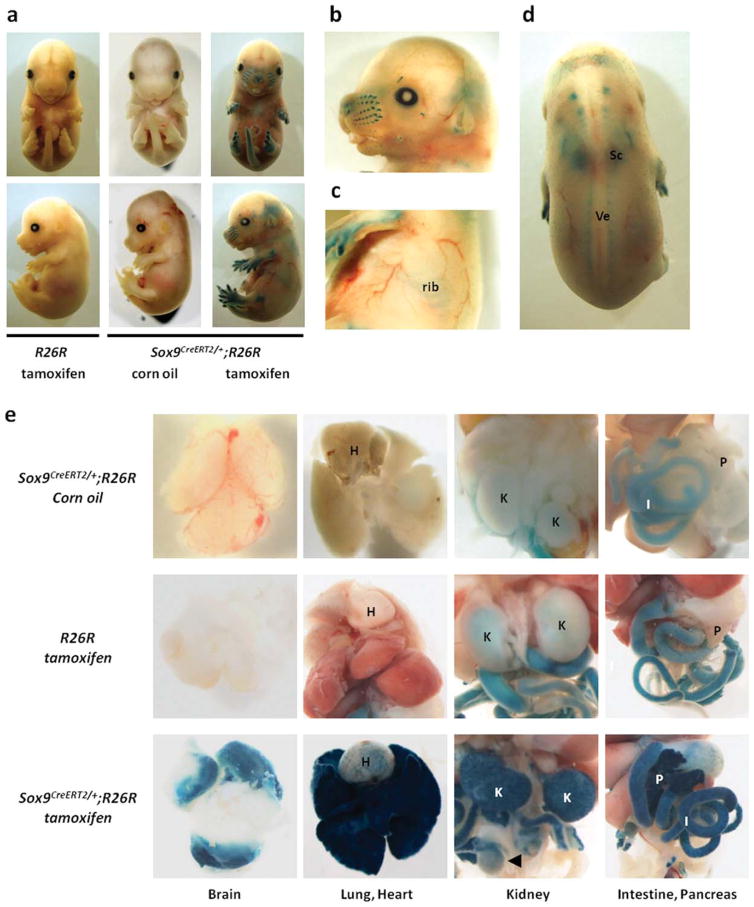
To test the inducible properties of the Cre recombinase, male Sox9CreERT2/+ mice were crossed with female R26R mice. R26R mice carry the LacZ gene inserted into the ubiquitously expressed Rosa26 locus, which is preceded by a transcriptional stop cassette flanked by loxP sites (Soriano, 1999). Pregnant females were injected once with tamoxifen intraperitoneally at embryonic day (E) 13.5, and were sacrificed 48 h after injection. The presence of Cre activity was monitored by detection of β-galactosidase expression using X-gal staining. Strong X-gal staining was detected in the cartilages of the appendicular and axial skeleton, as well as in the whisker follicles and ears of Sox9CreERT2/+;R26R embryos at E15.5. (Fig. 2a–d). The calvariae that were formed via intramembranous ossification were X-gal negative (Fig. 2b). No X-gal staining was observed in control R26R embryos harvested from pregnant females that were injected with tamoxifen and control Sox9CreERT2/+;R26R embryos harvested from pregnant females that were injected with the vehicle alone, i.e., corn oil (Fig. 2a). Sox9-expressing tissues, including brain, heart, lung, kidney, testis, intestine, and pancreas of E16.5 Sox9CreERT2/+;R26R embryos harvested from pregnant females that were injected with tamoxifen at E12.5 were X-gal positive, while weak endogenous β-galactosidase activity was detected in the kidney and intestine of control R26R embryos and control Sox9CreERT2/+;R26R embryos (Fig. 2e). This weak endogenous β-galactosidase activity was nonspecific X-gal staining.
FIG. 2.
Sox9-specific Cre induction by tamoxifen treatment. (a, b, c, d) Tamoxifen or vehicle control is injected into pregnant females at E13.5 and the females are sacrificed 48 h later. (a) X-gal positive staining in Sox9CreERT2/+;R26R embryos injected with tamoxifen is detected in appendicular and axial skeletons. No X-gal staining is observed in R26R embryos injected with tamoxifen and Sox9CreERT2/+;R26R embryos injected with corn oil. (b–d) Large magnification views of Sox9CreERT2/+;R26R embryos injected with tamoxifen. (b) X-gal positive staining is detected in whisker follicles, whereas X-gal staining is absent in calvariae. (c) X-gal positive staining is detected in ribs, (d) scapula (Sc), and vertebrae (Ve). (e) Pregnant females are injected once intraperitoneally with tamoxifen or corn oil at E12.5 and are sacrificed at E16.5. X-gal positive staining is detected in brain, lung, heart, kidney, intestine, pancreas, and testis of Sox9CreERT2/+;R26R embryos injected with tamoxifen. H, K, I, and P indicate heart, kidney, intestine, and pancreas, respectively. The arrow head indicates testis.
Tamoxifen-mediated Cre Induction in Osteo-chondroprogenitor Cells of Limb Buds
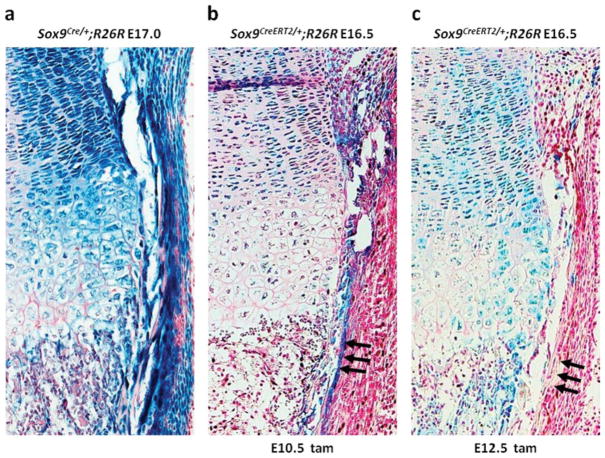
Our recent cell-lineage analysis using Sox9-Cre knock-in mice suggested that Sox9-expressing limb bud mesenchymal cells give rise to both chondrocytes and osteoblasts, indicating that Sox9 defines osteo-chondroprogenitors (Fig. 3a) (Akiyama et al., 2005). Strong X-gal staining was detected in all chondrocytes of the growth plate including hypertrophic chondrocytes of E16.5 Sox9CreERT2/+;R26R embryos harvested from pregnant females that were injected with tamoxifen at E10.5 before chondrogenic mesenchymal condensation and at E12.5 during chondrogenic mesenchymal condensation (Ng et al., 1997) (Fig. 3b, c). However, the positive X-gal staining detected in periosteum, in which preosteoblasts were located, was detected exclusively in embryos from females injected with tamoxifen at E10.5, and not in embryos from females injected at E12.5 (Fig. 3b, c, arrow). This suggests that segregation of osteoblast and chondrocyte lineages occurred in mesenchymal condensation.
FIG. 3.
Identification of the segregation of osteoblastic and chondrogenic lineages in osteo-chondroprogenitor cells using tamoxifen-inducible Cre recombinase. Sagittal section of the tibia harvested at E17.0 from Sox9Cre/+;R26R embryos and at E16.5 from Sox9CreERT2/+;R26R embryos injected with tamoxifen at E10.5 and E12.5. (a) Positive X-gal staining is detected in all chondrocytes of the growth plate and in the periosteum of Sox9Cre/+;R26R embryos. (b) Positive X-gal staining is detected in all chondrocytes of the growth plate and in the periosteum of Sox9CreERT2/+;R26R embryos injected with tamoxifen at E10.5. (c) Positive X-gal staining is detected in all chondrocytes of the growth plate, but not in the periosteum, of Sox9CreERT2/+;R26R embryos injected with tamoxifen at E12.5. Arrows indicate the periosteum.
Stage-specific Cell-lineage Tracing of the Cruciate Ligaments of the Knee Joint Using Sox9CreERT2/+;R26R Mice
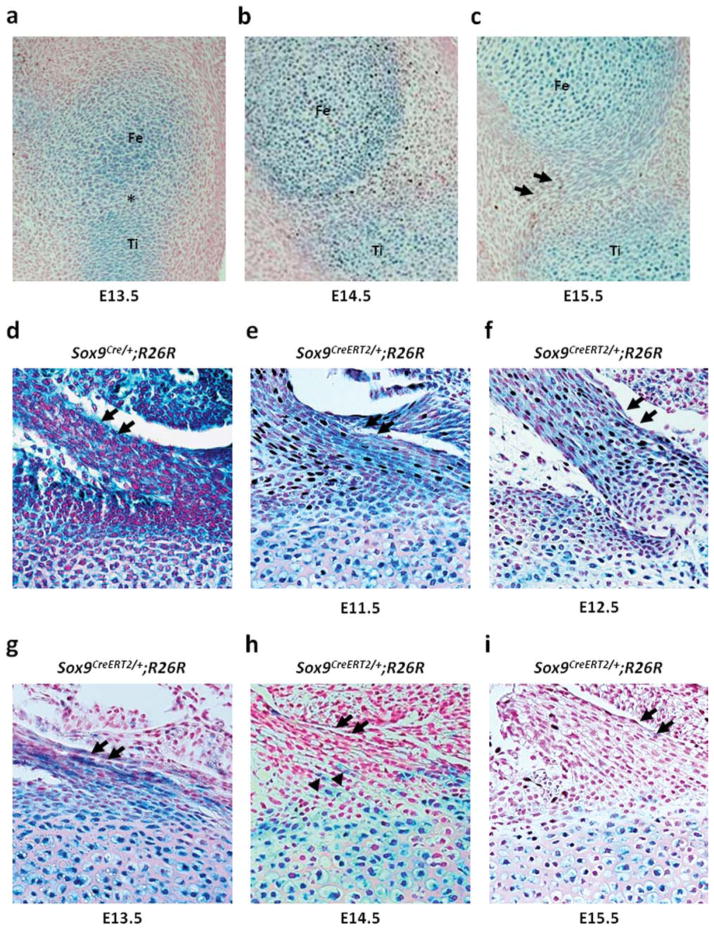
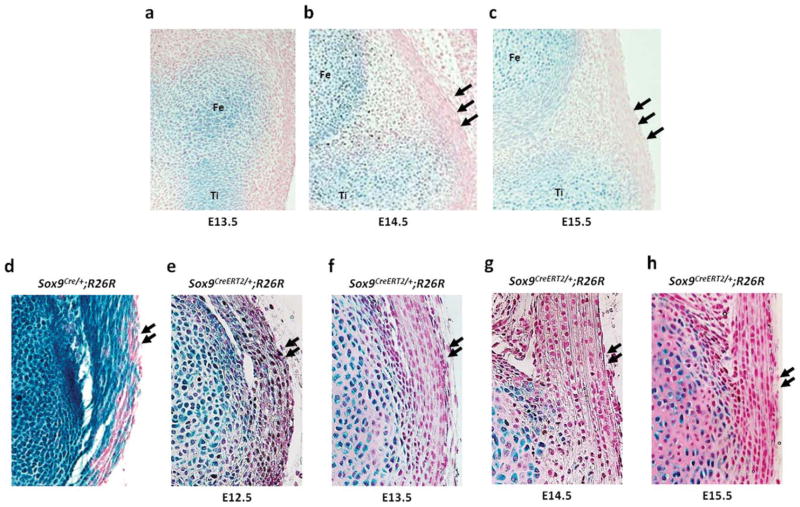
We reported previously that Sox9-expressing mesenchymal cells contribute to the formation of the ligaments, tendons and synovium in Sox9Cre/+;R26R embryos (Akiyama et al., 2005). In Sox9Cre/+;R26R embryos harvested at E17.0, strong X-gal staining was observed in the cruciate ligaments of the knee joint, the Achilles tendon and the patellar tendon (Figs. 4d, 5d, and 6d). In addition, a recent report using Col2a1-Cre/ R26R mice showed that the cruciate ligaments of the knee joint are derived from Col2a1-expressing cells (Hyde et al., 2008). First, we analyzed the expression of Sox9 in the cruciate ligaments via X-gal staining using Sox9-LacZ mice that were generated previously by our group (Bi et al., 1999). At E13.5, Sox9 was expressed in cartilage primordia in the presumptive knee joint of Sox9LacZ/+ embryos. At this stage, the interzone started to form, and Sox9 was expressed in some cells of the interzone (Fig. 4a). At E14.5, Sox9 expression was restricted to chondrocytes, and no expression of Sox9 was observed in the developing joint (Fig. 4b). At E15.5, when the cruciate ligaments form (Hyde et al., 2008), Sox9 expression was absent in the cells of the cruciate ligaments (Fig. 4c).
FIG. 4.
Cell-lineage tracing in the cruciate ligaments of the knee joint. Sagittal sections of the knee joint of Sox9LacZ/+embryos and Sox9CreERT2/+;R26R newborn mice from the pregnant females that are injected once with tamoxifen at different time points between E11.5 and E15.5. (a) Sox9 is detected in cartilage primordia of the femur, tibia, and fibula in the knee joint of E13.5 Sox9LacZ/+embryos. The cells of the interzone between the cartilage primordia exhibit Sox9 expression. (b) At E14.5, Sox9 expression is restricted to chondrocytes. (c) At E15.5, the cruciate ligaments form and the ligament cells do not express Sox9. (d) The cruciate ligament cells are positive in the knee joint of E17.0 Sox9Cre/+;R26R embryos. (e–g) The cruciate ligament cells are positive for X-gal after tamoxifen injection at E11.5, E12.5, and E13.5. (h) The number of labeled cells is markedly decreased in newborn mice that are injected with tamoxifen at E14.5. (i) No labeled cells are detected when tamoxifen is injected at E15.5. Fe, Ti, and Fi indicate the cartilage primordia of the femur, tibia, and fibula, respectively. Arrows indicate the cruciate ligaments. Arrow heads indicate X-gal positive cells.
FIG. 5.
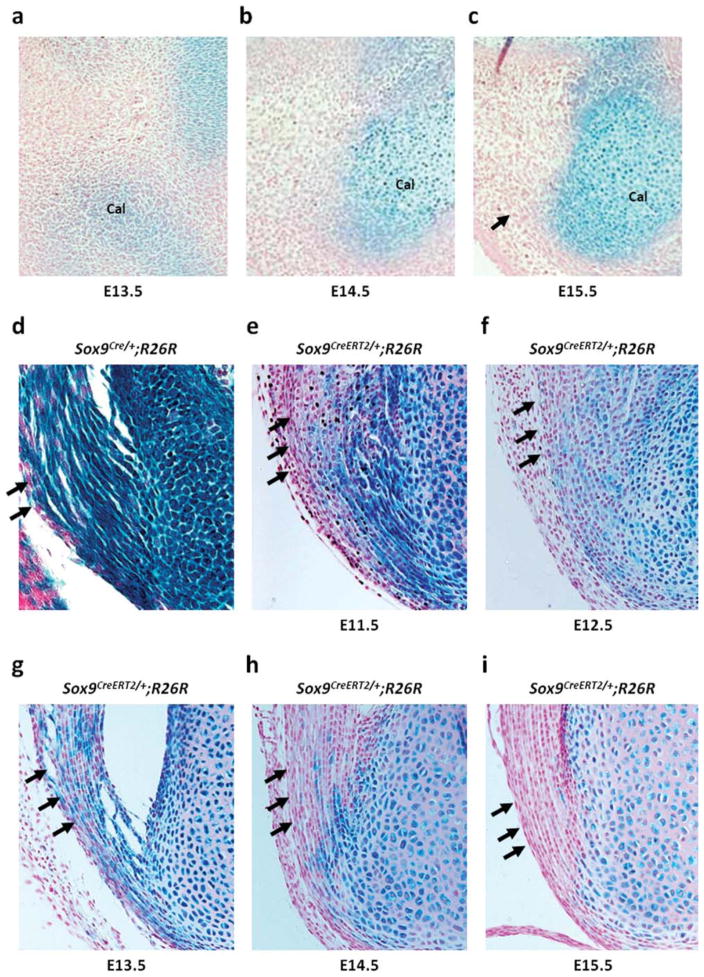
Cell-lineage tracing of the Achilles tendon. Sagittal section of the zeugopod/autopod of the hindlimb of Sox9LacZ/+embryos and Sox9CreERT2/+;R26R new-born mice from pregnant females that are injected once with tamoxifen at different time points from E11.5 to E15.5. (a) Sox9 expression is detected in cartilage primordia of calcaneus of Sox9LacZ/+embryos at E13.5. (b) At E14.5, Sox9 expression is restricted to chondrogenic cells of cartilage primordia. (c) At E15.5, the Achilles tendon form and the cells of the tendon do not express Sox9. (d) The cells of the Achilles tendon of E17.0 Sox9Cre/+;R26R embryos are positive. (e–g) The cells of the Achilles tendon of Sox9CreERT2/+;R26R newborn mice that are injected with tamoxifen at E11.5, E12.5 and E13.5 are stained with X-gal. (h) The number of labeled cells of the Achilles tendon is markedly decreased in newborn mice injected with tamoxifen at E14.5. (i) No labeled cells of the Achilles tendon are detected in mice injected with tamoxifen at E15.5. Cal indicates cartilage primordia of the calcaneus. Arrows indicate the Achilles tendon.
FIG. 6.
Cell-lineage tracing of the patellar tendon. Sagittal section of the knee joint of Sox9LacZ/+embryos and Sox9CreERT2/+;R26R new-born mice from pregnant females that are injected once with tamoxifen at different time points from E12.5 to E15.5. (a) Sox9 expression is detected in cartilage primordia of tibia of Sox9LacZ/+embryos at E13.5. (b, c) At E14.5 and E15.5, no X-gal positive cells in the patellar tendon. (d) The cells of the patellar tendon of E17.0 Sox9Cre/+;R26R embryos are positive. (e) Bony insertions of the patellar tendon to tibia of Sox9CreERT2/+;R26R newborn mice that are injected with tamoxifen at E12.5 is stained with X-gal. (f–h) No labeled cells of the patellar tendon are detected in mice injected with tamoxifen at E13.5, E14.5, and E15.5. Fe and Ti indicate the cartilage primordia of the femur and tibia, respectively. Arrows indicate the patellar tendon.
Next, to determine the origins of the cruciate ligament cells, we examined Sox9CreERT2/+;R26R newborn mice using X-gal staining after single injection of tamoxifen at various time points between E11.5 and E15.5. X-gal positive cells were detected in the cruciate ligaments of Sox9CreERT2/+;R26R newborn mice that were injected at E11.5 before chondrogenic mesenchymal condensation (Fig. 4e). The cruciate ligament cells of Sox9CreERT2/+; R26R newborn mice that were injected with tamoxifen at E12.5 and E13.5 were also positive for X-gal staining (Fig. 4f, g). Tamoxifen injection at E14.5 led to marked decrease in the number of X-gal positive cells which were detected only in and around the bony insertions of the cruciate ligaments (Fig. 4h). No labeled cells were detected in newborn mice that were injected with tamoxifen at E15.5 (Fig. 4i). Thus, these results suggest that the cells in the cruciate ligaments of the knee joint originated from Sox9-expressing condensed mesenchymal cells and that these cells are committed to ligament cells before the joint cavity is formed.
Stage-specific Cell-lineage Tracing of the Limb Tendons of Sox9CreERT2/+;R26R Mice
Tendons are interconnective tissues between muscles and bones. Although specific molecules, including Scler-axis and Tenomodulin, play important roles in tendon development, the cellular origins of the tendons have not been defined. First, we performed cell-lineage tracing of the Achilles tendon, which is the largest tendon in the zeugopod/autopod. Sox9-expressing cells were detected exclusively in cartilage primordia in the auto-pod of the hind limbs of Sox9LacZ/+ embryos at E13.5 and E14.5, at which stages the Achilles tendon was not yet established (Fig. 5a, b). Sox9 expression was negative in the calcaneus with the exception of chondrocytes at E15.5, which is the stage of formation of the Achilles tendon (Fig. 5c). The Achilles tendon cells of Sox9CreERT2/+; R26R newborn mice that were injected tamoxifen at E11.5, E12.5, and E13.5 were X-gal positive (Fig. 5e–g), whereas only a few cells were labeled after tamoxifen injection at E14.5 and X-gal positive cells were only detected at the bony insertions of the Achilles tendon (Fig. 5h). No labeled cells were detected in newborn mice that were injected with tamoxifen at E15.5 (Fig. 5i).
Similar results were observed in the other tendon. The cartilage primordium of tibia was observed in Sox9LacZ/+ embryos at E13.5 (Fig. 6a). At E14.5 the patellar tendon in the stylopod/zeugopod were established, but no X-gal positive cells were observed in this tendon and at a later stage. (Fig. 6b, c). The patellar tendon cells of Sox9CreERT2/+; R26R newborn mice injected tamoxifen at E12.5 were X-gal positive (Fig. 6e). No labeled cells were detected in newborn mice that were injected with tamoxifen at E13.5 and later stages (Fig. 6f–h). Interestingly, X-gal positive cells were extensively found in the Achilles tendon and in patellar tendon when tamoxifen was injected before and just at mesenchymal condensations (Figs. 5g and 6e), but the number of X-gal positive cells rapidly decreased when tamoxifen was injected after mesenchymal condensations. These results indicate that the cells of the tendon originated from Sox9-expressing chondrogenic mesenchymal cells in the cartilage primordia. These cells are committed to the tendon cells at, and just after, mesenchymal condensation. Once the tendon forms and attaches to the cartilage, Sox9-expressing cells in the cartilage primordia no longer contribute to it.
DISCUSSION
Recent mouse genetic approaches using the conditional alleles of the Sox9 gene revealed essential roles for Sox9 in the development of a variety of tissues during mouse embryogenesis, including cartilage, spinal cord, testis, and pancreas (Akiyama et al., 2002; Chabossier et al., 2004; Mori-Akiyama et al., 2003; Seymour et al., 2007; Stolt et al., 2003). In these tissues, Sox9 defines progenitor cells and determines cell-lineages at the initial step of organogenesis. During the skeletal development, genetic ablation of the Sox9 gene results in complete absence of endochondral skeletal elements (Akiyama et al., 2002). In the developing spinal cord, Sox9 expression is detected throughout the ventricular neuroepithelial layer, in which neural progenitor cells are located (Stolt et al., 2003). During the formation of testis, Sox9 is detected in mesenchymal progenitors (Akiyama et al., 2005). In vitro culture of the urogenital ridges of Sox9-null gonads results in gonads that lack testicular cords that initiate ovarian development (Kobayashi et al., 2005). During pancreas development, Sox9 inactivation induces severe pancreatic hypoplasia, which results from the depletion of the progenitor pool (Seymour et al., 2007). On the basis of these mouse genetic studies, we previously generated mice carrying the Cre recombinase gene inserted into the 3′UTR of the Sox9 gene. This mouse line has been useful for the tracing of Sox9-expressing precursors and for specific gene ablation in progenitor cells at the early stage of tissue development (Akiyama et al., 2005).
The expression and functions of Sox9 are also restricted at later stages of tissue development: Sox9 is expressed exclusively in chondrocytes in the skeletons, Sertoli cells in the testis, epithelial cells in the intestine, and ductal cells in the pancreas. Thus, we took advantage of the developmental stage-specific expression of Sox9, to generate mice carrying an inducible form of the Cre recombinase gene, CreERT2, under the control of the endogenous Sox9 promoter. The properties of tamoxifen-inducible Cre recombinase expression were assessed by crossing Sox9CreERT2/+ mice with the Cre-dependent R26R strain (Soriano, 1999). As expected, intra-peritoneal injection of tamoxifen induced genomic recombination exclusively in Sox9-expressing tissues, which included cartilage, brain, lung, heart, intestine, pancreas, kidney and testis. To determine whether the Sox9CreERT2/+ mouse line is useful for cell-lineage tracing at different developmental stages, we confirmed the properties of X-gal positive cells in the limb buds of Sox9CreERT2/+;R26R embryos by tamoxifen treatment at different time points. Positive X-gal staining was observed in all chondrocytes of the growth plate and periosteum, in which the preosteoblasts are located, of E16.5 Sox9CreERT2/+;R26R embryos harvested from pregnant females that were injected with tamoxifen at E10.5, whereas positive X-gal staining was also observed in all chondrocytes and in the cruciate ligaments, but not in periosteum, of E16.5 Sox9CreERT2/+;R26R embryos harvested from pregnant females that were injected with tamoxifen at E12.5. Tamoxifen treatment at different developmental stages of limb buds resulted in the identification of cell-lineage segregation in Sox9CreERT2/+;R26R. Thus, tamoxifen-inducible Cre recombinase expression in Sox9CreERT2/+;R26R mice is useful for developmental-stage-specific cell-lineage tracing in various tissues.
We used the advantage of Sox9CreERT2/+ mice to perform cell-lineage tracing in the cruciate ligaments of the knee joint via stage-specific single injection of tamoxifen into pregnant females. During knee joint formation, the cartilage primordia are separated by the formation of an interzone at E13.5, which becomes a joint cavity (Hyde et al., 2007). The cells in the interzone are flattened and cease to express Col2a1 (Hyde et al., 2008). In Sox9LacZ/+ embryos, Sox9 expression was detected in the interzone cells of the knee joint up to E13.5 and, thereafter, Sox9 expression was detected exclusively in chondrocytes. In contrast, in Sox9CreERT2/+;R26R newborn mice, the cells in the cruciate ligaments were labeled when tamoxifen was injected before E14.5. Thus, these results suggest that the cruciate ligament cells of the knee joint originate from Sox9-expressing chondrogenic cells of the cartilage primordia. This conclusion is in keeping with a recent study using Col2a1-Cre/R26R mice (Hyde et al., 2008). Although many cells in the presumptive joint interzone of these mice do not express Col2a1 at E13.5, all the cells in this zone were derived from resident anlagen cells that expressed Col2a1 previously. These authors concluded that the cruciate ligaments originate from Col2a1-positive cells.
Tendon progenitor cells express Scleraxis (Scx) and are induced between E9.5 and E12.5 in the syndetome and limb bud mesenchyme. During limb bud development, all of the limb tendons are derived from lateral plate mesenchyme, and tendon progenitors are thought to be localized under the ectoderm, in locations that follow the proximal-to-distal outgrowth of the limb bud (Schweitzer et al., 2001). By E12.5, the Scx-expressing cells align as loosely organized progenitors between differentiating muscles and corresponding cartilage condensations, and then condense and differentiate to give rise to overtly distinct tendons, which form by E13.5. However, this assertion is based on the continuity of Scx-expressing cells, and not based on a direct lineage from Scx-expressing cells. (Brent et al., 2003; Murchison et al., 2007; Pryce et al., 2009). In this study, we injected tamoxifen once into Sox9CreERT2/+;R26R pregnant females to determine the origins of the cells of the limb tendons. We observed previously that positive X-gal cells are present in tendons of Sox9Cre/+;R26R embryos (Akiyama et al., 2005), which suggests that Sox9-expressing cells differentiate into tendon cells. Interestingly, although Sox9 expression was not detected in the cells of the limb tendons in Sox9LacZ/+ embryos, tendon cells were labeled in Sox9CreERT2/+;R26R newborn mice if tamoxifen was injected before and at the stage of chondrogenic mesenchymal condensation. The tendon cells of the Achilles tendon were still labeled in Sox9CreERT2/+;R26R newborn mice injected tamoxifen at E14.5, which is the stage of the formation of these tendons. Thus, our results strongly suggest that at least part of the cells of the tendons originate from Sox9-expressing condensed mesenchymal cells of the cartilage primordia and possibly contribute to the formation of the tendon.
We concluded that the Sox9-CreERT2 knock-in mouse line is a very valuable tool for the temporal genetic tracing of cellular progeny and for the inducible genetic activation or inactivation of Sox9-expressing cells.
METHODS
Generation of Sox9-CreERT2 Knock-in Mice
A Sox9 clone was isolated from a mouse 129/SvEv genomic DNA library (Bi et al., 1999; Chen and Behringer, 1995). The targeting vector spanned a 7.7 kb fragment of the Sox9 gene and a 5′IRES-CreERT2-pA/FRT-flanked PGK-neo-bpA cassette was inserted into HpaI site located within the 3′UTR of exon3. An MC1-tk-pA herpes simplex virus thymidine kinase expression cassette was added onto the 3′arm of homology to enrich for homologous recombinants via negative selection with 1-(2′-deoxy-2-fluoro′-β-D-arabinofuranosyl)-5-iodouracil (FIAU). The targeting vector was introduced into 129SvEv AB1 mouse ES cells and G418/FIAU-resistant ES cell clones were initially screened by Southern blot analysis of BamHI-digested genomic DNA by using a 3′ probe that was external to the region of vector homology. Mouse chimeras were generated by injection of mutant ES cell clones into C57BL/6 host blastocysts, and the chimeras obtained were crossed with C57BL/6 mice to generate heterozygous Sox9CreERT2/+ mice. Mouse-tail DNA was used for PCR genotyping. The following primer pairs were used: Cre, 5′-TCCAATTTACTGACCGTACAC CAA-3′ and 5′-CCTGATCCTGGCAATTTCGGCTA-3′ (545 bp fragment) (Ovchinnkov et al., 2000); and R26R, 5-AATGGCTTTCGCTACCTGGAG-3′ and 5′-TGGTGTTTT GCTTCCGTCAGC-3′ (200 bp fragment) (Nakamura et al., 2006).
Tamoxifen Treatment
Tamoxifen-inducible Cre activity was evaluated using the R26R mice. Tamoxifen (T5648, Sigma, St. Louis, MO) was dissolved in ethanol and diluted in corn oil (C8267, Sigma) at a concentration of 10 mg/ml, as described previously (Danielian et al., 1998). Three milligrams of tamoxifen were injected into the peritoneal cavity of pregnant females at the indicated time points.
Histological Analysis
Whole-mount X-gal staining was performed as described previously (Hogan et al., 1994). Decalcification of newborn mice was performed over the period of 1 week using 20% EDTA at 4°C. For the histological analyses, embryos and newborn mice were embedded in paraffin, sectioned at a thickness of 5 μm, and counter-stained with nuclear fast red.
Acknowledgments
We thank Dr. Ronen Schweitzer, Oregon Health and Science University, for critical discussions and reading of the manuscript.
Contract grant sponsor: Japanese Ministry of Education, Culture, Sports, Science, and Technology, Contract grant number: 21249078; Contract grant sponsor: National Institutes of Health, Contract grant number: HD30284
LITERATURE CITED
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somatic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lützkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Goodfellow J, David B, Alan JS. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Hyde G, Boot-Handford RP, Wallis GA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J Anat. 2008;213:531–538. doi: 10.1111/j.1469-7580.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Chang H, Chaboissier MC, Schedl A, Behringer RR. Sox9 in testis determination. Ann N Y Acad Sci. 2005;1061:9–17. doi: 10.1196/annals.1336.003. (Review) [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scler-axis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreERT to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Müller W, Gu H, Rajewsky K. Cre-loxP mediated gene replacement: A mouse strain producing humanized antibodies. Curr Biol. 1994;4:1099–1103. doi: 10.1016/s0960-9822(00)00248-7. [DOI] [PubMed] [Google Scholar]