Abstract
Many poikilothermic vertebrate lineages, especially among amphibians and fishes, possess a rapid turnover of sex chromosomes, while in endotherms there is a notable stability of sex chromosomes. Reptiles in general exhibit variability in sex-determining systems; as typical poikilotherms, they might be expected to have a rapid turnover of sex chromosomes. However, molecular data which would enable the testing of the stability of sex chromosomes are lacking in most lineages. Here, we provide molecular evidence that sex chromosomes are highly conserved across iguanas, one of the most species-rich clade of reptiles. We demonstrate that members of the New World families Iguanidae, Tropiduridae, Leiocephalidae, Phrynosomatidae, Dactyloidae and Crotaphytidae, as well as of the family Opluridae which is restricted to Madagascar, all share homologous sex chromosomes. As our sampling represents the majority of the phylogenetic diversity of iguanas, the origin of iguana sex chromosomes can be traced back in history to the basal splitting of this group which occurred during the Cretaceous period. Iguanas thus show a stability of sex chromosomes comparable to mammals and birds and represent the group with the oldest sex chromosomes currently known among amniotic poikilothermic vertebrates.
Keywords: Anolis, lizard, male heterogamety, molecular sexing, Pleurodonta, qPCR
1. Introduction
In vertebrates, the gonad is not differentiated early in ontogeny and only later develops into testicular or ovarian structures. Although the genetic framework for the differentiation of testes or ovaries is highly conserved [1], the process called sex determination, responsible for the decision whether the undifferentiated gonad will turn into a testis or ovary, is surprisingly variable. However, the rate of turnover of sex-determining mechanisms is notably different among particular vertebrate lineages. Some poikilothermic lineages, such as several well-studied groups of fish or frogs, possess a rapid turnover of sex chromosomes [2,3], while endotherms, i.e. mammals and birds, have highly conserved sex chromosomes. As poikilotherms, reptiles (the term ‘reptiles’ denotes here the paraphyletic group, i.e. sauropsids with the exclusion of their inner avian group) are usually considered as a group with a rapid turnover of sex-determining mechanisms [4,5] and as a whole, they indeed exhibit a large variability in sex-determining systems [6,7]. However, we should keep in mind that reptiles are a very diverse group, with many ancient lineages, and therefore we should be cautious with generalizations. Some lineages of reptiles, e.g. dragon lizards or geckos [7–9], exhibit a large variability in sex determination, but based on the phylogenetic distribution of male and female heterogamety, other lineages may possess conserved sex chromosomes [7,10]. Molecular or molecular-cytogenetic data which would enable the testing of the evolutionary stability of sex chromosomes are, however, lacking for most reptilian lineages. Colubroid snakes represent a notable exception with a demonstration of sex chromosome conservation for at least 40 Ma [11,12].
Here, we focus on the testing of the homology of sex chromosomes across iguanas (Pleurodonta)—the ancient, species-rich (more than 1080 recent species [13]) and highly diversified group of lizards. Karyotype data have shown that, wherever sex chromosomes are known, male heterogamety is present among iguanas, which suggests that iguanas may have conserved sex chromosomes [7]. At the same time, the sex chromosomes are very small (microchromosomes) and morphologically poorly differentiated in many species, so that it is impossible to evaluate the homology of sex chromosomes based only on their appearance. Moreover, other species have exhibited multiple and/or relatively large sex chromosomes, and in many other lineages sex chromosomes (if any) have remained undetected [14]. It is therefore possible that different chromosomes were independently co-opted for the function of sex chromosomes in various lineages of iguanas. Consequently, molecular evidence is needed to test the homology of sex chromosomes and for the evaluation of the evolutionary stability of sex-determining systems across iguanas.
2. Material and methods
For the testing of the homology of sex chromosomes across iguanas, we adopted the strategy recently used by Nguyen et al. [15], which is based on the comparison of the differences in gene dose between male and female genomes using qPCR. The partial genetic content of X chromosomes is known in a single species, Anolis carolinensis (ACA, Dactyloidae) [16], where the Y chromosome is degenerated and known X-linked genes are thus lacking from the Y chromosome. Therefore, females (XX) have twice as many copies of genes linked to the X-specific part of sex chromosomes as males (XY), while genes in autosomal or pseudoautosomal regions have equal copy numbers in both sexes. qPCR procedures including primers, and equations used for estimation of the gene dose of target genes relative to the reference gene and for male-to-female ratio in gene dose, are summarized in the electronic supplementary material, S1.
First, we tested the technique by comparison of sexual differences in gene dose in five genes (ATP2A2, CCDC92, FZD10, PEBP1 and TMEM132D) known to be present on the X chromosome and missing from the Y chromosome in ACA [16]. As a control, we tested a gene (ADARB2) located on chromosome 6 in ACA (ACA6). As an additional control, we tested the male-to-female ratio in gene dose for the same genes in the bearded dragon (Pogona vitticeps), a member of Acrodonta, the first outgroup to iguanas [17]. Both ACAX and ACA6 are homologous to autosomes of the bearded dragon [18], so that no sexual differences in gene doses should be revealed by qPCR in any tested gene in this species. Furthermore, we tested the male-to-female gene dose ratio for the whole set of genes in members of the iguana families Iguanidae, Tropiduridae, Leiocephalidae, Phrynosomatidae, Corytophanidae, Opluridae and Crotaphytidae. In ACA and in the species of basilisks (Corytophanidae), we tested five additional genes (CUX2, GAL3ST1, PPM1F, SH2B3 and ZCCHC8) located on non-assembled scaffolds in ACA (Ensembl database, http://www.ensembl.org), but on chromosome 15 of Gallus gallus (GGA15), which is at least partially homologous to the ACAX chromosome (see the electronic supplementary material, S1). Altogether we tested 32 individuals of known sex (sex was determined by external morphology) of 14 species representing eight families of iguanas (see the electronic supplementary material, S1, for details).
3. Results
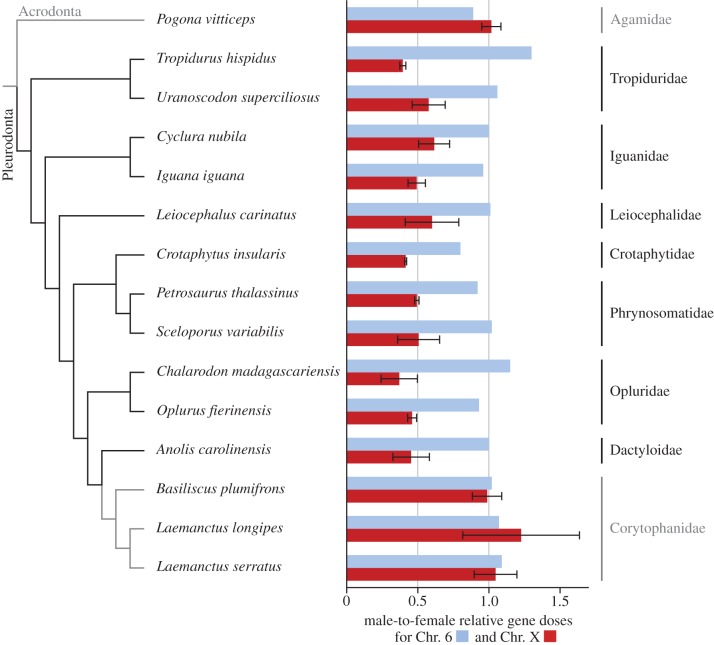
The qPCR results (figure 1; electronic supplementary material, table S1) confirmed the autosomal position of the ADARB2 gene and the X-linkage of the genes ATP2A2, CCDC92, FZD10, PEBP1 and TMEM132D in ACA (Ensembl database, http://www.ensembl.org, [16]). No gene exhibited sexual differences in dose in bearded dragons, which is consistent with their reported autosomal position [18]. As we designed the primers used in the qPCR from the known sequences in ACA and applied them to phylogenetically distant species of iguanas, not all primer pairs bound efficiently in all species. However, the control gene ADARB2 and at least two of the genes linked to the X chromosome in ACA were successfully amplified in each species. Our reference single copy gene EF1a was successfully amplified in all but one of the studied species. The exception was Cyclura nubila (Iguanidae), forcing us to use the autosomal gene ADARB2 for normalization of qPCR values in this species. Among the effectively working genes, the qPCR results of the ACA6 gene were consistent with autosomal or pseudoautosomal positions in other iguanas and, with the exception of basilisks, all ACAX-linked genes appeared to be linked to the X chromosome in other iguanas. The qPCR results are consistent with the X-linkage of the five genes previously located on non-assembled scaffolds in ACA; however, even these additional genes exhibit an autosomal or pseudoautosomal pattern in all three species of basilisks (electronic supplementary material, table S2).
Figure 1.
Relative gene dose ratios between a male and female in 14 species of iguanas and in the bearded dragon. Means ± s.d. for X-linked genes in ACA are depicted in red, values for the control autosomal locus in blue. Value 1.0 is expected for autosomal or pseudoautosomal genes, while the value 0.5 is consistent with X-linkage. Grey colour indicates lineages with autosomal or pseudoautosomal pattern in X-linked genes in ACA. Phylogenetic relationships follow [17]. These data suggest that the sex chromosomes in iguanas evolved in the Cretaceous period and, with the exception of basilisks, have been conserved across iguana evolution. (Online version in colour.)
4. Discussion
Our qPCR results confirm the occurrence of previously reported male heterogamety in the families Dactyloidae, Leiocephalidae, Phrynosomatidae and Tropiduridae [14]. The results are consistent with the presence of male heterogamety in the families Iguanidae, Opluridae and Crotaphytidae, where sex chromosomes have not been previously detected [14]. We can now present evidence that at least part of the X chromosome shares gene content in the overwhelming majority of iguana families and that consequently the sex chromosomes of iguanas are widely conserved across the group (figure 1). Unfortunately, owing to problems with material availability, we were not able to include members of the families Liolaemidae, Hoplocercidae, Polychrotidae and Leiosauridae. Although phylogenetic relationships among iguana families are not stable (cf. very different topologies in [17,19,20]), all of these recent molecular phylogenies consistently place these unsampled families into terminal positions. On the other hand, the three clades (Phrynosomatidae, Tropiduridae, Leiocephalidae) represented in our sample were assigned as sister to all the other families in respective phylogenetic analyses [17,19,20]. As far as known, neither the first (Acrodonta = dragon lizards and chameleons) nor the second (Anguimorpha) outgroup of iguanas [17] possesses a sex-determining system involving sex chromosomes homologous with ACA [7,18]. Therefore, we can conclude that the presence of an X chromosome homologous with ACAX and at least a partially degenerate Y chromosome represents the ancestral situation for recent iguanas, and that these sex chromosomes most likely evolved before the basal splitting of iguanas (reconstructed to ca 73 Ma [19]) and after the divergence of Acrodonta and Pleurodonta (ca 123 Ma [19]).
Basilisks were identified as the only group of iguanas where ACAX-linked genes showed a pattern typical for autosomal or pseudoautosomal positions, although we expanded our analyses to the wider region of their genomes homologous with the ACAX chromosome. Basilisks are deeply nested among iguana families with ancestral sex chromosomes [17,19,20]. Therefore, the situation in basilisks may reflect a rare event of dedifferentiation or the turnover of already differentiated sex chromosomes [21]; however, further research is needed to evaluate this possibility.
In summary, qPCR proved to be extremely useful for testing the homology of sex chromosomes in iguanas. Moreover, the approach described here is suitable for the reliable molecular sexing of a wide spectrum of iguana species. Despite their species richness, wide distribution and enormous ecological and morphological variability, iguanas possess a large degree of stability of sex chromosomes, which most likely evolved during the Cretaceous period. This stability is comparable to the well-known situation in birds and mammals. Although the stability of sex chromosomes has been demonstrated or inferred for other poikilothermic amniotes [10–12], to the best of our knowledge, iguanas represent the case of the oldest sex chromosomes among amniotic poikilothermic vertebrates. The highly unequal rate of turnover of sex-determining mechanisms among different lineages of lizards makes them a fascinating group for studies of the evolution of sex determination.
Acknowledgements
We thank Jan Červenka, Jan Hříbal, Jiří Moravec, František Marec and Romana Stopková for help. Chris Organ and an anonymous referee provided useful comments. This paper represents the eighth part of our series ‘Evolution of sex determining systems in lizards’.
The work was approved by the Ethical Committee of the Czech Republic.
Data accessibility
All results are uploaded as electronic supplementary material.
Funding statement
The project was supported by GAČR (506/10/0718) and GAUK (591712).
References
- 1.Morrish BC, Sinclair AH. 2002. Vertebrate sex determination: many means to an end. Reproduction 124, 447–457 (doi:10.1530/rep.0.1240447) [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi K, Hamaguchi S. 2013. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 242, 339–353 (doi:10.1002/dvdy.23927) [DOI] [PubMed] [Google Scholar]
- 3.Miura I. 2007. An evolutionary witness: the frog Rana rugosa underwent change of heterogametic sex from XY male to ZW female. Sex. Dev. 1, 323–331 (doi:10.1159/000111764) [DOI] [PubMed] [Google Scholar]
- 4.Sarre SD, Georges A, Quinn A. 2004. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. BioEssays 26, 639–645 (doi:10.1002/bies.20050) [DOI] [PubMed] [Google Scholar]
- 5.Organ CL, Janes DE. 2008. Evolution of sex chromosomes in Sauropsida. Integr. Comp. Biol. 48, 512–519 (doi:10.1093/icb/icn041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzuela N, Lance VA, Beggs K, Bull JJ. 2004. Temperature-dependent sex determination in vertebrates. Washington, DC: Smithsonian Institution Scholarly Press [Google Scholar]
- 7.Pokorná M, Kratochvíl L. 2009. Phylogeny of sex-determining mechanisms in squamate reptiles: are sex chromosomes an evolutionary trap? Zool. J. Linn. Soc. Lond. 156, 168–183 (doi:10.1111/j.1096-3642.2008.00481.x) [Google Scholar]
- 8.Ezaz T, Quinn AE, Sarre SD, O'Meally D, Georges A, Graves JAM. 2009. Molecular marker suggests rapid changes of sex determining mechanisms in Australian dragon lizards. Chromosome Res. 17, 91–98 (doi:10.1007/s10577-008-9019-5) [DOI] [PubMed] [Google Scholar]
- 9.Gamble T. 2010. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex. Dev. 4, 88–103 (doi:10.1159/000289578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badenhorst D, Stanyon R, Engstrom T, Valenzuela N. 2013. A ZZ/ZW microchromosome system in the spiny softshell turtle, Apalone spinifera, reveals an intriguing sex chromosome conservation in Trionychidae. Chromosome Res. 21, 137–147 (doi:10.1007/s10577-013-9343-2) [DOI] [PubMed] [Google Scholar]
- 11.Matsubara K, Tarui H, Toriba M, Yamada K, Nishida-Umehara C, Agata K, Matsuda Y. 2006. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and stepwise differentiation of snake sex chromosomes. Proc. Natl Acad. Sci. USA 103, 18 190–18 195 (doi:10.1073/pnas.0605274103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicoso B, Emerson JJ, Zektser Y, Mahajan S, Bachtrog D. 2013. Comparative sex chromosome genomics in snakes: differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biol. 11, e1001643 (doi:10.1371/journal.pbio.1001643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uetz P. (ed.). 2013. The reptile database http://www.reptile-database.org (accessed 8 December).
- 14.Olmo E, Signorino G. 2013. Chromorep: a reptiles chromosomes database http://chromorep.univpm.it (accessed 1 December).
- 15.Nguyen P, Sýkorová M, Šíchová J, Kůta V, Dalíková M, Čapková Frydrychová R, Neven LG, Sahara K, Marec F. 2013. Neo-sex chromosomes and adaptive potential in tortricid pests. Proc. Natl Acad. Sci. USA 110, 6931–6936 (doi:10.1073/pnas.1220372110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alföldi J, et al. 2011. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477, 587–591 (doi:10.1038/nature10390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 (doi:10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young MJ, O'Meally D, Sarre SD, Georges A, Ezaz T. 2013. Molecular cytogenetic map of the central bearded dragon, Pogona vitticeps (Squamata: Agamidae). Chromosome Res. 21, 361–374 (doi:10.1007/s10577-013-9362-z) [DOI] [PubMed] [Google Scholar]
- 19.Townsend TM, Mulcahy DG, Noonan BP, Sites JW, Kuczynski CA, Wiens JJ, Reeder TW. 2011. Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation. Mol. Phylogenet. Evol. 61, 363–380 (doi:10.1016/j.ympev.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 20.Wiens JJ, Hutter CR, Mulcahy DG, Noonan BP, Townsend TM, Sites JW, Jr, Reeder TW. 2012. Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol. Lett. 8, 1043–1046 (doi:10.1098/rsbl.2012.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicoso B, Bachtrog D. 2013. Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499, 332–335 (doi:10.1038/nature12235) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All results are uploaded as electronic supplementary material.