Abstract
The development of hydrogel-based biomaterials represents a promising approach to generating new strategies for tissue engineering and regenerative medicine. In order to develop more sophisticated cell-seeded hydrogel constructs, it is important to understand how cells mechanically interact with hydrogels. In this paper, we review the mechanisms by which cells remodel hydrogels, the influence that the hydrogel mechanical and structural properties have on cell behaviour and the role of mechanical stimulation in cell-seeded hydrogels. Cell-mediated remodelling of hydrogels is directed by several cellular processes, including adhesion, migration, contraction, degradation and extracellular matrix deposition. Variations in hydrogel stiffness, density, composition, orientation and viscoelastic characteristics all affect cell activity and phenotype. The application of mechanical force on cells encapsulated in hydrogels can also instigate changes in cell behaviour. By improving our understanding of cell–material mechano-interactions in hydrogels, this should enable a new generation of regenerative medical therapies to be developed.
Keywords: hydrogel, tissue engineering, adhesion, contraction, mechanobiology, bioreactor
1. Introduction
Over the past several decades, changes in population demographics within the developed world have shown an increase in the percentage of elderly people. It is believed these trends will continue into the future owing to increases in life expectancy coupled with declining fertility [1]. Attempts at improving the wellbeing of this ageing population have led to the investigation of new medical therapies to improve people's health and quality of life as they get older. The advent of tissue engineering and regenerative medical therapies represents a potentially exciting approach to tissue repair and regeneration [2–4]. Several strategies have been adopted to promote these therapies. These strategies tend to involve the isolation of cells from a patient or donor and the encapsulation of these cells in a three-dimensional scaffold. The scaffold acts as a temporary support matrix that can be remodelled by cells to generate a tissue-like structure. There are several different approaches to designing and manufacturing these scaffolds [5–8]. Ideally, the scaffold structure should replicate the native tissue's extracellular matrix composition and structure or allow cells to remodel it so as to provide a suitable environment for the cells to generate new tissue or repair existing tissue.
Among the most promising biomaterials under investigation for use as scaffolds in regenerative medicine are hydrogels. These consist of water-swollen networks of cross-linked polymer chains. Cross-links may be established via ionic, covalent or physical bonding of the polymer [9]. Hydrophilic functional groups attached to the polymer enable the hydrogels to retain a high percentage water content. The type of polymers and cross-linking used gives rise to a potentially wide range of properties and applications. Hydrogels are particularly attractive materials to be used as a structure for encapsulating cells for regenerative medicine because of their biocompatibility [10–12], their permeability to oxygen, nutrient growth factors and metabolic waste [13,14], their ability to be remodelled by cells [15–17] and their tissue-like viscoelastic characteristics [18,19].
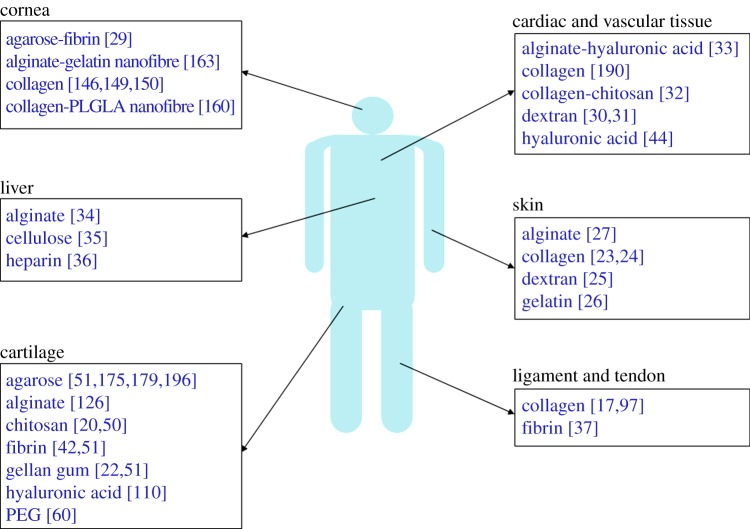
Cells encapsulated in biomimetic hydrogels are capable of remodelling their surrounding matrix and producing new extracellular matrix molecules. This remodelling behaviour is influenced by several factors, in particular, the type of hydrogel, the type of cell and the absence or presence of biochemical and mechanical stimuli. To date, cell-seeded hydrogels have been under investigation to repair or replace several tissue types, including cartilage [20–22], skin [23–27], cornea [28,29] and vascular tissues [30–33] among other tissues [34–37] (figure 1). As this field has grown, it has become evident that a greater understanding of the cell–material interactions in hydrogel systems is required to properly explain the mechanical mechanism that cells use to remodel their surroundings and to exploit this behaviour to develop functional tissues and tissue repair strategies. The aim of this paper is to give an overview of how cells interact and remodel hydrogels, the influence that the physical properties of hydrogels have on cell behaviour and how external mechanical stimuli affect the cell–hydrogel mechano-relationship.
Figure 1.
Examples of cell-seeded hydrogels that have been under investigation to engineer tissues and organs. (Online version in colour.)
2. Cell-mediated remodelling of hydrogels
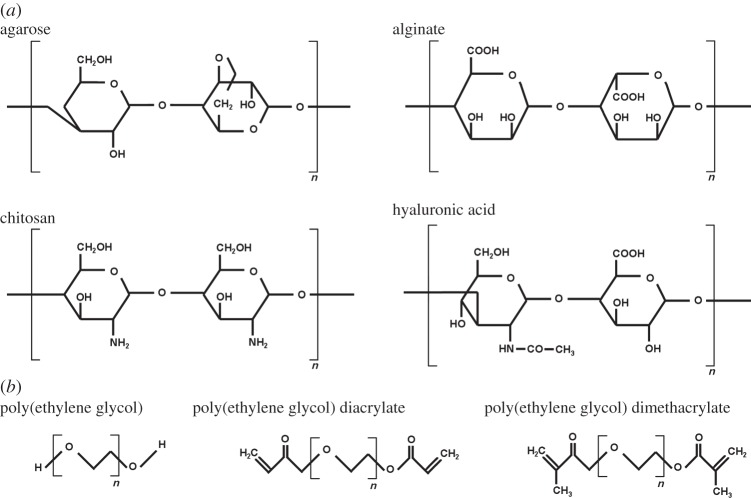
Cells have the ability to restructure and re-engineer their surroundings through several mechanical and biochemical mechanisms. This behaviour is vital for tissue growth and development, maintenance and function of healthy tissues and the repair of damaged tissues. The ability of cells to mechanically interact with their surrounding matrix in this manner can be exploited to allow cells embedded in hydrogels to remodel those hydrogels into tissue-like structures. This ability is primarily governed by the type of hydrogels and type of cells in addition to biochemical signalling molecules and mechanical cues. The types of hydrogels used in regenerative medicine can broadly be split into two categories: natural polymer hydrogels and synthetic polymer hydrogels [14]. Among the most extensively used natural hydrogels that have been under examination for use in tissue engineering and regenerative medicine are collagen [38–40], fibrin [41–43], hyaluronic acid [44–46], alginate [47,48], chitosan [49,50] and agarose [51–53]. These hydrogels differ in polymer structure (figure 1), ability to retain water, availability of binding sites and mechanical characteristics. For example, collagen consists of long chains of amino acids that form triple helix tropocollagens of approximately 300 nm in length [54,55]. The amino acids include glycine, proline or hydroxyproline and one other peptide such as alanine or arginine. These tropocollagens form strong, stable collagen fibrils [56,57] that are found in many load-bearing tissues and can form stable hydrogels with high water contents. By contrast, alginate is a copolymer consisting of β-d-mannuronic acid and α-l-guluronic acid monomer units. The ratio of these acid blocks controls the physical properties of the hydrogel. Unlike collagen which forms long stable fibrils, alginate hydrogels consist of chains of units that are ionically cross-linked by specific ions (such as calcium or barium) to form a network of chains resulting in hydrogel gelation [58,59]. This leads to a less stable and less organized structure than that found in collagen. The most extensively examined synthetic hydrogel material is poly(ethylene glycol) and its derivatives (figure 2) that can be chemically modified by attaching other polymer groups thus allowing a wide range of physical characteristics that can affect how the hydrogel interacts with cells [60–63].
Figure 2.
Chemical structure of (a) the natural polymers agarose, alginate (showing one β-d-mannuronic acid and one α-l-guluronic acid unit), chitosan and hyaluronic acid and (b) the synthetic polymer poly(ethylene glycol) and two of its derivatives (poly(ethylene glycol) diacrylate and poly(ethylene glycol) dimethacrylate).
In recent years, several peptide-based hydrogels have been developed to create a three-dimensional environment in which to study cell behaviour. Specific peptide chains can undergo a self-assembly process to produce a hydrogel with a high water content (over 99%). An alternating sequence of hydrophobic and hydrophilic amino acid sequences and positively and negatively charged peptides results in the formation of a stable hydrogel under controlled pH and ionic conditions [64–67]. These types of hydrogel are particularly useful in examining cellular behaviour owing to their biocompatibility, biodegradability and biofunctionality [68].
A key factor when examining cell–material mechano-interactions is the mechanisms of cell adhesion to the hydrogel. Cell surface receptors such as integrins bind to ligands within the hydrogel if such adhesion sites exist. Integrins, a collection of transmembrane glycoproteins intracellularly connected to the cell cytoskeleton, are among the most studied cell surface receptors and are associated with cell attachment, cell migration and extracellular matrix remodelling in addition to cell signalling [69–72]. They consist of α- and β-subunits, each combination of which forms a dimer with a specific function [73]. Other receptors such as syndecans, a collection of membrane-intercalated proteoglycans, work in synergy with integrins to provide cellular adhesion to their surrounding matrix [74]. Cadherin molecules facilitate cell–cell adhesion that in addition to influencing adhesion to the extracellular matrix can dictate cell behaviour and extracellular matrix production [75,76].
The availability of binding sites for cells to attach largely depends on the type of hydrogel. Collagen and fibrin hydrogels allow cells to bind directly to ligands on those proteins, whereas other hydrogels such as agarose, alginate and poly(ethylene glycol) lack binding sites thus preventing direct cell adhesion. For those hydrogels, oligopeptides such as arginine–glycine–aspartic acid (RGD) or various matrix proteins may be incorporated into the hydrogel to facilitate cell attachment [58,77] although incorporation of such peptides would also affect the physical properties of the hydrogels [78]. The availability of binding sites has a significant impact on cell behaviour and morphology within the hydrogel (figure 3). Cells embedded in hydrogels that facilitate binding have a different cytoskeletal structure compared with hydrogels lacking available binding sites [79,80]. Cells that attach to the hydrogel tend to have a spread morphology, high in actin stress fibres that pass through the cell (figure 3a). The spacing between binding sites can also influence the cell shape and cytoskeletal configuration [81]. Different approaches to quantifying the density of adhesion sites in hydrogels have previously been discussed [82]. By contrast, cells embedded in hydrogels that do not facilitate binding tend to have a spherical morphology and lack actin stress fibres (figure 3b). Hydrogels without binding sites may still enable cell adhesion through the production of extracellular matrix proteins by entrapped cells that can form a pericellular matrix onto which the cells can adhere over time [79].
Figure 3.
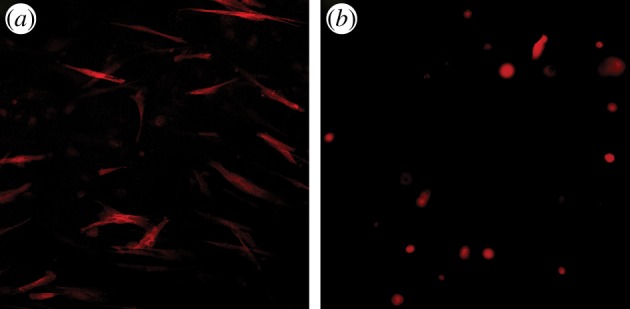
Images of fibroblasts in (a) collagen and (b) agarose hydrogels stained using phalloidin-TRITC and recorded using a fluorescent microscope. (Online version in colour.)
Cell mobility through a hydrogel is dependent on several factors, including the contractile and adhesive forces exerted by the cell, the availability of binding sites within the hydrogel, proteolysis of matrix constituents and the hydrogel matrix stiffness [83,84]. Cell mobility is vital for several physiological processes [85] and is important in enabling the integration of a hydrogel construct with its surrounding host tissue post-implantation. Several studies have examined the mechanisms by which cells can migrate in both two-dimensional culture [86–88] and in three-dimensional hydrogels [83,85]. The actin cytoskeleton plays a vital role in allowing cells to migrate through a hydrogel. Cellular protrusions such as filopodia and lamellipodia, which consist of polymerized actin networks at the front of the cell, extend and attach to ligands within the hydrogel. Once adhered, myosin II generates sufficient force to translocate the cell. Adhesions dissemble at the trailing edge, releasing the cell and allowing it to move through the hydrogel.
Several different approaches have been explored to control cell migration in hydrogels. One such mechanism is chemotaxis where biochemical stimuli are incorporated into the hydrogel to enhance cell migration in a particular direction [89]. An alternative mechanism is durotaxis where substrate rigidity regulates the direction of cell migration [90,91]. Localized variations in the stiffness of hydrogels can enable cells to preferentially migrate from one region of a hydrogel to another [92]. Most adherent cells will migrate from soft to stiffer regions within a hydrogel. One of the practical applications of enhancing cell migration is that it allows cell-free hydrogels to be implanted in a patient that can recruit host cells to infiltrate them from neighbouring tissue. Another application is the induction of angiogenesis where migrating endothelial cells form blood vessels within hydrogels [93]. Angiogenesis is important for maintaining the health and functionality of many tissue types that require oxygen and nutrients via a blood supply when implanted in vivo.
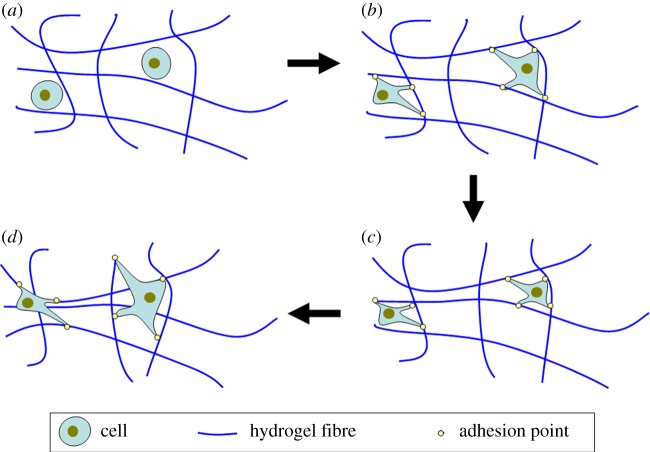
Contraction is one of the key mechanisms used by cells to remodel their surroundings. Cellular contraction of hydrogels follows a similar principle to cell migration. For example, in collagen hydrogels, fibroblasts bind and release via the α2β1 integrin using a ‘hand over hand’ cyclical motion to pull themselves along the collagen fibres [94,95]. However, if the fibres in the hydrogels have insufficient strength to withstand the applied force, then these fibres would buckle resulting in contractile remodelling of the hydrogel as shown schematically in figure 4. This can be a problem when trying to use hydrogels that have specific architectures incorporated into the structure to replicate a native tissue, because contraction will disrupt these structures [40]. Originally, it was envisioned that hydrogel contraction by cells could be used to generate new tissue [96] although this would require a comprehensive understanding of how the cells would remodel the hydrogel. Contraction also reduces the overall size of the hydrogels with some hydrogels such as collagen having been shown to contract to less than 20% of their original volume [97]. The rate of contraction may be controlled by altering the degree and mechanism of cross-linking, altering the number of binding sites and by changing the polymer concentration [98]. Hydrogel contraction can also be used to promote cell and tissue alignment in a particular direction by tethering the hydrogels at opposite ends, limiting the cellular contraction of the hydrogel in particular planes [99,100]. As the hydrogel contracts, the cells align along the direction of principal strain as they try to reach tensional homeostasis [101,102].
Figure 4.
Schematic of the contraction process in a cell-seeded hydrogel: (a) cells are embedded in a hydrogel matrix, (b) cells elongate and adhere to fibres, (c) cells pull fibres causing them to buckle, (d) cells release and reattach to new fibres resulting in contraction of the hydrogel. (Online version in colour.)
There are several mechanisms by which hydrogels can undergo degradation, including hydrolysis and enzymatic proteolysis. The digestion of matrix by matrix metalloproteinases (MMPs) and production of other extracellular matrix proteins allow the cells to reorganize and restructure their surroundings and form a new matrix. It is important that degradation products do not have a negative effect on cell viability or cell behaviour [9]. The precise degradation kinetics depends on several factors, including the type and activity of the cells present, the type of hydrogel and the degree of cross-linking. Ideally, the rate of degradation of the hydrogel would be optimized to match the degree of new tissue formation without any significant loss in mechanical strength. Incorporation of degradable moieties such as degradable polymer backbones, side groups or cross-link chains can be used to tailor degradation rate of the hydrogel [103]. The release of MMPs is common particularly among fibroblastic cells and is vital for cell translocation [104]. The cleavage of collagen by MMPs is dependent on the structure of the collagen with some collagens more resistant to degradation enzymatically than others [105]. It has been shown that fibroblasts embedded in a collagen hydrogel matrix release MMPs such as collagenase and gelatinase [106], resulting in the degradation of collagen and other proteins. MMP-1 (collagenase type 1) degrades collagen type 1 by attacking and cleaving the collagen triple helix chain. Other MMPs such as MMP-2 are also capable of cleaving the collagen 1 triple helix [107]. The sensitivity of proteins to MMPs has been used to develop hydrogel constructs capable of undergoing controlled remodelling by cells [108,109]. The release and activity of MMPs can be controlled biochemically by the application of reagents such as tissue inhibitors of metalloproteinases. An alternative application for enzymatic proteolysis is to liberate chemically bound biochemical molecules in hydrogels, such as growth factors. The degradation profile of the hydrogel or growth factor-loaded microspheres within the hydrogel can be controlled, so that there is a consistent release of growth factors over a particular time period. The growth factors, in turn, can promote cell activity or elicit a desired cell phenotype. This approach is particularly important when developing hydrogels for in vivo implantation as many growth factors necessary for tissue development or repair may not be present at the implant site. For example, transforming growth factor beta 1 and 3 (TGF-β1, TGF-β3) are known to elicit a chondrogenic phenotype from mesenchymal and adipose-derived stem cells. Because TGF is not present in sufficient quantities at cartilage defect sites, these growth factors can be incorporated into enzymatically degradable microspheres within a cell-seeded hydrogel to promote cartilage formation post-implantation [51,110,111]. The degradation rate of the microspheres can be tailored to allow growth factor release over a prolonged period of time to enable cartilage formation.
The rate of collagen breakdown by MMPs can be regulated by mechanical force applied to the collagen either by adjoining cells or neighbouring tissues. Several studies have found that mechanical force can either increase [112,113] or decrease [114] the rate of collagen degradation. The structure of the collagen appears to be vital in determining its susceptibility to enzymatic breakdown. Chang et al. [114] have suggested that when a strain is applied to homotrimer collagens, this resulted in unwinding of the collagen molecule thus increasing its ability to be cleaved by MMPs, whereas strain applied to heterotrimer collagen resulted in folding of collagen triple helix reducing its ability to be cleaved by MMPs [114]. In addition to affecting the susceptibility of collagen to enzymatic digestion, mechanical force can also affect the rate of release of MMPs by cells. Several studies using different cell sources have found that inducing mechanical stress on cells can regulate MMP production leading to changes in matrix remodelling [115–117].
Several studies have linked extracellular matrix production to changes in mechanical properties. Mineralization by cells of tissues or constructs through the release of extracellular products has led to an increase in stiffness of the surrounding matrix [118,119]. Mineralization is a key requirement for bone tissue engineering and regeneration, and it may be achieved in hydrogels by copolymerization with specific functional groups via the addition of calcium and phosphate solutions or alkaline phosphatase, combining with acid peptides or though promotion of cellular osteogenic activity by the addition of growth factors or adhesion molecules [120–124]. In addition to mineralization, other extracellular matrix products may also affect the mechanical characteristics of the hydrogels. Hu & Athanasiou [125] found the production of collagen and glycosaminoglycans (GAGs) by chondrocytes led to a threefold increase in the aggregate modulus of agarose constructs over an eight-week culture period. However, they also found that synthesis of matrix products has little effect on the modulus of poly(glycolic acid) constructs. This suggests that in addition to increased matrix production, the interaction of newly formed matrix proteins and the hydrogel affects the bulk mechanical properties of the construct. Williams et al. [126] found that increasing the chondrocyte density in an alginate construct led to both increased matrix production and increased mechanical strength [126]. Wan et al. [127] found that varying the seeding concentration and culture time affected the stiffness and viscoelastic characteristics of alginate hydrogels owing to differences in matrix accumulation [127].
3. Influence of the hydrogel on cell behaviour
The mechanical interactions between cells and extracellular matrix can be considered symbiotic. While cells have the ability to remodel their surrounding matrix, the mechanical, structural and chemical composition of these surroundings also regulates intracellular processes. This mutually dependent relationship between cells and their surrounding matrix is often referred to as dynamic reciprocity [128,129]. Cells respond to changes in their mechanical environment in a number of ways [130]. Changes in the cell phenotype [131], cytoskeleton [132], proliferation [16] and mobility [132,133] have been associated with differing matrix stiffnesses or matrix structures. This influence is most notable when examining the effect material stiffness has on the phenotypic behaviour of stem cells. Engler et al. [131] showed that the ability of stem cells to differentiate towards specific lineages was dependent on the substrate stiffness of the materials on which the cells were cultured [131]. They noted that neurogenic differentiation was optimal at a stiffness of 0.1–1 kPa, myogenic differentiation at 8–17 kPa and osteogenic differentiation at 25–40 kPa. Several subsequent studies have shown how substrate stiffness affects several different cell types, including neuronal cells [134,135], chondrocytes [136], cardiomyocytes [137,138], dermal fibroblasts [139] and limbal stem cells [140]. Recently, Trappmann et al. [141] suggested that substrates of different stiffness have differing protein anchorage densities and configurations that regulate stem cell fate through the mechanical feedback of cells attached to anchored proteins [141]. For this study, epidermal stem cells and mesenchymal stem cells were cultured on hydrogels of varying stiffness and protein anchorage densities. Because the theory linking protein anchorage and stem cell behaviour is relatively new, further studies are required to corroborate these findings.
In three-dimensional hydrogels, material stiffness plays a key role in influencing cell behaviour and mediating remodelling. Hydrogel stiffness is dependent on the concentration and arrangement of the polymer and the cross-linking density. Mesenchymal stem cells have demonstrated an increase in proliferation and cell spreading when cultured on stiff hydrogels compared with softer hydrogels [142]. By contrast, the proliferation of neuronal stem cells has been shown to decrease as hydrogel stiffness increases [143]. These findings suggest that the stiffness of the hydrogel affects different cell types in different manners. The hydrogel stiffness also affects the ability of cells to remodel their surroundings. In collagen hydrogels seeded with corneal fibroblasts the ability of the cells to contract and alter the stiffness of the hydrogel was found to be dependent on the hydrogel initial stiffness [15]. Even though such findings demonstrate the importance of stiffness in determining the suitability of a particular hydrogel material for a tissue engineering application, this cannot be considered in isolation as stiffness is generally dependent on other factors such as the hydrogel concentration and cross-linking. Changes to the stiffness of a hydrogel can affect the oxygen and nutrient permeability, availability of binding sites and overall water content. These factors would have a significant influence on cellular activities [144].
Several approaches have been investigated to increase the stiffness of hydrogels to improve their suitability for use in regenerative medicine. Plastic compression is one method that has been developed to produce collagen hydrogels with superior mechanical properties [38,92,145,146]. In addition to increasing the bulk stiffness of the hydrogel, this technique also reduces the water content and increases the overall material concentration without any adverse effect on cell viability. This increase in concentration leads to an increase in the number of available binding sites in close proximity to each cell and influences the ability of molecules and other products to diffuse through the hydrogel. The compression technique can be manipulated to allow fluid flow out of the hydrogels in a particular direction resulting in a more aligned fibre arrangement [147,148]. Plastic compression has several applications including the development of hydrogels with incremental increases in stiffness to study the effect of stiffness on cell migration and proliferation [92,145] and it has been under examination for cornea regeneration [146,149] and dermal tissue engineering and repair [23,24]. To date, this technique has primarily been used on collagen hydrogels, because most other hydrogels are incompressible under high strains, in part, owing to their hydrophilic nature.
Cross-linking techniques may also be used to improve the stiffness and mechanical strength of cell-seeded hydrogels. The increase in cross-links stabilizes the hydrogel, reducing its ability undergo enzymatic degradation. Ultraviolet cross-linking in the presence of riboflavin has been demonstrated to improve the stiffness of collagen hydrogels without impairing cell viability [150]. Chemical cross-linking using agents such as glutaraldehyde has also been used to cross-link hydrogels, although glutaraldehyde is highly toxic and needs to be completely removed from the hydrogel prior to cells being implanted. Some alternative cross-linking agents that are less toxic such as genepin [151] may represent a more suitable approach to cross-linking hydrogels for use in tissue engineering and regenerative medicine.
In addition to stiffness, the spatial arrangement within a hydrogel can influence the cell behaviour. Photopolymerization is a technique that can be used to chemically alter specified regions of a hydrogel to induce a spatial organization [52,152]. Ultraviolet light in the presence of cross-linking agents can be used to create regions of varying stiffness. By using a template, the light will only cross-link specified regions and can be used to create complex patterns within the hydrogel. This approach can affect cell migration, alignment and phenotype. Magnetic field alignment is an alternative method of introducing spatial organization into collagen-based hydrogels. Under a high magnetic field, collagen fibrils will assemble along the direction of the magnetic field and this results in hydrogels with aligned collagen fibres [153,154]. These techniques are particularly useful in replicating tissues with highly organized fibre orientations such as tendon or cornea.
As stated previously, hydrophilic polymer networks enable hydrogels to retain a large volume of water. This high water content gives hydrogels their viscoelastic characteristics. These characteristics are dependent on the percentage water content, porosity of the hydrogel and the polymer arrangement. Several studies have examined the viscoelastic properties of hydrogels [19,155–158], although few have examined in detail the influence these properties have on cell behaviour. The viscoelastic characteristic of cell-seeded hydrogels can change over time owing to a combination of contraction [97], enzyme-associated degradation and extracellular matrix deposition [53,127]. This, in turn, can lead to reciprocal change in the cell behaviour. Increased matrix deposition can lead to a reduction in percentage water content in hydrogels and alter the diffusion kinetics. The diffusion of oxygen and nutrients is vital for maintaining cell viability in the centre of hydrogel constructs [159].
An alternative approach to manipulating the structure of hydrogels and thus the cell behaviour within those hydrogels is to incorporate nanomaterials such as nanofibres, nanoparticles or nanotubes. Cells may be influenced by these materials either through contact or ingestion. The incorporation of electrospun nanofibres into hydrogels should increase the bulk elastic modulus and strength of these hydrogels [160,161] and can be used to dictate cell orientation and matrix production [160,162]. Nanofibres can be spun into aligned sheets that are then encapsulated by the cell-seeded hydrogels forming a nanofibre–cell–hydrogel composite. The embedded cells bind to the nanofibres and align themselves according to the fibre orientation. This phenomenon is particularly useful for engineering certain tissues such as cornea [160,163] that in situ have a high degree of cell alignment and distinct fibre orientations. Nanoparticles may also be incorporated into hydrogels as a drug release mechanism or for growth factor delivery [164,165]. Nanoparticles can also be used to alter the mechanical characteristics of hydrogels [166]. Carbon nanotubes have become popular in recent years as a method of reinforcing the mechanical properties of materials. They can be functionalized with side groups to enhance tissue regeneration [167]. For example, poly(aminobenzene sulfonic acid) has been bound to carbon nanotubes to promote bone formation [168]. The inclusion of carbon nanotubes has been shown to improve the overall mechanical strength of hydrogels [169,170]. In addition, it has been shown that nanotubes can affect cell behaviour by altering the cell morphology and increasing global stiffness [171].
4. Mechano-stimulation of cell-seeded hydrogels
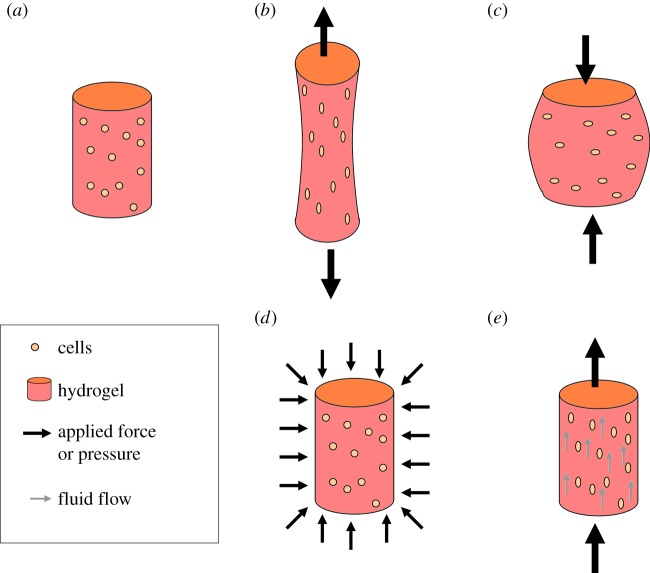
Mechanical stimulation of cells in hydrogels is an area of increasing interest for those developing new tissue engineering and regenerative medicine therapies and those who want to understand the mechanotransduction pathways that control cell activity. In the body, cells are constantly being subjected to mechanical forces that are believed to play a vital role in controlling their behaviour. These forces can be replicated and applied to cell-seeded hydrogels in vitro using a bioreactor system [172–174]. Depending on the type of bioreactor used, there are several mechanisms by which force can be imposed onto the hydrogel (as shown in figure 5) including by direct contact such as those found in compressive [175,176] or tensile bioreactor systems [177,178] or by indirect forces such as hydrostatic pressure [179,180] or via fluid flow [181,182].
Figure 5.
Schematic of different methods of applying force to cells in hydrogels: (a) no force, (b) tensile, (c) compression, (d) hydrostatic pressure and (e) fluid flow. (Online version in colour.)
When mechanical force is applied directly to the hydrogel, the resulting strain can be translated onto the cells either through focal adhesions for cells attached to their surrounding hydrogel matrix or through the cell membrane for encapsulated cells. Once strain is applied to the cell, it leads to changes in the cell's cytoskeletal configuration, disruption of the cell's nucleus, activation of ion channels and phosphorylation [183]. Forces generated on the cytoskeleton are transferred to the cell nucleus across the nuclear envelope through molecular tethers referred to as linkers of the nucleoskeleton to the cytoskeleton complex consisting of Sad1, UNC-84 and Klarsicht, ANC-1 and Syne homology proteins [184–187]. Mechanically induced activation of ion channels results in the release of signalling molecules that, in turn, influence the behaviour of neighbouring cells [188]. Phosphorylation involves the covalent binding of phosphate groups to proteins that leads to the activation of the protein and can induce other cellular processes. Mechanical stimulation of cells in hydrogels via compression has been investigated for regenerating several types of tissue, most prominently cartilage because it mimics the in vivo situation. Compressive force has been shown to promote or inhibit the chondrogenic capacity of chondrocytes and mesenchymal stem cells depending on the loading regime used [175,176,189]. Tensile loading of hydrogels has been shown to exhibit a wide range of effects on cells in hydrogels, including to promote fibroblastic differentiation of mesenchymal stem cells [177], induce matrix alignment in engineered ligaments [17], enhance cell organization in engineered cardiac tissue [190] and regulate mesenchymal stem cell gene expression [191]. The combination of these changes to cell behaviour after force has been applied demonstrates the importance of mechanical cues and mechanical stimulation for cell-seeded hydrogels.
An alternative to physically applying force directly onto the hydrogel is to use hydrostatic pressure to increase the pressure surrounding the hydrogel. Unlike the application of direct contact forces, hydrostatic pressure does not result in deformation of the hydrogel in a particular geometry. The force applied to the cells in the hydrogel should be more uniformly distributed around the cell body. Like direct force, exposure to increased hydrostatic pressure led to changes in cytoskeleton [79] and ion-channel activation [192]. Hydrostatic pressure appears to be particularly useful in initiating cartilage formation in hydrogels [193]. Hydrostatic pressure has been shown to up-regulate aggrecan and collagen II gene expression and increase sulfated GAG production in chondrocytes cultured in agarose hydrogels [194,195]. The dynamic modulus of mesenchymal stem cell-seeded hydrogels has also been shown to increase when cultured under hydrostatic pressure in a chemically defined chondrogenic differentiation media [180]. This increase was the result of increased extracellular deposition in the core of the hydrogel. Steward et al. [79] have suggested that vimentan may play a role in the chondrogenic differentiation of mesenchymal stem cells under hydrostatic pressure as part of a mechanotransduction pathway [79].
Fluid flow is another mechanism by which force can be applied to cells in hydrogels. The porous, water-swollen nature of hydrogels allows liquid to penetrate and pass through them. As this liquid passes through the hydrogels, cells attached to the matrix components of the hydrogel may experience shear stress from the fluid flow. The forces applied to the cell body have a similar effect on the cell behaviour as cells subjected to compressive or tensile force. Interestingly, Malone et al. [196] showed that cells subjected to a constant flow produced an increase in stress fibre formation while this was not present under interstitial flow conditions [196]. This suggests that the nature of the fluidic force applied has an impact on the cell response.
5. Conclusion
Understanding the interaction between cells and their surrounding matrix in hydrogels is vital in the development of new tissue engineering and regenerative medicine therapies. The main challenges to be overcome in using hydrogels are improving the control of cell behaviour; determining appropriate mechanical cues to initiate specific cell activities and developing hydrogels with properties similar to native tissue and that promote tissue formation. Success in completing these challenges will enable clinicians, scientists and engineers to develop new tissue engineering and regenerative medicine treatments.
Funding statement
This publication has emanated from research conducted with the financial support of Science Foundation Ireland and the Marie Curie Action COFUND under grant number 11/SIRG/B2104.
References
- 1.Lutz W, Sanderson W, Scherbov S. 2008. The coming acceleration of global population ageing. Nature 451, 716–719. ( 10.1038/nature06516) [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. 1993. Tissue engineering. Science 260, 920–926. ( 10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Shi Y, Ding S. 2008. A chemical approach to stem-cell biology and regenerative medicine. Nature 453, 338–344. ( 10.1038/nature07042) [DOI] [PubMed] [Google Scholar]
- 4.Mano JF, et al. 2007. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface 4, 999–1030. ( 10.1098/rsif.2007.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch AP, Ahearne M. 2013. Strategies for developing decellularized corneal scaffolds. Exp. Eye Res. 108, 42–47. ( 10.1016/j.exer.2012.12.012) [DOI] [PubMed] [Google Scholar]
- 6.Sukmana I. 2012. Bioactive polymer scaffold for fabrication of vascularized engineering tissue. J. Artif. Organs 15, 215–224. ( 10.1007/s10047-012-0644-6) [DOI] [PubMed] [Google Scholar]
- 7.Hutmacher DW. 2000. Scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529–2543. ( 10.1016/S0142-9612(00)00121-6) [DOI] [PubMed] [Google Scholar]
- 8.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. 2002. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 60, 613–621. ( 10.1002/jbm.10167) [DOI] [PubMed] [Google Scholar]
- 9.Nicodemus GD, Bryant SJ. 2008. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B 14, 149–165. ( 10.1089/ten.teb.2007.0332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhon MS, Andrade JD. 1973. Water and hydrogels. J. Biomed. Mater. Res. 7, 509–522. ( 10.1002/jbm.820070604) [DOI] [PubMed] [Google Scholar]
- 11.Molinaro G, Leroux JC, Damas J, Adam A. 2002. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials 23, 2717–2722. ( 10.1016/S0142-9612(02)00004-2) [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura Y, Hyon S, Ikadat Y. 1991. Poly(vinyl alcohol) hydrogel as an artificial articular cartilage: evaluation of biocompatibility. J. Appl. Biomater. 2, 101–107. ( 10.1002/jab.770020205) [DOI] [PubMed] [Google Scholar]
- 13.Jen AC, Wake MC, Mikos AG. 1996. Review: hydrogels for cell immobilization. Biotechnol. Bioeng. 50, 357–364. () [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Mooney DJ. 2001. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1879. ( 10.1021/cr000108x) [DOI] [PubMed] [Google Scholar]
- 15.Ahearne M, Wilson SL, Liu KK, Rauz S, El Haj AJ, Yang Y. 2010. Influence of cell and collagen concentration on the cell–matrix mechanical relationship in a corneal stroma wound healing model. Exp. Eye Res. 91, 584–591. ( 10.1016/j.exer.2010.07.013) [DOI] [PubMed] [Google Scholar]
- 16.Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell JA, Lutolf MP, Rizzi SC. 2010. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 31, 8454–8464. ( 10.1016/j.biomaterials.2010.07.046) [DOI] [PubMed] [Google Scholar]
- 17.Noth U, et al. 2005. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy 7, 447–455. ( 10.1080/14653240500319093) [DOI] [PubMed] [Google Scholar]
- 18.Ahearne M, Yang Y, El Haj AJ, Then KY, Liu KK. 2005. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2, 455–463. ( 10.1098/rsif.2005.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bader RA. 2008. Synthesis and viscoelastic characterization of novel hydrogels generated via photopolymerization of 1,2-epoxy-5-hexene modified poly(vinyl alcohol) for use in tissue replacement. Acta Biomater. 4, 967–975. ( 10.1016/j.actbio.2008.02.015) [DOI] [PubMed] [Google Scholar]
- 20.Suh JK, Matthew HW. 2000. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials 21, 2589–2598. ( 10.1016/S0142-9612(00)00126-5) [DOI] [PubMed] [Google Scholar]
- 21.Bahney CS, Hsu CW, Yoo JU, West JL, Johnstone B. 2011. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 25, 1486–1496. ( 10.1096/fj.10-165514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira JT, Santos TC, Martins L, Silva MA, Marques AP, Castro AG, Neves NM, Reis RL. 2009. Performance of new gellan gum hydrogels combined with human articular chondrocytes for cartilage regeneration when subcutaneously implanted in nude mice. J. Tissue Eng. Regen. Med. 3, 493–500. ( 10.1002/term.184) [DOI] [PubMed] [Google Scholar]
- 23.Hu K, Shi H, Zhu J, Deng D, Zhou G, Zhang W, Cao Y, Liu W. 2010. Compressed collagen gel as the scaffold for skin engineering. Biomed. Microdevices 12, 627–635. ( 10.1007/s10544-010-9415-4) [DOI] [PubMed] [Google Scholar]
- 24.Hartmann-Fritsch F, et al. In press. Collagen hydrogels strengthened by biodegradable meshes are a basis for dermo-epidermal skin grafts intended to reconstitute human skin in a one-step surgical intervention. J. Tissue Eng. Regen. Med. ( 10.1002/term.1665) [DOI] [PubMed] [Google Scholar]
- 25.Sun G, et al. 2011. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl Acad. Sci. USA 108, 20 976–20 981. ( 10.1073/pnas.1115973108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reno F, Rizzi M, Cannas M. 2012. Effect of a gelatin hydrogel incorporating epiregulin on human keratinocyte growth. J. Biomater. Sci. Polym. Ed. 23, 2025–2038. ( 10.1163/092050611X603872) [DOI] [PubMed] [Google Scholar]
- 27.Hunt NC, Shelton RM, Grover L. 2009. An alginate hydrogel matrix for the localised delivery of a fibroblast/keratinocyte co-culture. Biotechnol. J. 4, 730–737. ( 10.1002/biot.200800292) [DOI] [PubMed] [Google Scholar]
- 28.Li FF, et al. 2003. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl Acad. Sci. USA 100, 15 346–15 351. ( 10.1073/pnas.0136820100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alaminos M, Del Carmen Sanchez-Quevedo M, Munoz-Avila JI, Serrano D, Medialdea S, Carreras I, Campos A. 2006. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest. Ophthalmol. Vis. Sci. 47, 3311–3317. ( 10.1167/iovs.05-1647) [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Chan-Park MB. 2009. Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials 30, 196–207. ( 10.1016/j.biomaterials.2008.09.041) [DOI] [PubMed] [Google Scholar]
- 31.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. 2007. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28, 2706–2717. ( 10.1016/j.biomaterials.2007.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng C, Zhang P, Vulesevic B, Kuraitis D, Li F, Yang AF, Griffith M, Ruel M, Suuronen EJ. 2010. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng. A 16, 3099–3109. ( 10.1089/ten.tea.2009.0504) [DOI] [PubMed] [Google Scholar]
- 33.Dahlmann J, et al. 2013. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials 34, 940–951. ( 10.1016/j.biomaterials.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 34.Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska AM, Prakash S. 2005. In vitro study of alginate-chitosan microcapsules: an alternative to liver cell transplants for the treatment of liver failure. Biotechnol. Lett. 27, 317–322. ( 10.1007/s10529-005-0687-3) [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya M, et al. 2012. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 164, 291–298. ( 10.1016/j.jconrel.2012.06.039) [DOI] [PubMed] [Google Scholar]
- 36.Kim M, Lee JY, Jones CN, Revzin A, Tae G. 2010. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 31, 3596–3603. ( 10.1016/j.biomaterials.2010.01.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, et al. 2013. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng. A 19, 2439–2451. ( 10.1089/ten.tea.2012.0453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. 2005. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 15, 1762–1770. ( 10.1002/adfm.200500042) [DOI] [Google Scholar]
- 39.Lee JH, Yu HS, Lee GS, Ji A, Hyun JK, Kim HW. 2011. Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells. J. R. Soc. Interface 8, 998–1010. ( 10.1098/rsif.2010.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillette BM, Jensen JA, Tang B, Yang GJ, Bazargan-Lari A, Zhong M, Sia SK. 2008. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 7, 636–640. ( 10.1038/nmat2203) [DOI] [PubMed] [Google Scholar]
- 41.Janmey PA, Winer JP, Weisel JW. 2009. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 6, 1–10. ( 10.1098/rsif.2008.0327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahearne M, Buckley CT, Kelly DJ. 2011. A growth factor delivery system for chondrogenic induction of infrapatellar fat pad-derived stem cells in fibrin hydrogels. Biotechnol. Appl. Biochem. 58, 345–352. ( 10.1002/bab.45) [DOI] [PubMed] [Google Scholar]
- 43.Ahmed TA, Dare EV, Hincke M. 2008. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. B 14, 199–215. ( 10.1089/ten.teb.2007.0435) [DOI] [PubMed] [Google Scholar]
- 44.Hanjaya-Putra D, Shen YI, Wilson A, Fox-Talbot K, Khetan S, Burdick JA, Steenbergen C, Gerecht S. 2013. Integration and regression of implanted engineered human vascular networks during deep wound healing. Stem Cells Transl. Med. 2, 297–306. ( 10.5966/sctm.2012-0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. 2013. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465. ( 10.1038/nmat3586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baier Leach J, Bivens KA, Patrick CW, Jr, Schmidt CE. 2003. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 82, 578–589. ( 10.1002/bit.10605) [DOI] [PubMed] [Google Scholar]
- 47.Kuo CK, Ma PX. 2001. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 22, 511–521. ( 10.1016/S0142-9612(00)00201-5) [DOI] [PubMed] [Google Scholar]
- 48.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. 2001. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 80, 2025–2029. ( 10.1177/00220345010800111501) [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, et al. 2012. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 33, 3093–3106. ( 10.1016/j.biomaterials.2011.12.044) [DOI] [PubMed] [Google Scholar]
- 50.Hong Y, Song H, Gong Y, Mao Z, Gao C, Shen J. 2007. Covalently crosslinked chitosan hydrogel: properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 3, 23–31. ( 10.1016/j.actbio.2006.06.007) [DOI] [PubMed] [Google Scholar]
- 51.Ahearne M, Kelly DJ. 2013. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed. Mater. 8, 035004 ( 10.1088/1748-6041/8/3/035004) [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Shoichet MS. 2004. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 3, 249–253. ( 10.1038/nmat1092) [DOI] [PubMed] [Google Scholar]
- 53.Buckley CT, Vinardell T, Thorpe SD, Haugh MG, Jones E, McGonagle D, Kelly DJ. 2010. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J. Biomech. 43, 920–926. ( 10.1016/j.jbiomech.2009.11.005) [DOI] [PubMed] [Google Scholar]
- 54.Orgel JP, Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ. 2001. The in situ supermolecular structure of type I collagen. Structure 9, 1061–1069. ( 10.1016/S0969-2126(01)00669-4) [DOI] [PubMed] [Google Scholar]
- 55.Bella J, Eaton M, Brodsky B, Berman HM. 1994. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 266, 75–81. ( 10.1126/science.7695699) [DOI] [PubMed] [Google Scholar]
- 56.Buehler MJ. 2006. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc. Natl Acad. Sci. USA 103, 12 285–12 290. ( 10.1073/pnas.0603216103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buehler MJ. 2008. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 1, 59–67. ( 10.1016/j.jmbbm.2007.04.001) [DOI] [PubMed] [Google Scholar]
- 58.Rowley JA, Madlambayan G, Mooney DJ. 1999. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53. ( 10.1016/S0142-9612(98)00107-0) [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Shelton RM, Cooper PR, Lawson M, Triffitt JT, Barralet JE. 2003. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 24, 3475–3481. ( 10.1016/S0142-9612(03)00167-4) [DOI] [PubMed] [Google Scholar]
- 60.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. 2002. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J. Biomed. Mater. Res. 59, 429–437. ( 10.1002/jbm.1259) [DOI] [PubMed] [Google Scholar]
- 61.Burdick JA, Anseth KS. 2002. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23, 4315–4323. ( 10.1016/S0142-9612(02)00176-X) [DOI] [PubMed] [Google Scholar]
- 62.Zhu J. 2010. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639–4656. ( 10.1016/j.biomaterials.2010.02.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu YC, Kocagoz S, Larson JC, Brey EM. 2013. Evaluation of physical and mechanical properties of porous poly (ethylene glycol)-co-(l-lactic acid) hydrogels during degradation. PLoS ONE 8, e60728 ( 10.1371/journal.pone.0060728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. 2002. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl Acad. Sci. USA 99, 9996–10 001. ( 10.1073/pnas.142309999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. 2000. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl Acad. Sci. USA 97, 6728–6733. ( 10.1073/pnas.97.12.6728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S, Holmes T, Lockshin C, Rich A. 1993. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl Acad. Sci. USA 90, 3334–3338. ( 10.1073/pnas.90.8.3334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. 1995. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 16, 1385–1393. ( 10.1016/0142-9612(95)96874-Y) [DOI] [PubMed] [Google Scholar]
- 68.Dasgupta A, Mondal JH, Das D. 2013. Peptide hydrogels. RSC Adv. 3, 9117–9149. ( 10.1039/c3ra40234g) [DOI] [Google Scholar]
- 69.Hynes RO. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. ( 10.1016/0092-8674(92)90115-S) [DOI] [PubMed] [Google Scholar]
- 70.Hynes RO. 1987. Integrins: a family of cell surface receptors. Cell 48, 549–554. ( 10.1016/0092-8674(87)90233-9) [DOI] [PubMed] [Google Scholar]
- 71.Ruoslahti E, Pierschbacher MD. 1987. New perspectives in cell adhesion: RGD and integrins. Science 238, 491–497. ( 10.1126/science.2821619) [DOI] [PubMed] [Google Scholar]
- 72.Cukierman E, Pankov R, Stevens DR, Yamada KM. 2001. Taking cell–matrix adhesions to the third dimension. Science 294, 1708–1712. ( 10.1126/science.1064829) [DOI] [PubMed] [Google Scholar]
- 73.Hynes RO. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. ( 10.1016/S0092-8674(02)00971-6) [DOI] [PubMed] [Google Scholar]
- 74.Morgan MR, Humphries MJ, Bass MD. 2007. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969. ( 10.1038/nrm2289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gates J, Peifer M. 2005. Can 1000 reviews be wrong? Actin, alpha-catenin, and adherens junctions. Cell 123, 769–772. ( 10.1016/j.cell.2005.11.009) [DOI] [PubMed] [Google Scholar]
- 76.Chen S, Lewallen M, Xie T. 2013. Adhesion in the stem cell niche: biological roles and regulation. Development 140, 255–265. ( 10.1242/dev.083139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. 2005. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 26, 5991–5998. ( 10.1016/j.biomaterials.2005.03.018) [DOI] [PubMed] [Google Scholar]
- 78.Zustiak SP, Durbal R, Leach JB. 2010. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 6, 3404–3414. ( 10.1016/j.actbio.2010.03.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steward AJ, Wagner DR, Kelly DJ. 2013. The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. Eur. Cell Mater. 25, 167–178. [DOI] [PubMed] [Google Scholar]
- 80.Steward AJ, Thorpe SD, Vinardell T, Buckley CT, Wagner DR, Kelly DJ. 2012. Cell–matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater. 8, 2153–2159. ( 10.1016/j.actbio.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 81.Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP. 2007. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 92, 2964–2974. ( 10.1529/biophysj.106.089730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tzeranis DS, Roy A, So PT, Yannas IV. 2010. An optical method to quantify the density of ligands for cell adhesion receptors in three-dimensional matrices. J. R. Soc. Interface 7(Suppl. 5), S649–S661. ( 10.1098/rsif.2010.0321.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gobin AS, West JL. 2002. Cell migration through defined, synthetic ECM analogs. FASEB J. 16, 751–753. ( 10.1096/fj.01-0759fje) [DOI] [PubMed] [Google Scholar]
- 84.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. 2006. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell–matrix adhesion and proteolysis. Proc. Natl Acad. Sci. USA 103, 10 889–10 894. ( 10.1073/pnas.0604460103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burgess BT, Myles JL, Dickinson RB. 2000. Quantitative analysis of adhesion-mediated cell migration in three-dimensional gels of RGD-grafted collagen. Ann. Biomed. Eng. 28, 110–118. ( 10.1114/1.259) [DOI] [PubMed] [Google Scholar]
- 86.Mattila PK, Lappalainen P. 2008. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454. ( 10.1038/nrm2406) [DOI] [PubMed] [Google Scholar]
- 87.Wehrle-Haller B, Imhof BA. 2003. Actin, microtubules and focal adhesion dynamics during cell migration. Int. J. Biochem. Cell Biol. 35, 39–50. ( 10.1016/S1357-2725(02)00071-7) [DOI] [PubMed] [Google Scholar]
- 88.Pantaloni D, Le Clainche C, Carlier MF. 2001. Mechanism of actin-based motility. Science 292, 1502–1506. ( 10.1126/science.1059975) [DOI] [PubMed] [Google Scholar]
- 89.Cheng SY, Heilman S, Wasserman M, Archer S, Shuler ML, Wu M. 2007. A hydrogel-based microfluidic device for the studies of directed cell migration. Lab Chip 7, 763–769. ( 10.1039/b618463d) [DOI] [PubMed] [Google Scholar]
- 90.Choi YS, Vincent LG, Lee AR, Kretchmer KC, Chirasatitsin S, Dobke MK, Engler AJ. 2012. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials 33, 6943–6951. ( 10.1016/j.biomaterials.2012.06.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vincent LG, Choi YS, Alonso-Latorre B, del Alamo JC, Engler AJ. 2013. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol. J. 8, 472–484. ( 10.1002/biot.201200205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hadjipanayi E, Mudera V, Brown RA. 2009. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil. Cytoskeleton 66, 121–128. ( 10.1002/cm.20331) [DOI] [PubMed] [Google Scholar]
- 93.Miller JS, Shen CJ, Legant WR, Baranski JD, Blakely BL, Chen CS. 2010. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials 31, 3736–3743. ( 10.1016/j.biomaterials.2010.01.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. 2005. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat. Cell Biol. 7, 157–164. ( 10.1038/ncb1216) [DOI] [PubMed] [Google Scholar]
- 95.Wakatsuki T, Elson EL. 2003. Reciprocal interactions between cells and extracellular matrix during remodeling of tissue constructs. Biophys. Chem. 100, 593–605. ( 10.1016/S0301-4622(02)00308-3) [DOI] [PubMed] [Google Scholar]
- 96.Bell E, Ivarsson B, Merrill C. 1979. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl Acad. Sci. USA 76, 1274–1278. ( 10.1073/pnas.76.3.1274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahearne M, Liu KK, El Haj AJ, Then KY, Rauz S, Yang Y. 2010. Online monitoring of the mechanical behavior of collagen hydrogels: influence of corneal fibroblasts on elastic modulus. Tissue Eng. C 16, 319–327. ( 10.1089/ten.tec.2008.0650) [DOI] [PubMed] [Google Scholar]
- 98.Chung C, Anderson E, Pera RR, Pruitt BL, Heilshorn SC. 2012. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3D cultures. Soft Matter 8, 10 141–10 148. ( 10.1039/c2sm26082d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahearne M, Bagnaninchi PO, Yang Y, El Haj AJ. 2008. Online monitoring of collagen fibre alignment in tissue-engineered tendon by PSOCT. J. Tissue Eng. Regen. Med. 2, 521–524. ( 10.1002/term.124) [DOI] [PubMed] [Google Scholar]
- 100.East E, de Oliveira DB, Golding JP, Phillips JB. 2010. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng. A 16, 3173–3184. ( 10.1089/ten.tea.2010.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rhee S, Grinnell F. 2007. Fibroblast mechanics in 3D collagen matrices. Adv. Drug Deliv. Rev. 59, 1299–1305. ( 10.1016/j.addr.2007.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. 1998. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 175, 323–332. () [DOI] [PubMed] [Google Scholar]
- 103.Kharkar PM, Kiick KL, Kloxin AM. 2013. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 42, 7335–7372. ( 10.1039/c3cs60040h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ragoowansi R, Khan U, Brown RA, McGrouther DA. 2003. Differences in morphology, cytoskeletal architecture and protease production between zone II tendon and synovial fibroblasts in vitro. J. Hand Surg. Br. 28, 465–470. ( 10.1016/S0266-7681(03)00140-2) [DOI] [PubMed] [Google Scholar]
- 105.Han S, et al. 2010. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J. Biol. Chem. 285, 22 276–22 281. ( 10.1074/jbc.M110.102079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mauch C, Adelmann-Grill B, Hatamochi A, Krieg T. 1989. Collagenase gene expression in fibroblasts is regulated by a three-dimensional contact with collagen. FEBS Lett. 250, 301–305. ( 10.1016/0014-5793(89)80743-4) [DOI] [PubMed] [Google Scholar]
- 107.Aimes RT, Quigley JP. 1995. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 270, 5872–5876. ( 10.1074/jbc.270.11.5872) [DOI] [PubMed] [Google Scholar]
- 108.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. 2003. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA 100, 5413–5418. ( 10.1073/pnas.0737381100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patterson J, Hubbell JA. 2010. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 31, 7836–7845. ( 10.1016/j.biomaterials.2010.06.061) [DOI] [PubMed] [Google Scholar]
- 110.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. 2011. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425–6434. ( 10.1016/j.biomaterials.2011.05.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeFail AJ, Chu CR, Izzo N, Marra KG. 2006. Controlled release of bioactive TGF-beta 1 from microspheres embedded within biodegradable hydrogels. Biomaterials 27, 1579–1585. ( 10.1016/j.biomaterials.2005.08.013) [DOI] [PubMed] [Google Scholar]
- 112.Adhikari AS, Chai J, Dunn AR. 2011. Mechanical load induces a 100-fold increase in the rate of collagen proteolysis by MMP-1. J. Am. Chem. Soc. 133, 1686–1689. ( 10.1021/ja109972p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ellsmere JC, Khanna RA, Lee JM. 1999. Mechanical loading of bovine pericardium accelerates enzymatic degradation. Biomaterials 20, 1143–1150. ( 10.1016/S0142-9612(99)00013-7) [DOI] [PubMed] [Google Scholar]
- 114.Chang SW, Flynn BP, Ruberti JW, Buehler MJ. 2012. Molecular mechanism of force induced stabilization of collagen against enzymatic breakdown. Biomaterials 33, 3852–3859. ( 10.1016/j.biomaterials.2012.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hou X, Han QH, Hu D, Tian L, Guo CM, Du HJ, Zhang P, Wang Y-S, Hui Y-N. 2009. Mechanical force enhances MMP-2 activation via p38 signaling pathway in human retinal pigment epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 247, 1477–1486. ( 10.1007/s00417-009-1135-1) [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Li X, Bi LJ. 2011. Alterations of collagen-I, MMP-1 and TIMP-1 in the periodontal ligament of diabetic rats under mechanical stress. J. Periodontal Res. 46, 448–455. ( 10.1111/j.1600-0765.2011.01359.x) [DOI] [PubMed] [Google Scholar]
- 117.Cummins PM, von Offenberg Sweeney N, Killeen MT, Birney YA, Redmond EM, Cahill PA. 2007. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: a force to be reckoned with. Am. J. Physiol. Heart Circ. Physiol. 292, H28–H42. ( 10.1152/ajpheart.00304.2006) [DOI] [PubMed] [Google Scholar]
- 118.Nauman EA, Ebenstein DM, Hughes KF, Pruitt L, Halloran BP, Bikle DD, Keaveny TM. 2002. Mechanical and chemical characteristics of mineral produced by basic fibroblast growth factor-treated bone marrow stromal cells in vitro. Tissue Eng. 8, 931–939. ( 10.1089/107632702320934038) [DOI] [PubMed] [Google Scholar]
- 119.Kazakia GJ, Nauman EA, Ebenstein DM, Halloran BP, Keaveny TM. 2006. Effects of in vitro bone formation on the mechanical properties of a trabeculated hydroxyapatite bone substitute. J. Biomed. Mater. Res. A 77, 688–699. ( 10.1002/jbm.a.30644) [DOI] [PubMed] [Google Scholar]
- 120.Gkioni K, Leeuwenburgh SC, Douglas TE, Mikos AG, Jansen JA. 2010. Mineralization of hydrogels for bone regeneration. Tissue Eng. B 16, 577–585. ( 10.1089/ten.teb.2010.0462) [DOI] [PubMed] [Google Scholar]
- 121.Zhong C, Chu CC. 2012. Biomimetic mineralization of acid polysaccharide-based hydrogels: towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 22, 6080–6087. ( 10.1039/c2jm15610e) [DOI] [Google Scholar]
- 122.Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, de Groot K. 2000. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 50, 518–527. () [DOI] [PubMed] [Google Scholar]
- 123.Douglas TE, et al. 2012. Enzymatic mineralization of hydrogels for bone tissue engineering by incorporation of alkaline phosphatase. Macromol. Biosci. 12, 1077–1089. ( 10.1002/mabi.201100501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. 2002. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J. Control. Release 83, 53–63. ( 10.1016/S0168-3659(02)00181-5) [DOI] [PubMed] [Google Scholar]
- 125.Hu JC, Athanasiou KA. 2005. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials 26, 2001–2012. ( 10.1016/j.biomaterials.2004.06.038) [DOI] [PubMed] [Google Scholar]
- 126.Williams GM, Klein TJ, Sah RL. 2005. Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate-chondrocyte constructs. Acta Biomater. 1, 625–633. ( 10.1016/j.actbio.2005.07.009) [DOI] [PubMed] [Google Scholar]
- 127.Wan LQ, Jiang J, Miller DE, Guo XE, Mow VC, Lu HH. 2011. Matrix deposition modulates the viscoelastic shear properties of hydrogel-based cartilage grafts. Tissue Eng. A 17, 1111–1122. ( 10.1089/ten.tea.2010.0379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bissell MJ, Aggeler J. 1987. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog. Clin. Biol. Res. 249, 251–262. [PubMed] [Google Scholar]
- 129.Roskelley CD, Bissell MJ. 1995. Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem. Cell Biol. 73, 391–397. ( 10.1139/o95-046) [DOI] [PubMed] [Google Scholar]
- 130.Discher DE, Janmey P, Wang YL. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143. ( 10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- 131.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 132.Peyton SR, Putnam AJ. 2005. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 204, 198–209. ( 10.1002/jcp.20274) [DOI] [PubMed] [Google Scholar]
- 133.Lo CM, Wang HB, Dembo M, Wang YL. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152. ( 10.1016/S0006-3495(00)76279-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Even-Ram S, Artym V, Yamada KM. 2006. Matrix control of stem cell fate. Cell 126, 645–647. ( 10.1016/j.cell.2006.08.008) [DOI] [PubMed] [Google Scholar]
- 135.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. 2008. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438. ( 10.1529/biophysj.108.132217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanz-Ramos P, Mora G, Vicente-Pascual M, Ochoa I, Alcaine C, Moreno R, Doblaré M, Izal-Azcárate I. 2013. Response of sheep chondrocytes to changes in substrate stiffness from 2 to 20 Pa: effect of cell passaging. Connect. Tissue Res. 54, 159–166. ( 10.3109/03008207.2012.762360) [DOI] [PubMed] [Google Scholar]
- 137.Forte G, et al. 2012. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng. A 18, 1837–1848. ( 10.1089/ten.tea.2011.0707) [DOI] [PubMed] [Google Scholar]
- 138.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. 2010. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 105, 1148–1160. ( 10.1002/bit.22647) [DOI] [PubMed] [Google Scholar]
- 139.Hopp I, Michelmore A, Smith LE, Robinson DE, Bachhuka A, Mierczynska A, Vasilev K. 2013. The influence of substrate stiffness gradients on primary human dermal fibroblasts. Biomaterials 34, 5070–5077. ( 10.1016/j.biomaterials.2013.03.075) [DOI] [PubMed] [Google Scholar]
- 140.Jones RR, Hamley IW, Connon CJ. 2012. Ex vivo expansion of limbal stem cells is affected by substrate properties. Stem Cell Res. 8, 403–409. ( 10.1016/j.scr.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 141.Trappmann B, et al. 2012. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649. ( 10.1038/nmat3339) [DOI] [PubMed] [Google Scholar]
- 142.Marklein RA, Burdick JA. 2010. Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter 6, 136–143. ( 10.1039/b916933d) [DOI] [Google Scholar]
- 143.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. 2009. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30, 4695–4699. ( 10.1016/j.biomaterials.2009.05.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cha C, Jeong JH, Shim J, Kong H. 2011. Tuning the dependency between stiffness and permeability of a cell encapsulating hydrogel with hydrophilic pendant chains. Acta Biomater. 7, 3719–3728. ( 10.1016/j.actbio.2011.06.017) [DOI] [PubMed] [Google Scholar]
- 145.Hadjipanayi E, Mudera V, Brown RA. 2009. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 3, 77–84. ( 10.1002/term.136) [DOI] [PubMed] [Google Scholar]
- 146.Levis HJ, Brown RA, Daniels JT. 2010. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 31, 7726–7737. ( 10.1016/j.biomaterials.2010.07.012) [DOI] [PubMed] [Google Scholar]
- 147.Alekseeva T, Hadjipanayi E, Abou Neel EA, Brown RA. 2012. Engineering stable topography in dense bio-mimetic 3D collagen scaffolds. Eur. Cells Mater. 23, 28–40. [DOI] [PubMed] [Google Scholar]
- 148.Kureshi A, Cheema U, Alekseeva T, Cambrey A, Brown R. 2010. Alignment hierarchies: engineering architecture from the nanometre to the micrometre scale. J. R. Soc. Interface 7(Suppl. 6), S707–S716. ( 10.1098/rsif.2010.0346.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mi S, Chen B, Wright B, Connon CJ. 2010. Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng. A 16, 2091–2100. ( 10.1089/ten.tea.2009.0748) [DOI] [PubMed] [Google Scholar]
- 150.Ahearne M, Yang Y, Then KY, Liu KK. 2008. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br. J. Ophthalmol. 92, 268–271. ( 10.1136/bjo.2007.130104) [DOI] [PubMed] [Google Scholar]
- 151.Moffat KL, Marra KG. 2004. Biodegradable poly(ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. J. Biomed. Mater. Res. B 71, 181–187. ( 10.1002/jbm.b.30070) [DOI] [PubMed] [Google Scholar]
- 152.Wylie RG, Shoichet MS. 2011. Three-dimensional spatial patterning of proteins in hydrogels. Biomacromolecules 12, 3789–3796. ( 10.1021/bm201037j) [DOI] [PubMed] [Google Scholar]
- 153.Girton TS, Dubey N, Tranquillo RT. 1999. Magnetic-induced alignment of collagen fibrils in tissue equivalents. Methods Mol. Med. 18, 67–73. ( 10.1385/0-89603-516-6:67) [DOI] [PubMed] [Google Scholar]
- 154.Torbet J, Ronziere MC. 1984. Magnetic alignment of collagen during self-assembly. Biochem. J. 219, 1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ahearne M, Siamantouras E, Yang Y, Liu KK. 2009. Mechanical characterization of biomimetic membranes by micro-shaft poking. J. R. Soc. Interface 6, 471–478. ( 10.1098/rsif.2008.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarvestani AS, He X, Jabbari E. 2007. Viscoelastic characterization and modeling of gelation kinetics of injectable in situ cross-linkable poly(lactide-co-ethylene oxide-co-fumarate) hydrogels. Biomacromolecules 8, 406–415. ( 10.1021/bm060648p) [DOI] [PubMed] [Google Scholar]
- 157.Hyland LL, Taraban MB, Feng Y, Hammouda B, Yu YB. 2012. Viscoelastic properties and nanoscale structures of composite oligopeptide-polysaccharide hydrogels. Biopolymers 97, 177–188. ( 10.1002/bip.21722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Y, Bagnaninchi PO, Ahearne M, Wang RK, Liu KK. 2007. A novel optical coherence tomography-based micro-indentation technique for mechanical characterization of hydrogels. J. R. Soc. Interface 4, 1169–1173. ( 10.1098/rsif.2007.1044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demol J, Lambrechts D, Geris L, Schrooten J, Van Oosterwyck H. 2011. Towards a quantitative understanding of oxygen tension and cell density evolution in fibrin hydrogels. Biomaterials 32, 107–118. ( 10.1016/j.biomaterials.2010.08.093) [DOI] [PubMed] [Google Scholar]
- 160.Wilson SL, Wimpenny I, Ahearne M, Ruaz S, El Haj AJ, Yang Y. 2012. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Funct. Mater. 22, 3641–3649. ( 10.1002/adfm.201200655) [DOI] [Google Scholar]
- 161.Kai D, Prabhakaran MP, Stahl B, Eblenkamp M, Wintermantel E, Ramakrishna S. 2012. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology 23, 095705 ( 10.1088/0957-4484/23/9/095705) [DOI] [PubMed] [Google Scholar]
- 162.Yang Y, Wimpenny I, Ahearne M. 2011. Portable nanofiber meshes dictate cell orientation throughout three-dimensional hydrogels. Nanomedicine 7, 131–136. ( 10.1016/j.nano.2010.12.011) [DOI] [PubMed] [Google Scholar]
- 163.Tonsomboon K, Oyen ML. 2013. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 21, 185–194. ( 10.1016/j.jmbbm.2013.03.001) [DOI] [PubMed] [Google Scholar]
- 164.Geng H, Song H, Qi J, Cui D. 2011. Sustained release of VEGF from PLGA nanoparticles embedded thermo-sensitive hydrogel in full-thickness porcine bladder acellular matrix. Nanoscale Res. Lett. 6, 312 ( 10.1186/1556-276X-6-312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lim SM, Oh SH, Lee HH, Yuk SH, Im GI, Lee JH. 2010. Dual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 21, 2593–2600. ( 10.1007/s10856-010-4118-1) [DOI] [PubMed] [Google Scholar]
- 166.Gaharwar AK, Dammu SA, Canter JM, Wu CJ, Schmidt G. 2011. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 12, 1641–1650. ( 10.1021/bm200027z) [DOI] [PubMed] [Google Scholar]
- 167.Tran PA, Zhang L, Webster TJ. 2009. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 61, 1097–1114. ( 10.1016/j.addr.2009.07.010) [DOI] [PubMed] [Google Scholar]
- 168.Zhao B, Hu H, Mandal SK, Haddon RC. 2005. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 17, 3235–3241. ( 10.1021/cm0500399) [DOI] [Google Scholar]
- 169.Shin SR, et al. 2012. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6, 362–372. ( 10.1021/nn203711s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xie F, Weiss P, Chauvet O, Le Bideau J, Tassin JF. 2010. Kinetic studies of a composite carbon nanotube-hydrogel for tissue engineering by rheological methods. J. Mater. Sci. Mater. Med. 21, 1163–1168. ( 10.1007/s10856-009-3984-x) [DOI] [PubMed] [Google Scholar]
- 171.Dong C, Kashon ML, Lowry D, Dordick JS, Reynolds SH, Rojanasakul Y, Sargent LM, Dinu CZ. 2013. Exposure to carbon nanotubes leads to changes in the cellular biomechanics. Adv. Healthcare Mater. 2, 945–951. ( 10.1002/adhm.201200430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martin I, Wendt D, Heberer M. 2004. The role of bioreactors in tissue engineering. Trends Biotechnol. 22, 80–86. ( 10.1016/j.tibtech.2003.12.001) [DOI] [PubMed] [Google Scholar]
- 173.Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. 2005. Bioreactor design for tissue engineering. J. Biosci. Bioeng. 100, 235–245. ( 10.1263/jbb.100.235) [DOI] [PubMed] [Google Scholar]
- 174.Chen HC, Hu YC. 2006. Bioreactors for tissue engineering. Biotechnol. Lett. 28, 1415–1423. ( 10.1007/s10529-006-9111-x) [DOI] [PubMed] [Google Scholar]
- 175.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122, 252–260. ( 10.1115/1.429656) [DOI] [PubMed] [Google Scholar]
- 176.Mauck RL, Byers BA, Yuan X, Tuan RS. 2007. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech. Model. Mechanobiol. 6, 113–125. ( 10.1007/s10237-006-0042-1) [DOI] [PubMed] [Google Scholar]
- 177.Doroski DM, Levenston ME, Temenoff JS. 2010. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng. A 16, 3457–3466. ( 10.1089/ten.tea.2010.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, Tresco PA. 2006. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J. Biomech. 39, 1136–1144. ( 10.1016/j.jbiomech.2004.08.026) [DOI] [PubMed] [Google Scholar]
- 179.Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. 2013. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng. A 19, 299–306. ( 10.1089/ten.tea.2012.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meyer EG, Buckley CT, Steward AJ, Kelly DJ. 2011. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 4, 1257–1265. ( 10.1016/j.jmbbm.2011.04.012) [DOI] [PubMed] [Google Scholar]
- 181.Chen T, Buckley M, Cohen I, Bonassar L, Awad HA. 2012. Insights into interstitial flow, shear stress, and mass transport effects on ECM heterogeneity in bioreactor-cultivated engineered cartilage hydrogels. Biomech. Model. Mechanobiol. 11, 689–702. ( 10.1007/s10237-011-0343-x) [DOI] [PubMed] [Google Scholar]
- 182.Eniwumide JO, Lee DA, Bader DL. 2009. The development of a bioreactor to perfuse radially-confined hydrogel constructs: design and characterization of mass transport properties. Biorheology 46, 417–437. ( 10.3233/BIR-2009-0552) [DOI] [PubMed] [Google Scholar]
- 183.Orr AW, Helmke BP, Blackman BR, Schwartz MA. 2006. Mechanisms of mechanotransduction. Dev. Cell 10, 11–20. ( 10.1016/j.devcel.2005.12.006) [DOI] [PubMed] [Google Scholar]
- 184.Starr DA, Fridolfsson HN. 2010. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 26, 421–444. ( 10.1146/annurev-cellbio-100109-104037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. 2006. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41–53. ( 10.1083/jcb.200509124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Khatau SB, et al. 2012. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci. Rep. 2, 488 ( 10.1038/srep00488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sosa BA, Rothballer A, Kutay U, Schwartz TU. 2012. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035–1047. ( 10.1016/j.cell.2012.03.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Boitano S, Sanderson MJ, Dirksen ER. 1994. A role for Ca(2+)-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. J. Cell Sci. 107, 3037–3044. [DOI] [PubMed] [Google Scholar]
- 189.Stojkovska J, Bugarski B, Obradovic B. 2010. Evaluation of alginate hydrogels under in vivo-like bioreactor conditions for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 21, 2869–2879. ( 10.1007/s10856-010-4135-0) [DOI] [PubMed] [Google Scholar]
- 190.Gonen-Wadmany M, Gepstein L, Seliktar D. 2004. Controlling the cellular organization of tissue-engineered cardiac constructs. Ann. N.Y. Acad. Sci. 1015, 299–311. ( 10.1196/annals.1302.025) [DOI] [PubMed] [Google Scholar]
- 191.Rathbone SR, Glossop JR, Gough JE, Cartmell SH. 2012. Cyclic tensile strain upon human mesenchymal stem cells in 2D and 3D culture differentially influences CCNL2, WDR61 and BAHCC1 gene expression levels. J. Mech. Behav. Biomed. Mater. 11, 82–91. ( 10.1016/j.jmbbm.2012.01.019) [DOI] [PubMed] [Google Scholar]
- 192.Hall AC. 1999. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J. Cell. Physiol. 178, 197–204. () [DOI] [PubMed] [Google Scholar]
- 193.Elder BD, Athanasiou KA. 2009. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng. B 15, 43–53. ( 10.1089/ten.teb.2008.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Toyoda T, Seedhom BB, Kirkham J, Bonass WA. 2003. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology 40, 79–85. [PubMed] [Google Scholar]
- 195.Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA. 2003. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum 48, 2865–2872. ( 10.1002/art.11250) [DOI] [PubMed] [Google Scholar]
- 196.Malone AM, Batra NN, Shivaram G, Kwon RY, You L, Kim CH, Rodriguez J, Jair K, Jacobs CR. 2007. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am. J. Physiol. Cell Physiol. 292, C1830–C1836. ( 10.1152/ajpcell.00352.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]