Abstract
Alpine meadows are one major type of pastureland on the Tibetan Plateau. However, few studies have evaluated the response of soil respiration (R s) to grazing along an elevation gradient in an alpine meadow on the Tibetan Plateau. Here three fenced enclosures were established in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) in July 2008. We measured R s inside and outside the three fenced enclosures in July–September, 2010-2011. Topsoil (0–20 cm) samples were gathered in July, August, and September, 2011. There were no significant differences for R s, dissolved organic C (DOC), and belowground root biomass (BGB) between the grazed and ungrazed soils. Soil respiration was positively correlated with soil organic C (SOC), microbial biomass (MBC), DOC, and BGB. In addition, both R s and BGB increased with total N (TN), the ratio of SOC to TN, ammonium N (NH4 +-N), and the ratio of NH4 +-N to nitrate N. Our findings suggested that the negligible response of R s to grazing could be directly attributed to that of respiration substrate and that soil N may indirectly affect R s by its effect on BGB.
1. Introduction
Soil respiration (R s) is an important flux in the C cycle [1–3]. Raich and Schlesinger [1] indicated that R s is the second in magnitude to gross primary production but equivalent to or even greater than net primary production. Root and microbial respiration are the two most important components of R s; thus the factors which affect root growth and microbial activity could all influence R s [4–6]. Water availability and temperature are the two most important abiotic factors controlling R s at various spatial and temporal scales [7, 8]. The positive relationship between R s and temperature could be weakened or masked by other factors (e.g., respiration substrate) [9, 10]. Previous studies have shown that R s increases with respiration substrate, including labile C (e.g., microbial biomass C, MBC; dissolved organic C, DOC) and belowground root biomass (BGB) [5, 10–12]. Soil N also affect R s by influencing plant growth and microbial activity [6, 13].
Grazing is a major type of land use in grasslands and previous studies have shown inconsistent results on the response of R s to grazing [14, 15]. Many studies have indicated that grazing significantly decreased R s [14, 16, 17], whereas other studies have shown quite the contrary result [18, 19]. The responses of soil C and N (e.g., MBC, DOC, microbial biomass N, and dissolved organic N) to grazing differ among previous studies [20–23]. There are also inconsistent results on the response of BGB to grazing [15, 24]. The response of R s to grazing is complex and may be dependent on the responses of respiration substrate [25] and soil N.
Alpine meadows are a major type of pastureland on the Tibetan Plateau [14, 22] and store 4.68 Pg soil organic C (SOC) with density of 9.05 kg m−2 at depth of 0–100 cm [26]. Few studies have evaluated the response of R s to grazing in an alpine meadow along an elevation gradient, although pasture for domestic sheep and yak is a common land use type on the Tibetan Plateau [22]. Here, we investigated the grazing effect on R s in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) on the Northern Tibetan Plateau.
The main objectives of this study were to examine (1) the effect of grazing on R s, soil C, and N and (2) the relationships between R s and respiration substrate (soil organic C, MBC, DOC, and BGB) and soil N (total N, microbial biomass N, dissolved organic N, ammonium N, and nitrate N) along an elevation gradient in an alpine meadow in Tibet.
2. Materials and Methods
2.1. Study Area
The study area (30°30′–30°32′N and 91°03′–91°04′E) was located at the Damxung Grassland Observation Station, Tibetan Autonomous Region in China. Annual mean solar radiation was 7527.6 MJ m−2 and sunlight was 2880.9 h [12]. Annual average precipitation was around 476.8 mm and annual potential evapotranspiration was about 1725.7 mm [27]. Annual mean air temperature was 1.3°C [12]. The soil was classified as a shallow sandy loam (~0.5–0.7 m), with organic matter of 0.3–11.2%, total N of 0.03–0.49%, and pH of 6.0–6.7 [22]. The vegetation surrounding the study site was Kobresia-dominated alpine meadow [28]. Roots are mainly concentrated in the topsoil layer (0–20 cm) [29].
Based on meteorological observations from 1963 to 2012 at the Damxung Station (4288 m, approximately 4 km from our study site), there was no significant change for annual precipitation, while annual mean air temperature increased at a rate of 0.04°C a−1 [12].
2.2. Experimental Design
Three sites (about 20 m × 20 m for each) were fenced in an alpine meadow on a south-facing slope on the Nyainqentanglha Mountains along an elevation gradient (i.e., 4313 m, 4513 m, and 4693 m) in July 2008. Before enclosure, the site at elevation 4313 m was winter pasture, while the other two sites were summer pasture [22]. A more detailed description of the experimental design can be found in Fu et al. [22].
Soil temperature (T s) at a depth of 5 cm, soil water content (SWC) at a depth of 10 cm, and air temperature and relative humidity at a height of 15 cm were continuously monitored using data loggers (HOBO weather station, Onset Computer Corporation, USA) at each elevation [22]. Both air temperature and T s increased with decreasing elevation [22].
2.3. Measurement of R s
Soil respiration was measured using a soil CO2 flux system (LI-8100, LI-COR Biosciences, Lincoln, NE, USA) [6, 30] during the period from July to September in 2010 and 2011 (Figure 1). Soil respirationat 9:00–11:00 am was close to daily average R s [6, 31]; thus R s was measured between 9:00 and 11:00 (local time) in this study. Four polyvinyl chloride (PVC) collars (20 cm in diameter and 5 cm in height) were inserted into the soil to depths of about 2-3 cm on each measuring date. All the PVC collars were installed and the aboveground biomass was removed at least 12 h before R s measurement in order to reduce disturbance [6, 12]. The opaque survey chamber was manually mounted on PVC collars for R s measurements [30]. One cycle was performed on each measuring date.
Figure 1.
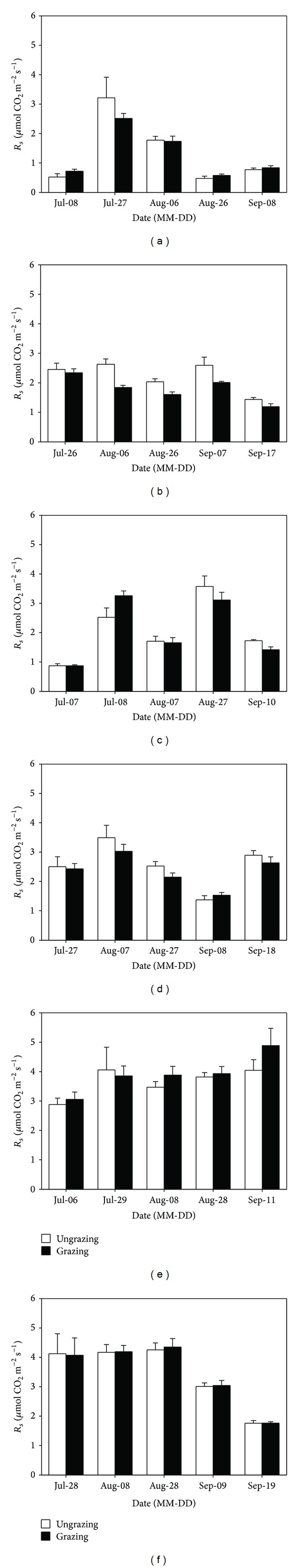
Effects of grazing on soil respiration (R s, μmol CO2 m−2 s−1) in an alpine meadow located at elevations of 4313 m (a, b), 4513 m (c, d), and 4693 m (e, f) on the Tibetan Plateau in 2010 (a, c, and e) and 2011 (b, d, and f), respectively. Error bars represent standard error (n = 4).
2.4. Soil Sampling and Analysis
Topsoil samples (0–20 cm depth) inside and outside the three fenced enclosures were collected (using a soil auger of 3.0 cm in diameter) on July 7, August 9, and September 10, 2011 [22]. Five soil subsamples were randomly sampled and composited into one soil sample for each of the four replicates. The composited soil samples were stored in an icebox and transferred to laboratory. We sieved soil samples (with a sieve of 1 mm diameter) and picked up any visible roots from the sieved soil. Subsamples of the sieved soil were used to measure NO3 −-N, NH4 +-N, DOC, and DON. All the roots in the soil samples were washed, dried at 65°C for 48 h, and weighed.
We extracted 20 g fresh soil samples using 100 mL K2SO4. The K2SO4 extracts were filtered through 0.45 μm filter membrane and then soil available N (SAN, NO3 −-N, and NH4 +-N) in the extracts were analyzed on a LACHAT Quickchem Automated Ion Analyzer.
The methods of Jones and Willett [32] were used to determine DOC and dissolved total N (DTN). Briefly, we extracted 20 g fresh soil samples using 100 mL ultrapure water and filtered the extracts through 0.45 μm filter membrane. We analyzed the extractable soil organic C and total N in the ultrapure water extracts using a Liqui TOC II elementar analyzer (Elementar Liqui TOC, Elementar Co., Hanau, Germany) and a UV-1700 PharmaSpec visible spectrophotometer (220 nm and 275 nm), respectively. Dissolved inorganic N (DIN) concentrations in the ultrapure water extracts were also determined on a LACHAT Quickchem Automated Ion Analyzer. Then, DON was calculated as the difference between DTN and DIN. Soil organic C, TN, MBC, and MBN data were obtained from Fu et al. [22].
2.5. Statistical Analysis
Repeated-measures analysis of variance (ANOVA) was used to estimate the main and interactive effects of measuring date and grazing on R s for each site (Table 1). Repeated-measures ANOVA was used to estimate the main and interactive effects of sampling date and grazing on DOC, DON, DOC/DON ratio, NO3 −-N, NH4 +-N, NH4 +-N/NO3 −-N ratio, SAN, and BGB(Table 2). Student-Newman-Keuls multiple comparisons were performed among the three sites. Linear relationships of R s with SOC, TN, SOC/TN ratio, MBC, MBN, DOC, DON, NH4 +-N, SAN, NH4 +-N/NO3 −-N ratio, and BGB were conducted, respectively. All the statistical tests were performed using the SPSS software (version 16.0; SPSS Inc., Chicago, IL).
Table 1.
Repeated-measures analysis of variance for the main and interactive effects of grazing (G) and measuring date (D) on soil respiration (R s, µmol CO2 m−2 s−1) in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) on the Tibetan Plateau (n = 4).
| Model | 4313 m | 4513 m | 4693 m | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| G | 5.94 | 0.051 | 2.39 | 0.173 | 1.22 | 0.311 |
| D | 34.12 | <0.001 | 29.08 | <0.001 | 10.02 | <0.001 |
| G × D | 1.60 | 0.249 | 1.39 | 0.277 | 0.31 | 0.837 |
Table 2.
Repeated-measures analysis of variance for the main and interactive effects of grazing (G) and sampling date (D) on dissolved organic C (DOC, mg kg−1) and N (DON, mg kg−1), the ratio of DOC and DON (DOC/DON ratio), nitrate N (NO3 −-N, mg kg−1), ammonium N (NH4 +-N, mg kg−1), the ratio of NH4 +-N and NO3 −-N (NH4 +-N/NO3 −-N ratio), soil available N (SAN, mg kg−1), and belowground root biomass (BGB, kg m−2) in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) on the Tibetan Plateau (n = 4).
| Elevation | Model | DOC | DON | DOC/DON ratio | NO3 −-N | NH4 +-N | NH4 +-N/NO3 −-N ratio | SAN | BGB |
|---|---|---|---|---|---|---|---|---|---|
| 4313 m | G | 0.77 | 1.65 | 0.46 | 27.10** | 26.05** | 1.05 | 95.61*** | 0.00 |
| D | 26.25*** | 4.80* | 5.00* | 57.55*** | 10.47** | 8.95** | 29.95*** | 2.79 | |
| G × D | 2.64 | 1.46 | 0.29 | 11.02** | 3.00 | 2.16 | 7.13* | 1.45 | |
|
| |||||||||
| 4513 m | G | 0.00 | 2.23 | 5.51 | 7.29* | 13.99** | 0.04 | 16.50** | 1.52 |
| D | 35.53*** | 4.38* | 0.40 | 51.70*** | 29.87*** | 12.29*** | 57.31*** | 2.74 | |
| G × D | 3.15 | 3.81 | 3.49 | 1.85 | 2.05 | 0.51 | 3.07 | 0.98 | |
|
| |||||||||
| 4693 m |
G | 0.00 | 0.16 | 0.12 | 0.82 | 0.27 | 0.08 | 0.65 | 0.64 |
| D | 1.33 | 3.67 | 3.03 | 9.24** | 15.27*** | 0.94 | 15.78*** | 3.85 | |
| G × D | 3.41 | 1.24 | 0.04 | 1.79 | 6.95** | 0.30 | 5.67* | 0.95 | |
*, **, and ***means P < 0.05, P < 0.01, and P < 0.001, respectively.
3. Results
At elevation 4313 m, NO3 −-N, NH4 +-N, and SAN under grazing were 31.78% (1.95 mg kg−1), 39.14% (2.34 mg kg−1), and 35.41% (4.29 mg kg−1) lower compared with ungrazed soils across all the three sampling dates (Figure 2 and Table 2). Similarly, at elevation 4513 m, NO3 −-N, NH4 +-N, and SAN under grazing were 22.00% (1.41 mg kg−1), 23.60% (1.33 mg kg−1), and 22.75% (2.75 mg kg−1) lower than that of ungrazed soils (Figure 2 and Table 2). In contrast, there were no significant differences of NO3 −-N, NH4 +-N, and SAN between ungrazed and grazed soils at elevation 4693 m (Figure 2 and Table 2).
Figure 2.
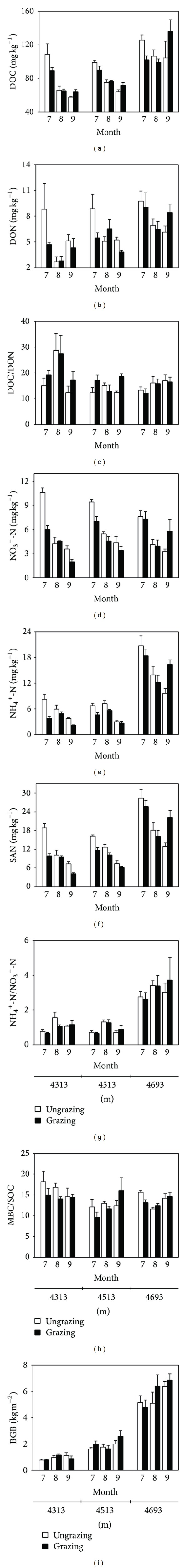
Effects of grazing on dissolved organic C (DOC, mg kg−1), dissolved organic N (DON, mg kg−1), the ratio of DOC and DON (DOC/DON ratio), nitrate N (NO3 −-N, mg kg−1), ammonium N (NH4 +-N, mg kg−1), soil available N (SAN, mg kg−1), the ratio of NH4 +-N and NO3 −-N (NH4 +-N/NO3 −-N ratio), and belowground root biomass (BGB, kg m−2) in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) on the Tibetan Plateau. Error bars represent standard error (n = 4). MBC and SOC data were obtained from Fu et al. [22].
In addition, grazing had no significant effects on NH4 +-N/NO3 −-N ratio, DOC, DON, DOC/DON ratio, and BGB for the three alpine meadow sites (Figure 2 and Table 2). Regardless of grazing, NO3 −-N, NH4 +-N, NH4 +-N/NO3 −-N ratio, SAN, DOC, DON, DOC/DON ratio, and BGB all showed similar seasonal dynamics among the three elevations (Figure 2).
No significant differences of DOC, NH4 +-N, and NH4 +-N/NO3 −-N ratio were found between elevations 4313 m and 4513 m, whereas they were significantly lower compared with elevation 4693 m whether or not grazing was present. Average DOC at elevations 4313 m and 4513 m across all the three sampling dates was 33.01% and 29.31% lower than that of elevation 4693 m, respectively, irrespective of grazing (P < 0.05). Average NH4 +-N at elevations 4313 m and 4513 m was 68.40% and 67.23% lower compared with elevation 4693 m, respectively (P < 0.05). Average NH4 +-N/NO3 −-N ratio at elevations 4313 m and 4513 m was 66.76% and 70.52% lower compared with elevation 4693 m, respectively (P < 0.05).
Average DON at elevations 4313 m and 4513 m across all the three sampling dates was 50.78% and 33.84% lower than that of elevation 4693 m under grazing, respectively (P < 0.05), whereas there was no significant difference between elevations 4313 m and 4513 m. There were no significant differences of average DON among the three sites when grazing was absent.
Average SAN at elevations 4313 m and 4513 m across all the three sampling dates was 38.69% and 38.88% lower than that of elevation 4693 m when grazing was absent, respectively (P < 0.05), while no significant difference between the two lower elevations was found. By contrast, average SAN increased with increasing elevation under grazing (F = 375.30, P < 0.001).
No significant differences of DOC/DON ratio and NO3 −-N were found among the three sites whether or not grazing was present.
The main effect of grazing and its interactive effect with measuring date on R s were not significant for each alpine meadow site (Figure 1 and Table 1). Grazing only tended to decrease the average R s across all the measuring dates by 14.02% (0.25 μmol CO2 m−2 s−1), 4.70% (0.11 μmol CO2 m−2 s−1), and −4.07% (−0.15 μmol CO2 m−2 s−1) at elevations 4313 m, 4513 m, and 4693 m, respectively. In contrast, there was significant seasonal variation for R s (Figure 1 and Table 1). Regardless of grazing, R s showed similar seasonal dynamics among the three elevations (Figure 1).
There were significant elevation effects on R s (F = 147.94, P < 0.001 for ungrazed condition; F = 227.25, P < 0.001 for grazed condition) and BGB (F = 315.20, P < 0.001 for ungrazed condition; F = 58.81, P < 0.001 for grazed condition) across all the measuring dates.
Belowground biomass was positively related to TN, NH4 +-N, SAN, SOC/TN ratio, and NH4 +-N/NO3 −-N ratio, respectively (Figure 3), but not to NO3 −-N (data not shown).
Figure 3.
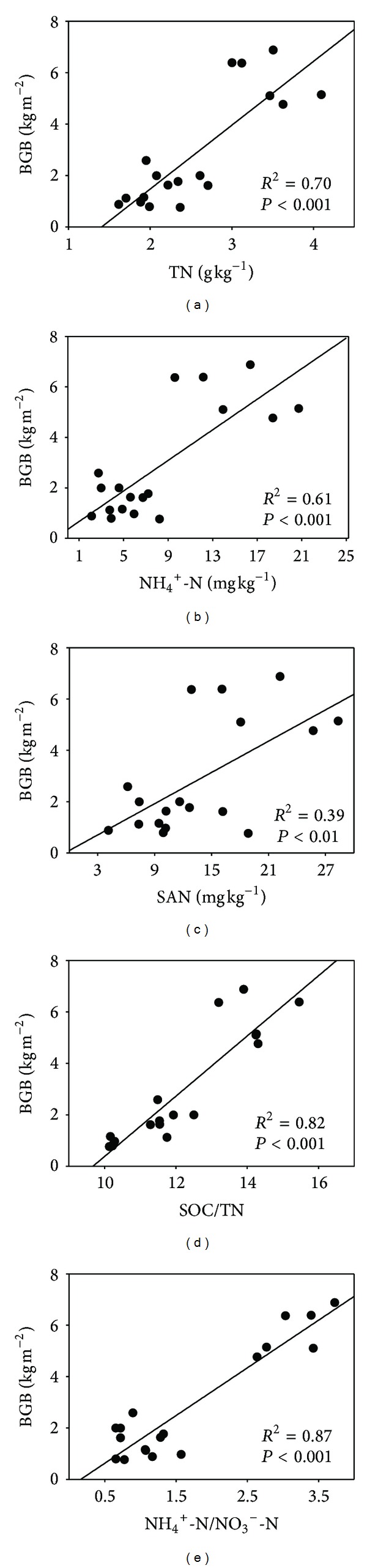
Relationships between belowground root biomass (BGB, kg m−2) and total N (TN, g kg−1), ammonium N (NH4 +-N, mg kg−1), soil available N (SAN, mg kg−1), the ratio of SOC and TN (SOC/TN ratio), and the ratio of NH4 +-N and nitrate N (NH4 +-N/NO3 −-N ratio), respectively. SOC and TN data were obtained from Fu et al. [22].
R s was positively correlated with SOC, TN, SOC/TN ratio, MBC, MBN, DOC, DON, NH4 +-N, SAN, NH4 +-N/NO3 −-N ratio, and BGB, respectively (Figure 4). However, R s was not linearly correlated with DOC/DON ratio, MBC/MBN ratio, and NO3 −-N (data not shown).
Figure 4.
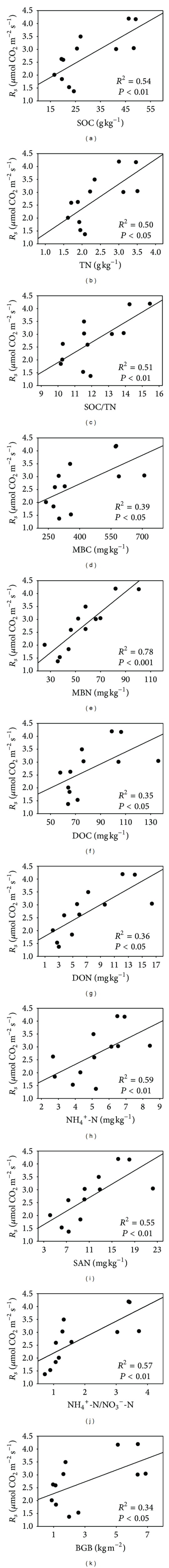
Relationships between soil respiration (R s, µmol CO2 m−2 s−1) measured early in August and September, 2011, and soil organic C (SOC, g kg−1), total N (TN, g kg−1), the ratio of SOC and TN (SOC/TN ratio), microbial biomass C (MBC, mg kg−1), microbial biomass N (MBN, mg kg−1), dissolved organic C (DOC, mg kg−1), dissolved organic N (DON, mg kg−1), ammonium N (NH4 +-N, mg kg−1), soil available N (SAN, mg kg−1), the ratio of NH4 +-N and nitrate N (NH4 +-N/NO3 −-N ratio), and belowground root biomass (BGB, kg m−2), respectively. SOC, TN, MBC, and MBN data were obtained from Fu et al. [22].
4. Discussion
Previous studies indicated that grazer urine and dung stimulated soil microbial activity and accelerated nutrient cycling in grasslands [33, 34]. However, this effect may be often weakened at the three alpine meadow sites because the dung of yak and goat was removed by local residents.
Generally, grazing did not alter the distributions of R s, DOC, DON, DOC/DON ratio, NO3 −-N, NH4 +-N, and BGB along the elevation gradient, which was in line with previous studies [22, 35, 36].
Soil microbial biomass N at elevations 4313 m and 4693 m and SAN (including NO3 −-N and NH4 +-N) at elevations 4313 m and 4513 m under grazing were significantly lower compared with ungrazed soils, while there were no significant differences of SOC, TN, DOC, DON, BGB, and R s between grazed and ungrazed soils (Tables 1 and 2, [22]). This suggests that soil microbial biomass and available N may respond more rapidly to grazing than SOC, TN, DOC, DON, BGB, and R s. The negligible response of R s to grazing was consistent with some previous studies conducted on the Tibetan Plateau (e.g., [15]).
Previous studies showed that BGB increased with increasing TN in alpine grasslands on the Tibetan Plateau [36, 37]. Our study confirmed this finding (Figure 3). Besides, BGB increased with increasing NH4 +-N, SAN, and NH4 +-N/NO3 −-N ratio, but not with NO3 −-N. Therefore, the positive relationship between BGB and SAN may be mainly attributed to that between BGB and NH4 +-N. In addition, the ratio of different soil available N forms could affect BGB.
Similar to BGB, R s increased with TN, SOC/TN ratio, NH4 +-N, SAN, and NH4 +-N/NO3 −-N ratio, respectively (Figure 4). Meanwhile, R s was positively correlated with BGB (Figure 4). Therefore, the effect of soil N availability and form on R s was probably associated with the effect of soil N availability and form on BGB. In addition, the negligible response of R s to grazing may be directly attributed to that of SOC, MBC, DOC, and BGB.
The positive relationships between DOC and SOC, DON and TN, DOC and MBC, DON and MBN, and SAN and MBN (data not shown) were in accordance with previous studies which were made in alpine meadows on the Tibetan Plateau [22, 38] and an upland grassland of northern England [39]. Previous studies found that DOC was a good index in reflecting C availability of soil microorganisms [40, 41]. Therefore, the variation of soil microbial biomass along the elevation gradient may be not only associated with that of SOC and TN [22] but also with that of DOC and DON [6, 11, 42]. In other words, soil microbial activity may regulate the balances of soil inorganic and organic C and N pools in this alpine meadow.
Many studies have found the positive relationship between R s and T s in various ecosystems [3, 15]. In contrast, R s increased significantly with increasing elevation, while both soil and air temperatures declined in the current study. In other words, the relationship between R s and T s was negative along the elevation gradient. This implied that other factors (e.g., respiration substrate) probably regulated or confounded the positive relationship between respiration and temperature [10, 25]. This viewpoint was as confirmed by the positive relationships between R s and BGB, SOC, MBC, and DOC (Figure 4).
5. Conclusions
In this study, we measured soil respiration under grazed and ungrazed conditions in an alpine meadow along an elevation gradient (4313–4693 m with approximate 200 m interval) in Tibet during the period from July to September in 2010-2011. We found that grazing did not significantly affect soil respiration, which was probably attributed to the insignificant response of respiration substrate (e.g., soil organic C and belowground root biomass) to grazing. Soil N availability and the ratio of ammonium to nitrate N might also influence soil respiration by affecting belowground root growth.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 40771121), National Science and Technology Plan Project of China (no. 2011BAC09B03), and the National Basic Research Program of China (nos. 2010CB951704 and 2010CB833502).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Raich JW, Schlesinger WH. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B. 1992;44(2):81–99. [Google Scholar]
- 2.Rustad LE, Campbell JL, Marion GM, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126(4):543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biology. 2011;17(2):927–942. [Google Scholar]
- 4.Singh JS, Gupta SR. Plant decomposition and soil respiration in terrestrial ecosystems. The Botanical Review. 1977;43(4):449–528. [Google Scholar]
- 5.Yan LM, Chen SF, Huang JH, Lin GH. Water regulated effects of photosynthetic substrate supply on soil respiration in a semiarid steppe. Global Change Biology. 2011;17(5):1990–2001. [Google Scholar]
- 6.Jiang J, Zonga N, Song M, et al. Responses of ecosystem respiration and its components to fertilization in an alpine meadow on the Tibetan Plateau. European Journal of Soil Biology. 2013;56:101–106. [Google Scholar]
- 7.Liu WX, Zhang Z, Wan SQ. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Global Change Biology. 2009;15(1):184–195. [Google Scholar]
- 8.Shi F, Chen H, Chen H, Wu Y, Wu N. The combined effects of warming and drying suppress CO2 and N2O emission rates in an alpine meadow of the eastern Tibetan Plateau. Ecological Research. 2012;27:725–733. [Google Scholar]
- 9.Luo YQ, Wan SQ, Hui DF, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413(6856):622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- 10.Frey SD, Drijber R, Smith H, Melillo J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biology and Biochemistry. 2008;40(11):2904–2907. [Google Scholar]
- 11.Iqbal J, Hu R, Feng M, Lin S, Malghani S, Ali IM. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: a case study at Three Gorges Reservoir Area, South China. Agriculture, Ecosystems and Environment. 2010;137(3-4):294–307. [Google Scholar]
- 12.Fu G, Zhang XZ, Zhou YT, Yu CQ, Shen ZX. Partitioning sources of ecosystem and soil respiration in an alpine meadow of Tibet Plateau using regression method. Polish Journal of Ecology. 2014;62:31–38. [Google Scholar]
- 13.Song M-H, Jiang J, Xu X-L, Shi P-L. Correlation between CO2 efflux and net nitrogen mineralization and its response to external C or N supply in an alpine meadow soil. Pedosphere. 2011;21(5):666–675. [Google Scholar]
- 14.Cao G, Tang Y, Mo W, Wang Y, Li Y, Zhao X. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biology and Biochemistry. 2004;36(2):237–243. [Google Scholar]
- 15.Lin XW, Zhang Z, Wang SQ, et al. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agricultural and Forest Meteorology. 2011;151(7):792–802. [Google Scholar]
- 16.Zhou X, Wan SQ, Luo YQ. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Global Change Biology. 2007;13(4):761–775. [Google Scholar]
- 17.Bahn M, Rodeghiero M, Anderson-Dunn M, et al. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems. 2008;11(8):1352–1367. doi: 10.1007/s10021-008-9198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank AB, Liebig MA, Hanson JD. Soil carbon dioxide fluxes in northern semiarid grasslands. Soil Biology and Biochemistry. 2002;34(9):1235–1241. [Google Scholar]
- 19.Li G, Sun S. Plant clipping may cause overestimation of soil respiration in a Tibetan alpine meadow, southwest China. Ecological Research. 2011;26(3):497–504. [Google Scholar]
- 20.Wright AL, Hons FM, Rouquette FM., Jr. Long-term management impacts on soil carbon and nitrogen dynamics of grazed bermudagrass pastures. Soil Biology and Biochemistry. 2004;36(11):1809–1816. [Google Scholar]
- 21.Shrestha G, Stahl PD. Carbon accumulation and storage in semi-arid sagebrush steppe: effects of long-term grazing exclusion. Agriculture, Ecosystems and Environment. 2008;125(1–4):173–181. [Google Scholar]
- 22.Fu G, Shen Z, Zhang X, Zhou Y, Zhang Y. Response of microbial biomass to grazing in an alpine meadow along an elevation gradient on the Tibetan Plateau. European Journal of Soil Biology. 2012;52:27–29. [Google Scholar]
- 23.Wu H, Wiesmeier M, Yu Q, Steffens M, Han X, Kögel-Knabner I. Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biology and Fertility of Soils. 2012;48(3):305–313. [Google Scholar]
- 24.Xu X, Niu S, Sherry RA, Zhou X, Zhou J, Luo Y. Interannual variability in responses of belowground net primary productivity (NPP) and NPP partitioning to long-term warming and clipping in a tallgrass prairie. Global Change Biology. 2012;18(5):1648–1656. [Google Scholar]
- 25.Wan SQ, Luo YQ. Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochemical Cycles. 2003;17(2):23–1. [Google Scholar]
- 26.Yang YH, Fang JY, Tang YH, et al. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Global Change Biology. 2008;14(7):1592–1599. [Google Scholar]
- 27.Fu G, Shen Z, Zhang X, Zhou Y. Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Applied Soil Ecology. 2012;61:158–160. [Google Scholar]
- 28.Fu G, Shen Z, Zhang X, et al. Calibration of MODIS-based gross primary production over an alpine meadow on the Tibetan Plateau. Canadian Journal of Remote Sensing. 2012;38:157–168. [Google Scholar]
- 29.Fu G, Zhang X, Zhang Y, et al. Experimental warming does not enhance gross primary production and above-ground biomass in the alpine meadow of Tibet. Journal of Applied Remote Sensing. 2013;7073505 [Google Scholar]
- 30.Fu G, Shen Z, Zhang X, Yu C, Zhou LI Y, Yang P. Response of ecosystem respiration to experimental warming and clipping at daily time scale in an alpine meadow of Tibet. Journal of Mountain Science. 2013;10:455–463. [Google Scholar]
- 31.Zong N, Shi P, Jiang J, et al. Responses of ecosystem CO2 fluxes to short-term experimental warming and nitrogen enrichment in an alpine meadow, Northern Tibet Plateau. Scientific World Journal. 2013;2013:11 pages. doi: 10.1155/2013/415318.415318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones DL, Willett VB. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry. 2006;38(5):991–999. [Google Scholar]
- 33.Augustine DJ, McNaughton SJ, Frank DA. Feedbacks between soil nutrients and large herbivores in a managed savanna ecosystem. Ecological Applications. 2003;13(5):1325–1337. [Google Scholar]
- 34.Bardgett RD, Wardle DA. Herbivore-mediated linkages between aboveground and belowground communities. Ecology. 2003;84(9):2258–2268. [Google Scholar]
- 35.Ohtsuka T, Hirota M, Zhang X, et al. Soil organic carbon pools in alpine to nival zones along an altitudinal gradient (4400–5300 m) on the Tibetan Plateau. Polar Science. 2008;2(4):277–285. [Google Scholar]
- 36.Wang Z, Luo TX, Li RC, Tang YH, Du MY. Causes for the unimodal pattern of biomass and productivity in alpine grasslands along a large altitudinal gradient in semi-arid regions. Journal of Vegetation Science. 2013;24:189–201. [Google Scholar]
- 37.Yang YH, Fang JY, Ji CJ, Han WX. Above- And belowground biomass allocation in Tibetan grasslands. Journal of Vegetation Science. 2009;20(1):177–184. [Google Scholar]
- 38.Rui Y, Wang S, Xu Z, et al. Warming and grazing affect soil labile carbon and nitrogen pools differently in an alpine meadow of the Qinghai-Tibet Plateau in China. Journal of Soils and Sediments. 2011;11(6):903–914. [Google Scholar]
- 39.Medina-Roldán E, Paz-Ferreiro J, Bardgett RD. Grazing exclusion affects soil and plant communities, but has no impact on soil carbon storage in an upland grassland. Agriculture, Ecosystems and Environment. 2012;149:118–123. [Google Scholar]
- 40.Boyer JN, Groffman PM. Bioavailability of water extractable organic carbon fractions in forest and agricultural soil profiles. Soil Biology and Biochemistry. 1996;28(6):783–790. [Google Scholar]
- 41.Kalbitz K, Solinger S, Park J-H, Michalzik B, Matzner E. Controls on the dynamics dissolved organic matter in soils:a review. Soil Science. 2000;165(4):277–304. [Google Scholar]
- 42.Song MH, Jiang J, Cao GM, Xu XL. Effects of temperature, glucose and inorganic nitrogen inputs on carbon mineralization in a Tibetan alpine meadow soil. European Journal of Soil Biology. 2010;46(6):375–380. [Google Scholar]


