Abstract
Epithelial–mesenchymal transition represents a key event in cancer progression and has emerged as a promising anticancer target. Estrogen-related receptor alpha (ERRα) is frequently elevated in advanced-stage ovarian cancer, but its potential role in tumor progression is not known. Here we show that ERRα functions in epithelial–mesenchymal transition and in subsequent stem cell traits responsible for the acquisition of high degree of aggressiveness and potential for metastasis that are characteristic of ovarian cancer. Importantly, targeted inhibition of ERRα also inhibited the expression of Snail, a repressor of E-cadherin and an inducer of epithelial–mesenchymal transition. Interestingly, induction of Snail resulted from not only changes in mRNA transcription rate but also mRNA stability. We thus identified the miR-200 family as a new player in the ERRα-mediated posttranscriptional regulation of Snail, and antagonism of miR-200a/b could revert the decreased expression of Snail and reversal of epithelial–mesenchymal transition and stem cell characteristics due to ERRα depletion. Finally, we showed that RNA interference–mediated inhibition of ERRα significantly reduced tumor burden, ascites formation, and metastatic peritoneal nodules in vivo in an orthotopic model of ovarian cancer. These results suggest ERRα activation as a mechanism of tumor aggressiveness and imply that targeting ERRα may be a promising approach in ovarian cancer treatment.
Introduction
Ovarian cancer is an aggressive disease, with 204,000 cases diagnosed worldwide each year, and is the leading cause of death among all gynecologic tumors.1 The late diagnosis, combined with widespread intraperitoneal metastasis and ascites formation, makes it extremely challenging to treat ovarian cancer in which current treatment options are largely ineffective, resulting in a dismal 5-year survival of <25%. Therefore, understanding the molecular mechanisms that mediate ovarian cancer progression is critically important in the search for novel therapeutic approaches.
Estrogen-related receptor alpha (ERRα) was among the first orphan members of the nuclear receptor superfamily to be discovered.2 Because of its structural similarities with estrogen receptor, initial studies on the possible roles of ERRα focused mainly on the potential cross talk between these two receptors. However, this concept has recently been revisited to reveal estrogen receptor–independent functions that are unique to ERRα in tumor biology. Of particular interest, levels of ERRα, but not those of other family members, are associated with a worse prognosis and have been reported to be elevated in the more-aggressive tumors in ovarian cancer.3,4 This opens the possibility that ERRα could directly regulate tumor progression of ovarian cancer cells. However, whether and how ERRα is involved in the process of metastasis remains unknown.
Epithelial-to-mesenchymal transition (EMT) is considered a key step in metastasis, including ovarian cancer, which endows carcinoma cells with enhanced migratory and survival abilities that facilitate malignant progression.5,6 Recent findings further illustrate a link between EMT and the gain of stem cell properties, and these studies provide a new concept for therapies that target cancer stem cells (CSCs).7 Understanding the molecular mechanisms that enable ovarian cancer cell dissemination, in particular characterizing EMT effectors, will yield important insights.
Loss or reduction of E-cadherin is a well-established hallmark of EMT, and the zinc finger transcription factors of the Snail/Slug family have been implicated in this repression.8 Although downstream effects of Snail/Slug activation are well defined, less is known about primary events that initiate EMT. Moreover, given that directly inhibiting transcription factors is currently infeasible,9,10 identifying their upstream regulators will also have great therapeutic significance.
In this study, we show for the first time that targeted inhibition of ERRα in highly metastatic ovarian cancer cells significantly attenuates EMT, CSC formation, and metastasis in vitro and in vivo. We also provide mechanistic insight suggesting that miR-200s, newly identified targets of ERRα action, is a critical upstream effector of Snail-dependent repression of E-cadherin of ERRα in this process.
Results
Targeted inhibition of ERRα suppresses EMT
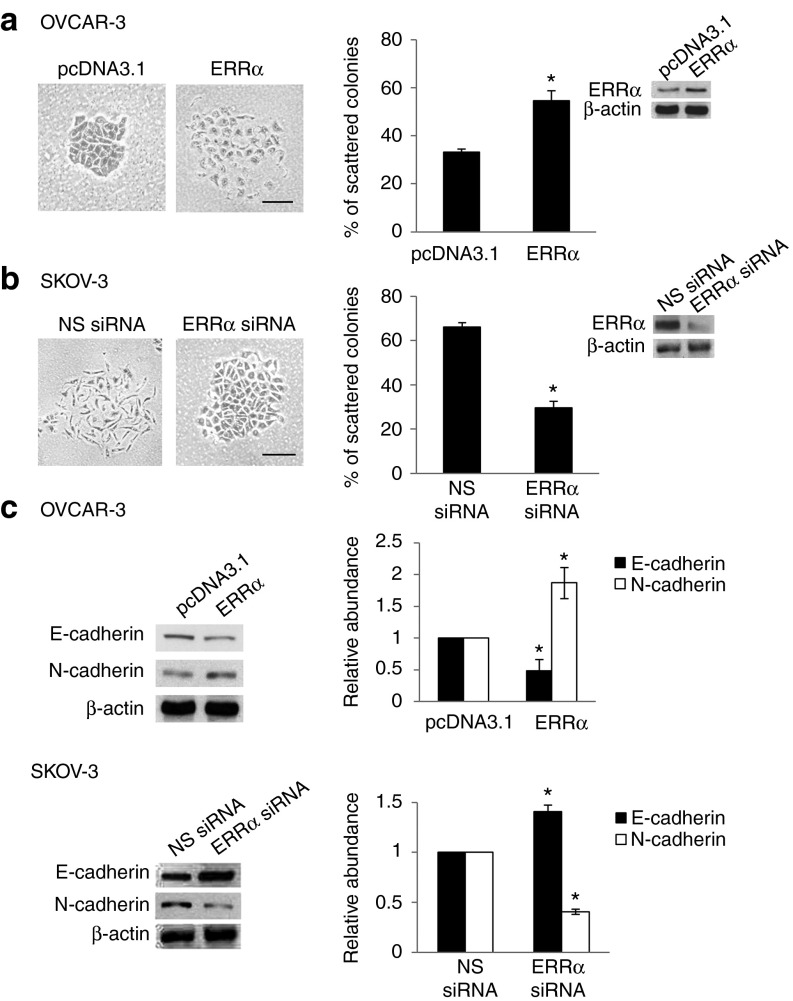
EMT is an important driver of cancer progression. To assess the functional role of ERRα in EMT, we first transfected OVCAR-3 cells with the wild-type ERRα construct, taking advantage of its low ERRα expression and epithelial morphology, and changes in cell behavior were followed by optical microscopy. Figure 1a shows that OVCAR-3 cells transfected with ERRα lost their cobblestone-like epithelial morphology but were dispersed and assumed a spindle-like fibroblast appearance. These changes are typical of cells with a mesenchymal phenotype. Conversely, we used RNA interference–mediated suppression of ERRα in SKOV-3, which had high ERRα expression and the cells in which were spindle-shaped and exhibited reduced cell–cell contact (Figure 1b). The effectiveness of this small interfering RNA (siRNA) in depleting ERRα expression was confirmed by western blotting (Figure 1b, inset). Knockdown of ERRα could revert the mesenchymal phenotype to an epithelial phenotype (Figure 1b). This was also confirmed by western blot analysis, which showed a decrease of the epithelial cell marker, E-cadherin expression, and an increase of the mesenchymal cell marker, N-cadherin expression, on ERRα overexpression (Figure 1c). Knocking down ERRα also stimulated the induction of E-cadherin and suppressed the expression of N-cadherin (Figure 1c). The use of nonspecific siRNA had no effect. In addition, there was a correlation between ERRα expression and metastatic phenotype in cell lines (Supplementary Figure S1). ERRα was highly expressed in CaOV-3, SKOV-3, and HEYA8 cells, all of which have been shown to frequently metastasize when inoculated into mice.11 OVCAR-3 and OV-90 cells, which possess less metastatic potential, showed lower ERRα expression.12 ERRα was absent in human ovarian surface epithelium (OSE), the tissue of origin of epithelial ovarian carcinomas. Together, these data show a critical role of ERRα in the induction of EMT and metastatic phenotypes.
Figure 1.
ERRα induces EMT in ovarian cells. (a) OVCAR-3 cells transfected with empty vector pcDNA3.1 or ERRα construct or (b) SKOV-3 cells transfected with NS siRNA or ERRα siRNA for 24 hours were fixed and assessed for morphologic changes consistent with EMT. Left, the presence of spindle-shaped cells with loss of epithelial cell morphology was noted in ERRα-overexpressing and NS siRNA–treated cells. Right, scattered colonies were scored. Inset, whole-cell lysates were analyzed for the levels of ERRα by western blot analysis. (c) Expression of the epithelial cell molecule E-cadherin and expression of mesenchymal marker N-cadherin were assessed by western blotting. β-actin was included as a loading control. The band intensities were quantified by densitometric analysis and expression levels relative to that of β-actin are indicated. Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. *P < 0.05, compared with pcDNA3.1 or NS siRNA. Bar = 50 µm. EMT, epithelial–mesenchymal transition; ERRα, estrogen-related receptor alpha; NS, nonspecific; siRNA, small interfering RNA.
ERRα represses E-cadherin expression through Snail
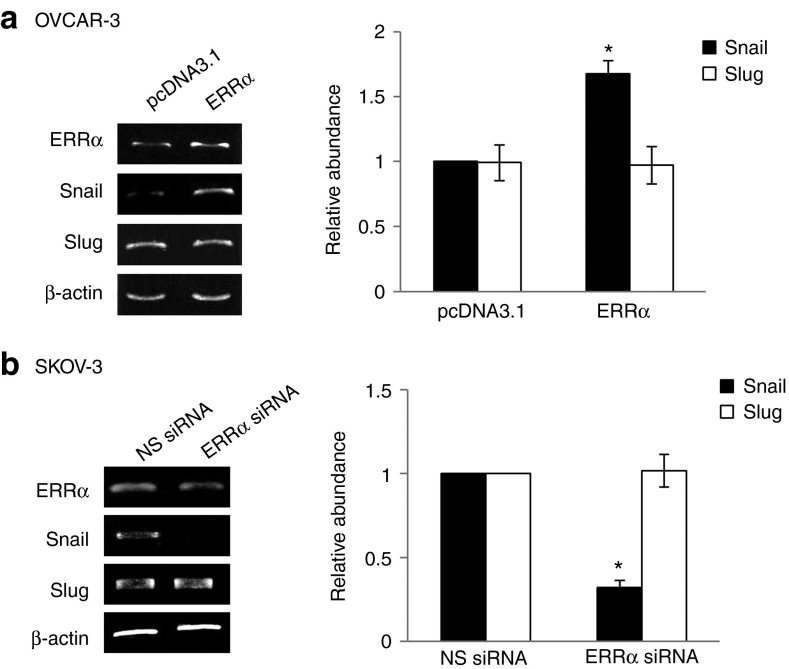
Snail and Slug are zinc finger transcription factors that induce EMT and repress E-cadherin gene transcription.13,14,15 To elucidate the mechanism of ERRα-regulated EMT, we examined changes in expression levels of these transcription factors. Our results showed that overexpression of ERRα was associated with an increase in the expression of Snail but it had no effect on Slug (Figure 2a). To further elucidate the involvement of ERRα in Snail upregulation, ERRα was repressed by the use of siRNA. ERRα-specific siRNA markedly reduced Snail mRNA (Figure 2b). No inhibition was observed with nonspecific siRNA (Figure 2b). Similarly, ERRα siRNA did not affect Slug expression (Figure 2b), suggesting a potent role of Snail in ERRα-mediated EMT regulation.
Figure 2.
ERRα induces the expression of Snail. (a) OVCAR-3 cells were transfected with empty vector pcDNA3.1 or ERRα construct or (b) SKOV-3 cells were transfected with nonspecific (NS) siRNA or ERRα siRNA for 24 hours. Total RNA was extracted and reverse transcription–polymerase chain reaction was performed using sequence-specific primers to ERRα, Snail, and Slug. β-actin was included as an internal control. The signal intensities were quantified by densitometric analysis and the amount was normalized for the amount of β-actin. Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. *P < 0.05, compared with pcDNA3.1 or NS siRNA. ERRα, estrogen-related receptor alpha; siRNA, small interfering RNA.
ERRα activates Snail via both transcriptional and posttranscriptional mechanisms
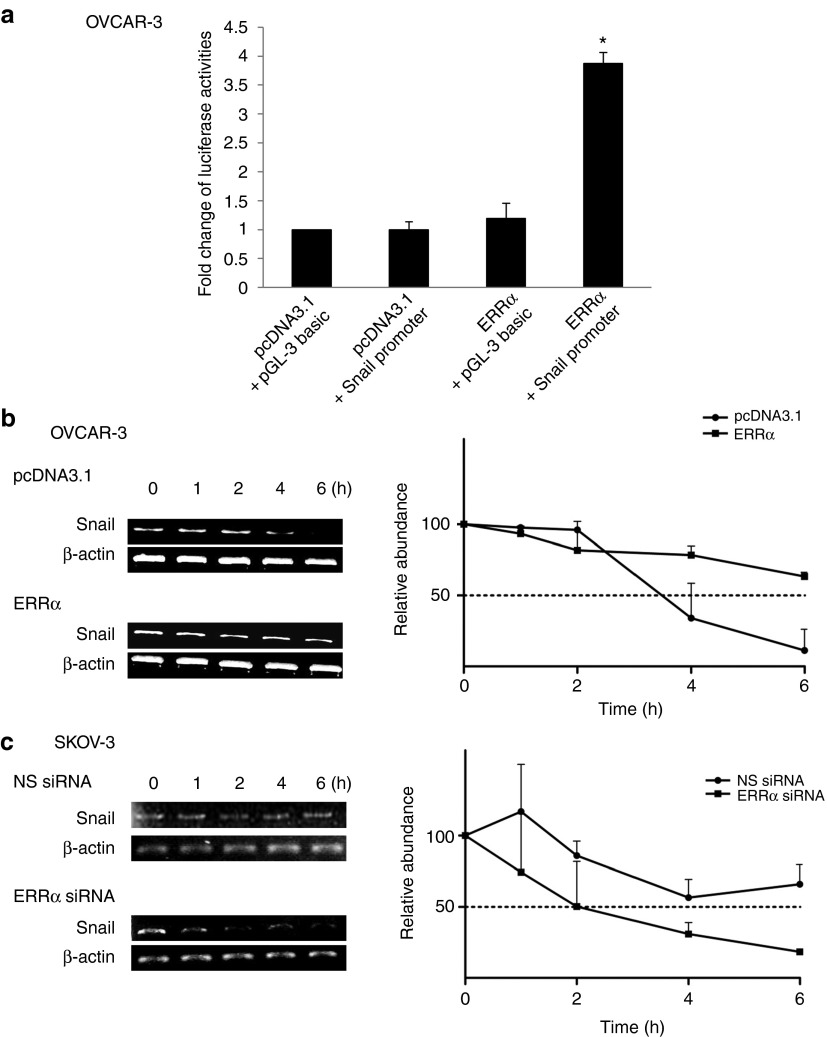
Next, we examined the mechanism by which ERRα increases Snail mRNA expression. This increase could be due to the result of increased synthesis of the transcript and/or stability. To examine whether Snail was activated at the transcriptional level, luciferase reporter containing the 5′ promoter region of Snail was expressed in ERRα-expressing cells. As shown, the expression of ERRα caused a significant increase in the activation of the Snail promoter (Figure 3a). To examine whether ERRα regulates Snail expression at the posttranscriptional level, we performed actinomycin D chase experiments to determine the half-life of Snail mRNA. Figure 3b shows that the half-life of Snail mRNA was dramatically prolonged by ERRα expression. However, in cells that were treated with ERRα siRNA, there was a substantial decrease in the half-life of Snail mRNA (Figure 3c), suggesting that the significant increase in Snail mRNA levels in response to ERRα could be due to the combined effects on both transcription rate and stability.
Figure 3.
ERRα activates the promoter of Snail and attenuates its mRNA degradation. (a) OVCAR-3 cells were transiently transfected with 1.5 µg of the Snail promoter and 15 ng of β-galactosidase plasmid for 24 hours. Luciferase and β-galactosidase activities were assayed, and the luciferase activity of each sample was normalized with β-galactosidase activity. The luciferase activity was calculated relative to that with promoter alone, which was arbitrarily assigned a value of one. (b) OVCAR-3 cells transfected with the empty vector pcDNA3.1 or ERRα construct or (c) SKOV-3 cells transfected with nonspecific (NS) siRNA or ERRα siRNA were incubated with actinomycin D (ActD; 5 µg/ml) over a time course of 0, 1, 2, 4, and 6 hours. Total RNA was then extracted and reverse transcription–polymerase chain reaction was performed using Snail sequence–specific primers. β-actin was included as an internal control. The signal intensities were quantified by densitometric analysis, and the amount was normalized for the amount of β-actin. Results are presented as the mean ± SD and were analyzed using Kruskal–Wallis test followed by Dunn's test for post hoc analysis. *P < 0.05, compared with pcDNA3.1. ERRα, estrogen-related receptor alpha; siRNA, small interfering RNA.
miR-200 family members are regulated by ERRα
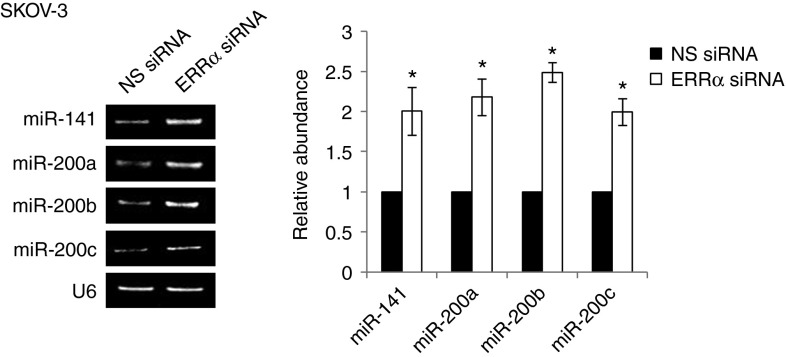
MicroRNAs (miRNAs), a class of 21- to 23-nucleotide-long noncoding RNAs, are emerging as an attractive mechanism to inhibit gene expression posttranscriptionally by either translational blockage or mRNA degradation.16 To identify miRNAs that are regulated by ERRα, we performed an unbiased screen using prediction softwares and the set of miRNAs known to be associated with ovarian cancer progression.17,18 We thus identified the miR-200 family (miR-141, miR-200a, miR-200b, and miR-200c) as Snail regulators. Although previously published data have shown that miR-200 family members can regulate Snail in epiblast differentiation during development,19 the regulation of Snail by miR-200 family members in tumor cells has never been investigated before. On examining the expression level of miR-141, miR-200a, miR-200b, and miR-200c, we found that the miRNAs are highly expressed in ERRα siRNA–treated cells, whereas nonspecific siRNA–treated cells showed no effect (Figure 4).
Figure 4.
ERRα-induced EMT requires miR-200 family members. SKOV-3 cells were transfected with NS siRNA or ERRα siRNA for 24 hours. Total RNA was extracted and reverse transcription–polymerase chain reaction was performed using sequence-specific primers to miR-141, miR-200a, miR-200b, and miR-200c. U6 was included as an internal control. The signal intensities were quantified by densitometric analysis and the amount was normalized for the amount of U6. Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. *P < 0.05, compared with NS siRNA. EMT, epithelial–mesenchymal transition; ERRα, estrogen-related receptor alpha; NS, nonspecific; siRNA, small interfering RNA.
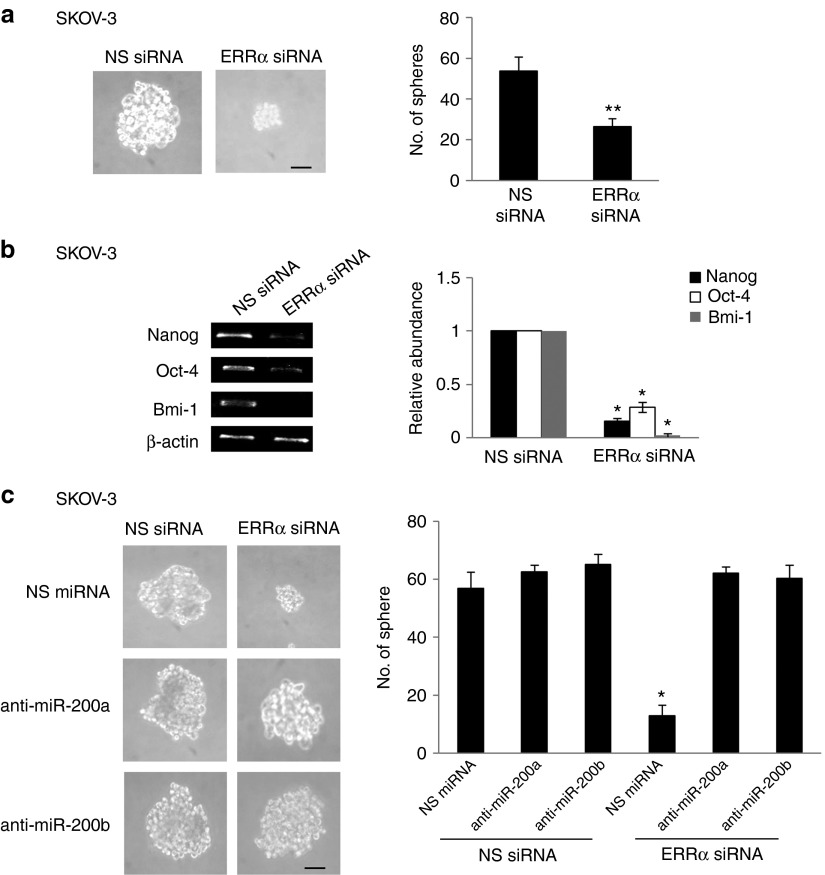
ERRα-regulated miR-200 family is involved in ERRα-mediated EMT and CSC
To assess whether miR-200 was required for ERRα-induced EMT regulation, we used hsa-miR-200a and hsa-miR-200b antagomirs. By inhibiting miR-200a and/or miR-200b, the decreased Snail expression and the reversal of EMT caused by ERRα depletion was significantly inhibited (Figure 5). Because cancer cells that undergo EMT can acquire stem cell properties that promote aggressive metastatic behavior, we determined the presence of CSCs in ERRα knockdown cells. Knockdown of ERRα significantly reduced formation of CSC-enriched spheres (Figure 6a). There was also a significant decrease in the expression of Nanog, Bmi-1, and Oct-4, which are established ovarian CSC markers,20 in ERRα knockdown cells (Figure 6b). We also observed that miR-200a and miR-200b antagomirs had a substantial function in reverting ERRα siRNA–mediated inhibition of sphere formation (Figure 6c). These results reveal a key role of the miR-200 family members in ERRα-mediated EMT regulation and show that miR-200 also has a role in EMT-regulated CSCs.
Figure 5.
ERRα-induced EMT requires miR-200 family members. (a) SKOV-3 cells were transfected with nonspecific (NS) siRNA or ERRα siRNA in combination with NS miRNA, anti-miR-200a, or anti-miR-200b for 24 hours. Morphologic changes were assessed by light microscopy (upper) and scattered colonies were scored (lower). (b) Total RNA was extracted and reverse transcription–polymerase chain reaction was performed using sequence-specific primers to Snail. β-actin was included as an internal control. The signal intensities were quantified by densitometric analysis and the amount was normalized for the amount of β-actin. Results are presented as the mean ± SD and were analyzed using Kruskal–Wallis test followed by Dunn's test for post hoc analysis. *P < 0.05, compared with NS siRNA. Bar = 50 µm. EMT, epithelial–mesenchymal transition; ERRα, estrogen-related receptor alpha; siRNA, small interfering RNA.
Figure 6.
Knockdown of ERRα inhibits CSC phenotype. (a) SKOV-3 cells were transfected with nonspecific (NS) siRNA or ERRα siRNA for 72 hours. The number of tumor spheres generated was photographed (left) and counted (right). Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. (b) Total RNA was extracted and reverse transcription–polymerase chain reaction was performed using sequence-specific primers to Nanog, Oct-4, and Bmi-1. β-Actin was included as an internal control. The signal intensities were quantified by densitometric analysis and the amount was normalized for the amount of β-actin. Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. (c) The number of tumor spheres generated was photographed (left) and counted (right). Bar = 50 µm. Results are presented as the mean ± SD and were analyzed using Kruskal–Wallis test followed by Dunn's test for post hoc analysis. *P < 0.05; **P < 0.01, compared with NS siRNA. Bar = 50 µm. CSC; cancer stem cells; ERRα, estrogen-related receptor alpha; siRNA, small interfering RNA.
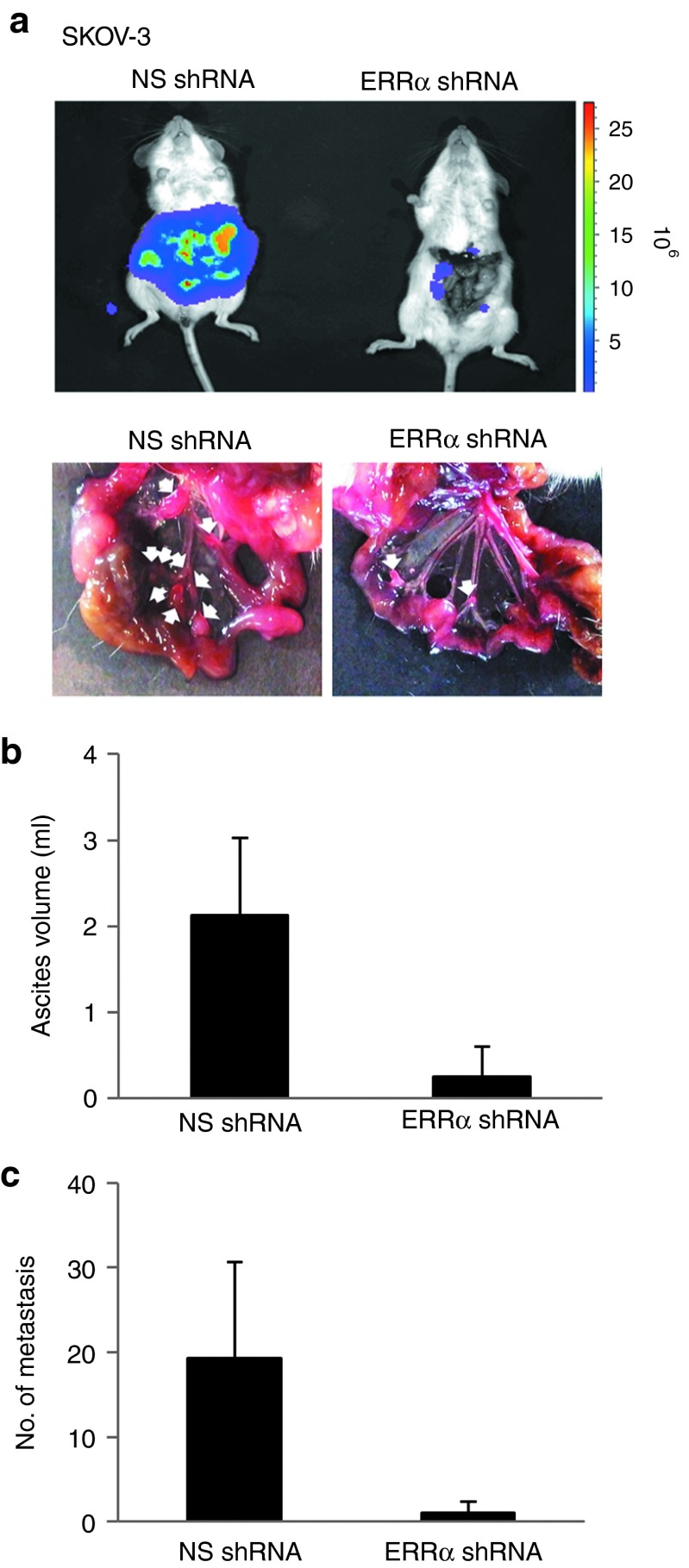
Silencing ERRα inhibits orthotopic ovarian cancer metastasis
To extend these studies in an animal model, we investigated the effect of ERRα silencing on ovarian cancer metastasis. In this in vivo study, luciferase-labeled nonspecific or ERRα siRNA–expressing SKOV-3 cells were orthotopically injected into the ovarian bursa, which emulates the clinical presentation of human patients with ovarian cancer, and subjected to bioluminescence imaging. The results showed that SKOV-3 cells expressing nonspecific siRNA grew as highly aggressive tumors and the tumor cells disseminated to the peritoneum—with visible tumor masses growing on the omentum, mesenteries, and small bowels—and developed ascites, reflecting characteristics commonly displayed by ovarian cancer lesions (Figure 7a). ERRα knockdown led to a marked reduction of the ascites volume compared with the volume in nonspecific siRNA–treated cells (Figure 7b). Similarly, the number and the size of tumor nodules were substantially decreased by treatment with ERRα siRNA than with nonspecific siRNA (Figure 7c), giving further support to the importance of ERRα in ovarian cancer metastasis.
Figure 7.
ERRα knockdown inhibits peritoneal dissemination of ovarian cancer cells in vivo. (a) NOD/SCID mice were orthotopically injected with SKOV-3 cells transduced by nonspecific (NS) shRNA or ERRα shRNA (three mice per group). At the time of sacrifice, bioluminescence imaging was again performed, and the peritoneal cavity was assessed for evidence of metastases. (b) Ascites fluid was collected and the volume was measured. (c) The metastatic lesions were excised and their total number was counted. Results are presented as the mean ± SD and were analyzed using Mann–Whitney U-test. ERRα, estrogen-related receptor alpha; NOD/SCID, nonobese diabetic/severe combined immunodeficient; shRNA, short hairpin RNA.
Discussion
As a crucial initial step in cancer metastasis, anti-EMT strategies hold great promise for the treatment of many cancers that currently lack effective therapies. As such, identifying factors that control EMT and the associated malignant features is critical. ERRα overexpression is correlated with poor outcome in ovarian cancer,3,4 but the molecular mechanism underlying the aggressive nature of these tumors remains largely unknown. Furthermore, although originally identified as a metabolic regulator, recent evidence suggests that its actions are not confined to metabolic regulation.21,22,23 The work presented here is significant in several ways. First, we show for the first time an additional and novel role for ERRα as a critical positive regulator of EMT and subsequent CSC-like properties and an inducer of ovarian cancer metastasis, both in vitro and in vivo. Moreover, we show a new mechanism of action by which ERRα regulates the miR-200 family to modulate the EMT-inducing transcription factor Snail to suppress E-cadherin expression. Given that EMT represents a fundamentally important process in ovarian cancer24 and that E-cadherin repression is associated with poor outcome in ovarian cancer patients,25 our result that ERRα is involved in the regulation of ovarian tumor progression and metastasis is clinically relevant.
There is increasing evidence that members of the Snail family of transcription factors can be differentially regulated.5 Thus, despite the many similarities between Snail and Slug, they might play important but distinct roles in cancer progression, and these roles are compatible with current models.26,27 Moreover, in addition to Snail, other members of the zinc finger transcription factors, including Zeb1, SIP1, E12/E47, and Twist, have also been shown to induce EMT and metastasis through the repression of E-cadherin. Although Snail is implicated in the early step of EMT, Zeb1 and other repressors are responsible for maintenance of the migratory phenotype.28 Our data showing that ERRα induces expression of Snail suggest that ERRα may regulate the first and necessary phase of the ovarian cancer dissemination process. These observations follow clinical reports in which primary ovarian tumors are believed to engage the EMT program at the initial dissemination stage.24 It is also relevant that Snail expression in ovarian carcinomas is associated with a worse prognosis and shows more aggressive biological behavior.29,30,31
We show that ERRα affects Snail expression not only through transcriptional regulation of Snail but also through posttranscriptional regulation that targets the 3′ untranslated region of Snail. This dual mechanism of regulation is similar to what has been recently described for insulin-like growth factor (IGF)-I signaling. EWS-Fli1 regulates the IGF-I promoter directly and the expression of IGF-I via miRNAs indirectly.32,33 The combined ability of ERRα in increasing Snail transcription and in preventing mRNA degradation indicates that it plays a central role in regulating Snail expression and thus EMT.
Here, we also describe a novel oncogenic role of ERRα in regulating miRNAs, although previously published data have shown that ERRα can be regulated by miR-137.34 The molecular mechanism by which ERRα might regulate miRNA-200s is not known. However, there are several plausible mechanisms: (i) In its capacity as a transcriptional regulator,35,36 ERRα may directly alter miR-200 gene expression. ERRα response elements were found at the promoter regions of the miR-200a/b cluster and miR-200c/141. (ii) Alternatively, ERRα may regulate miRNA biogenesis by downregulating Drosha and Dicer transcriptionally. Transcription is increasingly recognized as an important mechanism of Drosha and Dicer regulation,37 and ERRα-binding sites (TNAGGTCA) within the human Drosha (GenBank accession number NC_000005.9) and Dicer (GenBank accession number NG_016311.1) promoters have been identified. (iii) In addition, although the transcriptional mechanism is well recognized, there is now evidence that nuclear receptors can mediate rapid nongenomic signaling.38 These issues remain to be elucidated.
There is increasing evidence that the induction of EMT in transformed ovarian cancer cells in vitro or in mouse models generates cells with CSC characteristics, suggesting that ovarian epithelial cells can gain CSC characteristics through EMT.39,40 Moreover, CSCs derived from ovarian tumors and metastatic ovarian peritoneal effusions express EMT-associated markers.41 Similarly, EMT and CSC markers are frequently associated with ovarian carcinomas that have a propensity to metastasize, thus reinforcing the close relationship of these processes.40 Our studies reveal a role for ERRα in linking EMT and CSCs, which not only expands our understanding of the molecular mechanism but also sheds light on a new therapeutic concept that warrants clinical investigation.
This study further confirms an essential role of ERRα in ovarian cancer progression and positions it as an important target for cancer treatment. Because cells of the human OSE generally do not express ERRα (Supplementary Figure S1), its therapeutic targeting may offer a highly selective approach. It is also worth noting that high levels of ERRα are found in various cancers, including ovarian cancer, and high ERRα expression is associated with poor prognosis in breast, colon, and ovarian cancers,42,43,44,45 suggesting that the role of ERRα in EMT may have broader implications for other tumor cell types. Small molecules that modulate ERRα activity are not yet available. However, several orphan nuclear receptor antagonists have recently entered various clinical trials with success, but these also cause unwanted side effects.46 Therefore, seeking a safe and effective therapeutic method is crucial. In recent years, the use of siRNA, a powerful gene-silencing technology, has been widely used for silencing malignant genes, including nuclear receptors, and in many cases, it possesses advantages (e.g., safe, efficient, and specific) as compared with small molecule inhibitors.47 The peritoneal dissemination of ovarian cancer may offer significant targeting advantage for intraperitoneal gene delivery in that the therapeutic siRNA can be sensitive and specific to the target cells owing to the closed space of the peritoneal cavity.48
In summary, our studies identify a novel role for ERRα as a positive regulator of EMT. These studies not only provide insights into the roles and mechanisms for ERRα in ovarian cancer progression but also suggest that disruption of ERRα signaling could serve as a new strategy for reversing EMT and tumor metastasis, both major adverse events in ovarian cancer that cause mortality.
Materials and Methods
Cell culture and transfection. Human OSEs were collected with informed consent, and approval of the appropriate institution ethical committee was obtained before the study. OSE-527 and OSE-535 were obtained from ovaries during laparoscopy on women undergoing surgery for nonmalignant gynecologic diseases. These cells were transfected with SV40 large T antigen to increase the proliferative life span of the cells but remain nontransformed.49 The human ovarian cancer cells CaOV-3, OVCAR-3, and SKOV-3 (a gift from Dr. N. Auersperg, University of British Columbia, Vancouver, BC, Canada) were maintained in Media 199/MCDB105 (1:1), and the OV-90 (American Type Culture Collection, Rockville, MD) and HEYA8 (a gift from Dr. J. Liu, M. D. Anderson Cancer Center, Houston, TX) were maintained in RPMI 1640; both media contained 10% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin, and cells were cultured in a humidified incubator with 5% CO2 at 37 °C. All cell lines were regularly examined to monitor cellular morphology and tested to ensure the absence of mycoplasma contamination. Cell lines were authenticated with an AmpFLSTR Identifier Plus PCR Amplification Kit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. The data were analyzed by GeneMapper 4.1 Software (Foster City, CA). Cells were tested in November 2013. To express ERRα, cells were transiently transfected with 1 µg plasmid DNA per well in six-well plates, which was accomplished using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), and incubated for 24 hours according to the manufacturer's instructions. FLAG-tagged ERRα was purchased from Addgene (Cambridge, MA).
RNA interference-mediated gene silencing. ERRα gene–specific siRNA duplexes (5′-GGCCUUCGCUGAGGACUUA-3′) or nonspecific control siRNA oligonucleotides (5′-GGCTACGTCCAGGAGCGCA-3′) were purchased from Dharmacon (Lafayette, CO). Transfection was performed at a final concentration of 20 nmol/l using siLentFect (Bio-Rad, Hercules, CA). Stable transfection was performed first to produce viral particles constitutively expressing ERRα short hairpin RNA or nonspecific short hairpin RNA with packaging cell line 293T using Lipofectamine 2000 reagent (Invitrogen). The medium containing lentivirus was collected 48 hours later, SKOV-3 cells were infected with lentivirus, and the transfected cells were selected in 1 μg/ml puromycin (Invitrogen).
Reverse transcription–polymerase chain reaction analysis. Total RNA was isolated using Trizol reagent (Invitrogen). RNA was reverse transcribed to cDNA using the SuperScript III first-strand synthesis kit according to the manufacturer's instructions (Invitrogen). The primers used in this study were the following: ERRα 5′-TCCAGCTCCCACTCGCTGCC-3′ (sense) and 5′-ACACTCGTTGGAGGCCGGAC-3′ (antisense)); Snail, 5′-TTCCAGCAGCCCAACGACCAG-3′ (sense) and 5′-CGGACTCTTGGTGCTTGTGGA-3′ (antisense); Slug, 5′-ACGCCTCCAAAAAGCCAAAC-3′ (sense) and 5′-GGTAATGTGTGGGTCCGAAT-3′ (antisense); Nanog, 5′-AAGACAAGGTCCCGGTCAAG-3′ (sense) and 5′-CCTAGTGGTCTGCTGTATTAC-3′ (antisense); Oct-4, 5′-ATCCTGGGGGTTCTATTTGG-3′ (sense) and 5′-TCTCCAGGTTGCCTCTCACT-3′ (antisense); Bmi-1, 5′-ATGTGTGTGCTTTGTGGAG-3′ (sense) and 5′-AGTGGTCTGGTCTTGTGAAC-3′ (antisense); and β-actin, 5′-TCACCGAGGCCCCTCTGAACCCTAGA-3′ (sense) and 5′-GGCAGTAATCTCCTTCTGCATCCT-3′ (antisense). The number of amplification cycles, during which polymerase chain reaction product formation was limited by template concentration, was determined in pilot experiments. To measure the expression of mature miR-200s, total RNA from cells was isolated using Trizol reagent (Invitrogen). Reverse transcription–polymerase chain reaction reactions were carried out using Taqman microRNA reverse transcription kit (Applied Biosystems, Foster City, CA) and the following specific stem–loop primers: miR-141, 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCCATCT-3′; miR-200a, 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACATCG-3′; miR-200b, 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTCATCA-3′; and miR-200c, 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTCCATC-3′. U6 was used as an internal control.
Western blotting. Whole-cell lysates were prepared using sodium dodecyl sulfate lysis buffer (260 nmol/l Tris–HCl, pH 6.8, 0.8% sodium dodecyl sulfate, 40% glycerol), 1 µg/ml leupeptin, aprotinin, and 1 mmol/l phenylmethylsulfonyl fluoride (Roche Basel, Switzerland). Lysates (20 µg) were separated by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were electrotransferred to nitrocellulose membranes before being incubated with appropriate primary antibodies: anti-ERRα (1:1,000; Upstate, Lake Placid, NY), anti-N-cadherin (1:1,000; Zymed, San Francisco, CA), anti-E-cadherin (1:1,000; BD Transduction Laboratories, San Diego, CA), anti-FLAG (1:1,000), and anti-β-actin (1:2,000; Sigma, St. Louis, MO); the membranes were then processed for enhanced chemiluminescence detection (Amersham, Little Chalfont, UK) according to the manufacturer's protocol.
Luciferase reporter assay. Cells were transiently transfected with 1.5 µg of the 5′ promoter region of Snail (a kind gift of Dr. A. Garcia de Herreros, Universitat Pompeu Fabra, Barcelona, Spain) and cotransfected with 15 ng of β-galactosidase expression plasmid (pSV-β-gal; Promega, Madison, WI), using Lipofectamine 2000 reagent (Invitrogen). At 24 hours after transfection, luciferase activities were assayed using the Luciferase Reporter Assay kit according to the manufacturer's protocol (Promega). β-Galactosidase activity was used to normalize the transfection. The promoterless luciferase vector (pGL3-Basic) was used as an internal control.
miRNA target prediction and antagonism. Potential upstream regulators of Snail were predicted by three publicly available algorithms: miRanda (http://www.microrna.org/microrna/home.do), TargetScan (http://www.targetscan.org/), and RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/). Human hsa-miR-200a and hsa-miR-200b antagomirs or nonspecific control were allowed to form transfection complexes with Lipofectamine 2000 (Invitrogen) at a final concentration of 20 nmol/l, according to the manufacturer's protocol.
Cell scatter assay. To evaluate EMT, morphologic changes were assessed by optical microscopy. Briefly, 6,000 cells were plated in six-well plates, allowed to form small colonies, and were then transfected with the indicated plasmids and imaged. Scattered colonies were judged by a typical change in morphology characterized by cell–cell dissociation and the acquisition of a migratory fibroblast-like phenotype. Scattering activity was measured in the total number of scattered colonies from 50 colonies under light microscope.
Sphere-forming assay. Sphere-forming assays were conducted as previously described.50 Briefly, 5,000 cells/ml were plated in ultra-low-attachment 100-mm culturing dish in serum-free stem cell–selective medium. After 72 hours, each well was examined using a light microscope, and the number of spheres was counted.
Orthotopic metastatic ovarian cancer model. All animal care and experimental procedures were carried out according to institution-approved protocols in compliance with the Committee for the Use of Laboratory Animals. Female nonobese diabetic/severe combined immunodeficient mice (Charles River Laboratories, Wilmington, MA) were used. Luciferase-labeled cells (1 × 106) were inoculated under the ovarian bursa of 6- to 8-week-old female nonobese diabetic/severe combined immunodeficient mice (n = 3 mice per group, and the experiment was conducted twice). Mice were assessed weekly for metastasis via intraperitoneal injections with 200 µl of 30 mg/ml d-luciferin and in vivo bioluminescence was assessed using a Xenogen IVIS 100 cooled charge-coupled device camera (Xenogen, Alameda, CA). At the time of sacrifice, ascites volume was determined with a pipette, and the number of all visible (>0.1 cm diameter) tumor nodules in the peritoneal cavity was assessed as evidence of metastases.
Statistical analysis. Experiments were done in duplicate or triplicate and were repeated at least four or five times, with each experiment yielding essentially identical results. Data were expressed as mean ± SD. All statistical analyses were performed using IBM SPSS software (SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Expression of ERRα is human ovarian surface epithelium and ovarian cancer cells.
Acknowledgments
The authors acknowledge the assistance of the Faculty Core Facility of the Li Ka Shing Faculty of Medicine at the University of Hong Kong (HKU). This work was supported by HKU Committee on Research and Conference Grants grant 20091159079, Research Grants Council Collaborative Research Fund grant CUHK8/CRF/11R, and a Croucher Senior Research Fellowship to A. S. T. W. The authors declare no conflict of interest.
Supplementary Material
Expression of ERRα is human ovarian surface epithelium and ovarian cancer cells.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Giguère V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Sun P, Wei L, Denkert C, Lichtenegger W, Sehouli J. The orphan nuclear receptors, estrogen receptor-related receptors: their role as new biomarkers in gynecological cancer. Anticancer Res. 2006;26 2C:1699–1706. [PubMed] [Google Scholar]
- Fujimoto J, Alam SM, Jahan I, Sato E, Sakaguchi H, Tamaya T. Clinical implication of estrogen-related receptor (ERR) expression in ovarian cancers. J Steroid Biochem Mol Biol. 2007;104:301–304. doi: 10.1016/j.jsbmb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Huang RY, Chung VY, Thiery JP. Targeting pathways contributing to epithelial-mesenchymal transition (EMT) in epithelial ovarian cancer. Curr Drug Targets. 2012;13:1649–1653. doi: 10.2174/138945012803530044. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118 Pt 19:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- Groom CR, Hopkins AL. Protein kinase drugs–optimism doesn't wait on facts. Drug Discov Today. 2002;7:801–802. doi: 10.1016/s1359-6446(02)02359-0. [DOI] [PubMed] [Google Scholar]
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5:821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–3676. [PubMed] [Google Scholar]
- Langdon SP. Isolation and culture of ovarian cancer cell lines. Methods Mol Med. 2004;88:133–139. doi: 10.1385/1-59259-406-9:133. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116 Pt 3:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Leskelä S, Leandro-García LJ, Mendiola M, Barriuso J, Inglada-Pérez L, Muñoz I, et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- Gill JG, Langer EM, Lindsley RC, Cai M, Murphy TL, Kyba M, et al. Snail and the microRNA-200 family act in opposition to regulate epithelial-to-mesenchymal transition and germ layer fate restriction in differentiating ESCs. Stem Cells. 2011;29:764–776. doi: 10.1002/stem.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RA, Gaillard S, McDonnell DP. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:106–112. doi: 10.1016/j.jsbmb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois G, Hall JA, Perry MC, Laganière J, Ghahremani M, Park M, et al. Genome-wide identification of direct target genes implicates estrogen-related receptor alpha as a determinant of breast cancer heterogeneity. Cancer Res. 2009;69:6149–6157. doi: 10.1158/0008-5472.CAN-09-1251. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. doi: 10.1002/jcp.21240. [DOI] [PubMed] [Google Scholar]
- Sundfeldt K. Cell-cell adhesion in the normal ovary and ovarian tumors of epithelial origin; an exception to the rule. Mol Cell Endocrinol. 2003;202:89–96. doi: 10.1016/s0303-7207(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Côme C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Shields MA, Krantz SB, Bentrem DJ, Dangi-Garimella S, Munshi HG. Interplay between ß1-integrin and Rho signaling regulates differential scattering and motility of pancreatic cancer cells by snail and Slug proteins. J Biol Chem. 2012;287:6218–6229. doi: 10.1074/jbc.M111.308940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloul S, Elstrand MB, Nesland JM, Tropé CG, Kvalheim G, Goldberg I, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- Blechschmidt K, Sassen S, Schmalfeldt B, Schuster T, Höfler H, Becker KF. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br J Cancer. 2008;98:489–495. doi: 10.1038/sj.bjc.6604115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cironi L, Riggi N, Provero P, Wolf N, Suvà ML, Suvà D, et al. IGF1 is a common target gene of Ewing's sarcoma fusion proteins in mesenchymal progenitor cells. PLoS ONE. 2008;3:e2634. doi: 10.1371/journal.pone.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey EL, Parrish JK, Irwin AE, Niemeyer BF, Kern HB, Birks DK, et al. A novel oncogenic mechanism in Ewing sarcoma involving IGF pathway targeting by EWS/Fli1-regulated microRNAs. Oncogene. 2011;30:4910–4920. doi: 10.1038/onc.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y, et al. MiR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PLoS ONE. 2012;7:e39102. doi: 10.1371/journal.pone.0039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguère V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro S, Wang Z, Kotomura N, Niwa O, Ueda K, Kamada N. Nuclear proteins binding to the recombination hotspot region of the retinoic acid receptor alpha gene. Leukemia. 1997;11 Suppl 3:285–286. [PubMed] [Google Scholar]
- Davis BN, Hata A. Regulation of MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Kang KS, Choi YP, Gao MQ, Kang S, Kim BG, Lee JH, et al. CD24? ovary cancer cells exhibit an invasive mesenchymal phenotype. Biochem Biophys Res Commun. 2013;432:333–338. doi: 10.1016/j.bbrc.2013.01.102. [DOI] [PubMed] [Google Scholar]
- Latifi A, Abubaker K, Castrechini N, Ward AC, Liongue C, Dobill F, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–2864. doi: 10.1002/jcb.23199. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–6518. [PubMed] [Google Scholar]
- Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, et al. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Notarnicola M, Giannini R, Montemurro S, Lorusso D, Visconti A, et al. Oestrogen receptor-related receptor alpha (ERRalpha) and oestrogen receptors (ERalpha and ERbeta) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer. 2005;41:1487–1494. doi: 10.1016/j.ejca.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6:203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- Shi Y. Orphan nuclear receptors in drug discovery. Drug Discov Today. 2007;12:440–445. doi: 10.1016/j.drudis.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss WA, Taylor SS, Shokat KM. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat Chem Biol. 2007;3:739–744. doi: 10.1038/nchembio1207-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;8:441–447. doi: 10.1007/s11912-006-0073-x. [DOI] [PubMed] [Google Scholar]
- Maines-Bandiera SL, Kruk PA, Auersperg N. Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am J Obstet Gynecol. 1992;167:729–735. doi: 10.1016/s0002-9378(11)91579-8. [DOI] [PubMed] [Google Scholar]
- Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/ß-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of ERRα is human ovarian surface epithelium and ovarian cancer cells.