Abstract
Posttranscriptional gene silencing by RNA interference can be therapeutically exploited to inhibit pathophysiological gene expression. However, in contrast to the established effectiveness of RNAi in vitro, safe and effective delivery of siRNAs to specific organs and cell types in vivo remains the major hurdle. Here, we report the development and in vivo characterization of a novel siRNA delivery system (DACC lipoplex) suitable for modulating target gene expression specifically in the lung vasculature. Systemic administration of DACC in mice delivered siRNA cargo functionally to the lung pulmonary endothelium. A single dose of DACC lipoplexes administered by bolus injection or by infusion was sufficient to specifically silence genes expressed in pulmonary endothelial cells such as CD31, Tie-2, VE-cadherin, or BMP-R2. When tested in a mouse model for lung cancer, repeated treatment with DACC/siRNACD31 reduced formation of lung metastases and increased life span in a mouse model of experimental lung metastasis.
Introduction
Posttranscriptional gene silencing by RNA interference (RNAi) enables drastic reduction of de novo protein synthesis of a targeted gene, and holds great therapeutic promise.1,2 In principal, expression of any gene can be downregulated by RNAi, including disease targets currently not “druggable” by conventional methods. Therefore, RNAi-based therapies unlock a great potential in the treatment of a multitude of diseases such as respiratory infectious, cardiovascular and metabolic diseases and cancer.3 Ongoing or completed clinical trials include siRNA therapeutics for the treatment of cancer, TTR (transthyretin-mediated amyloidosis), age-related macular degeneration, diabetic macular edema, hypercholesterolemia, as well as for treatment of respiratory syncytical virus infections.4 However, the major difficulty encountered when applying RNAi is the stability of the therapeutic siRNA and its efficient intracellular delivery to target tissues and respective cell types in vivo. As previously reported, we were able to improve stability of the siRNA molecules by incorporating chemical backbone modifications such as alternating 2′-O-methyl modification patterns on both blunt ended siRNA strands to prevent degradation of these molecules by plasma-derived nucleases.5 Since synthetic siRNAs fail to cross biological membranes via passive diffusion due to their high molecular weight and polyanionic nature, transmembrane drug delivery technologies are required in order to access the cytoplasm of target cells for functional RNA interference to occur. Cationic lipids have been shown to be extremely useful as efficient and scalable delivery vehicles for siRNA for this purpose6,7 due to their ability to form nanostructured complexes: negatively charged RNA is thus condensed into so called lipoplex particles,8 thereby protecting it from ribonucleases and shear degradation in vitro and in vivo. Lipoplex particles thus aid in directing therapeutic siRNA through the bloodstream and extracellular matrix and transport it across cellular membranes into the cytoplasm of the target cells, a prerequisite for mediating gene silencing by the RNA interference (RNAi) mechanism. Apart from their siRNA and cationic lipid components, lipoplex formulations often contain neutral colipids like phosphatidylcholines and a certain amount of polyethylene glycol (PEG)-lipid conjugates in order to prolong blood-circulation time and to reduce uptake by the mononuclear phagocyte system.9 Compared to the developments of lipid-based formulations for gene silencing by RNAi in the last decade, recent improvements have increasingly been made employing rational approaches. This includes the rational design of new cationic lipids with improved delivery capacities,10 as well as chemical modifications of these lipids to ensure more biodegradable entities with improved biocompatibility and facilitated elimination from plasma and tissues.11 Furthermore, the correlation between, and the relevance of results from in vitro and in vivo knock down experiments are much better understood, especially with respect to the impact of PEG shielding, and hence use for the modulation of PK properties critical for in vivo delivery.12
We have previously reported on a newly synthesized cationic lipid, referred to as AtuFECT01, as part of the lipid system AtuPLEX, which exhibits more efficient siRNA binding activity and delivery to cells of the vascular endothelium as compared to other commercially available cationic lipids such as DOTAP or DOTMA.5 Nevertheless, such nanoparticles tend to accumulate in filtering organs of the reticuloendothelial system. These tissues, including lymph nodes, spleen and liver, trap foreign particles as part of the organisms' natural defense mechanism against invading viruses, bacteria and parasites. Thus, many liposomes and nanoparticles tend to concentrate in the liver and spleen, which limits their application to other target tissues.13 In an attempt to address the above questions, we experimented with different lipid components and their respective ratios in the lipoplex formulations in order to alter their physico-chemical characteristics, stability, target tissue accumulation, RNAi activity, therapeutic potential and tolerance in vivo. One of our goals was to identify a siRNA delivery system suitable for functional gene silencing in the lungs. Here, we report the discovery and development of a novel cationic lipoplex delivery system, DACC, which shows improved pharmacokinetic properties and functional cellular uptake by cells of the pulmonary endothelium. We describe and functionally characterize DACC formulation, composed of the cationic lipid AtuFECT01, cholesterol, mPEG2000-DSPE and double-stranded siRNA. Intravenous tail-vein injection in mice of the novel DACC/siRNA formulation was found to accumulate to a high degree in the lungs. The observed increase of siRNA uptake by the lungs was shown to correlate with efficacy of RNA interference (RNAi) in the pulmonary endothelium. Finally, we show one potential application of DACC in a proof-of-concept study in a mouse model for lung cancer.
Results
siRNA biodistribution using different siRNA-lipoplex formulations
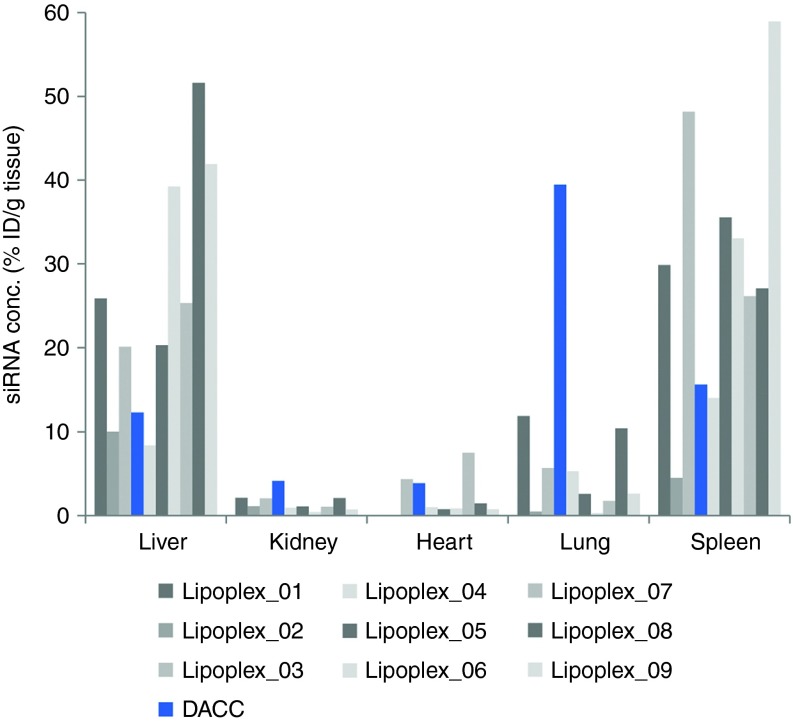
Different lipoplex formulations were evaluated in vivo in order to investigate their respective capacity to deliver siRNA cargo to select organs. All lipoplexes used to examine siRNA biodistribution pattern were formulated with the cationic lipid AtuFECT01 and siRNAPKN3 but contained different colipids and/or PEG-lipids at different ratios. Lipoplexes were administered intravenously via tail-vein injection, and siRNA concentrations were determined in liver, kidney, lung, heart, and spleen tissue samples one hour after systemic application using a siRNA specific quantitative ELISA-based capture-probe assay (Figure 1). Concentrations of siRNA delivered to the respective tissues varied depending on the lipoplex system used, whereas the DACC lipoplex system displayed the most efficient siRNA delivery to the lungs (Figure 1, blue bar).
Figure 1.
siRNA distribution in vivo 1 hour after systemic application using different lipoplex formulations. All lipoplexes were formulated with siRNAPKN3 containing different liposomal components and were administered intravenously via tail-vein injection. siRNA concentrations are given in percent initial dose per gram of tissue (%ID/g) and were determined by quantitative ELISA-based capture-probe assay. Concentrations of siRNA delivered to the respective tissues vary depending on the liposomal delivery system used. The DACC system shows most efficient siRNA delivery to the lungs as compared to all other formulations tested.
Lipid composition and physico-chemical characterization of the DACC/siRNA lipoplexes
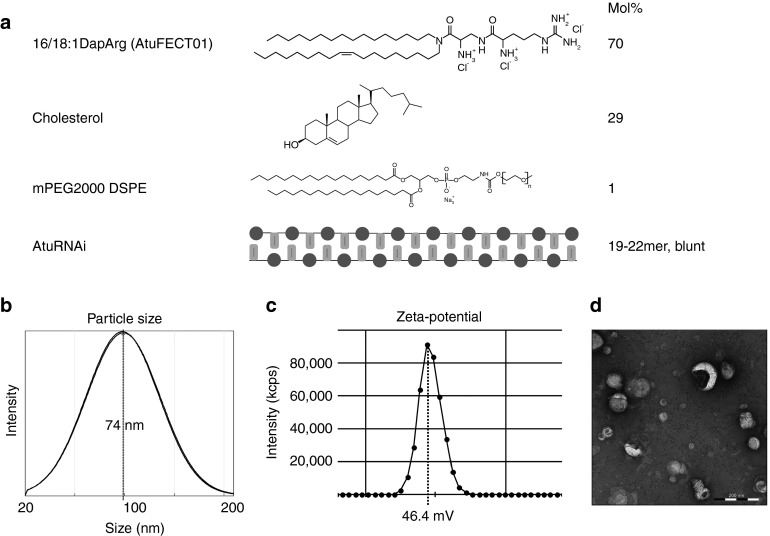
DACC lipoplexes are composed of the positively charged lipid system AtuFECT01 (β-L-arginyl-2,3-L-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride), cholesterol and mPEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanol amine-N (methoxy (polyethylene glycol)-2000)) in a molar ratio of 70:29:1 together with a blunt ended siRNA duplexes chemically stabilized by alternating 2′-O-methyl modifications on both strands5,14 (Figure 2a) in 270 mmol/l sucrose solution. The resulting DACC lipoplex particles were characterized regarding size and zeta potential. The Z-average size amounted to ~70 nm (polydispersity index (PDI) 0.25–0.35) as determined by dynamic light scattering (Figure 2b), and the zeta potential measured in 270 mmol/l sucrose was between 40 and 50 mV (Figure 2c). Electron microscopy of DACC lipoplex particles reveals predominantly lamellar structures in mostly spherical arrangements and confirms the particle sizes determined by dynamic light scattering (Figure 2d). The addition of sucrose enables formulation stability during freezing, drying and rehydration steps, thereby ensuring effective long-term storage as a lyophilized product (data not shown).
Figure 2.
Lipid composition and physico-chemical characterization of DACC lipoplex. (a) DACC lipoplex consisting of a positively charged lipid system (70 mol% cationic lipid AtuFECT01 (β-L-arginyl-2,3-L-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride), 1 mol% mPEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanol amine-N (methoxy (polyethylene glycol)-2000), and 29 mol% cholesterol) and blunt ended siRNA duplexes with alternating 2′-O-methyl modifications on both strands; (b) Particle size distribution: Z-average size ~70 nm as determined by dynamic light scattering (intensity distribution); (c) The Zeta potential of representative DACC lipoplexes lies between 40 and 50 mV as measured at 270 mmol/l sucrose; (d) Electron micrograph of DACC lipoplex particles showing predominantly lamellar structures in mostly spherical arrangements. Bar = 200 nm.
DACC delivers siRNAs primarily to the lung endothelium
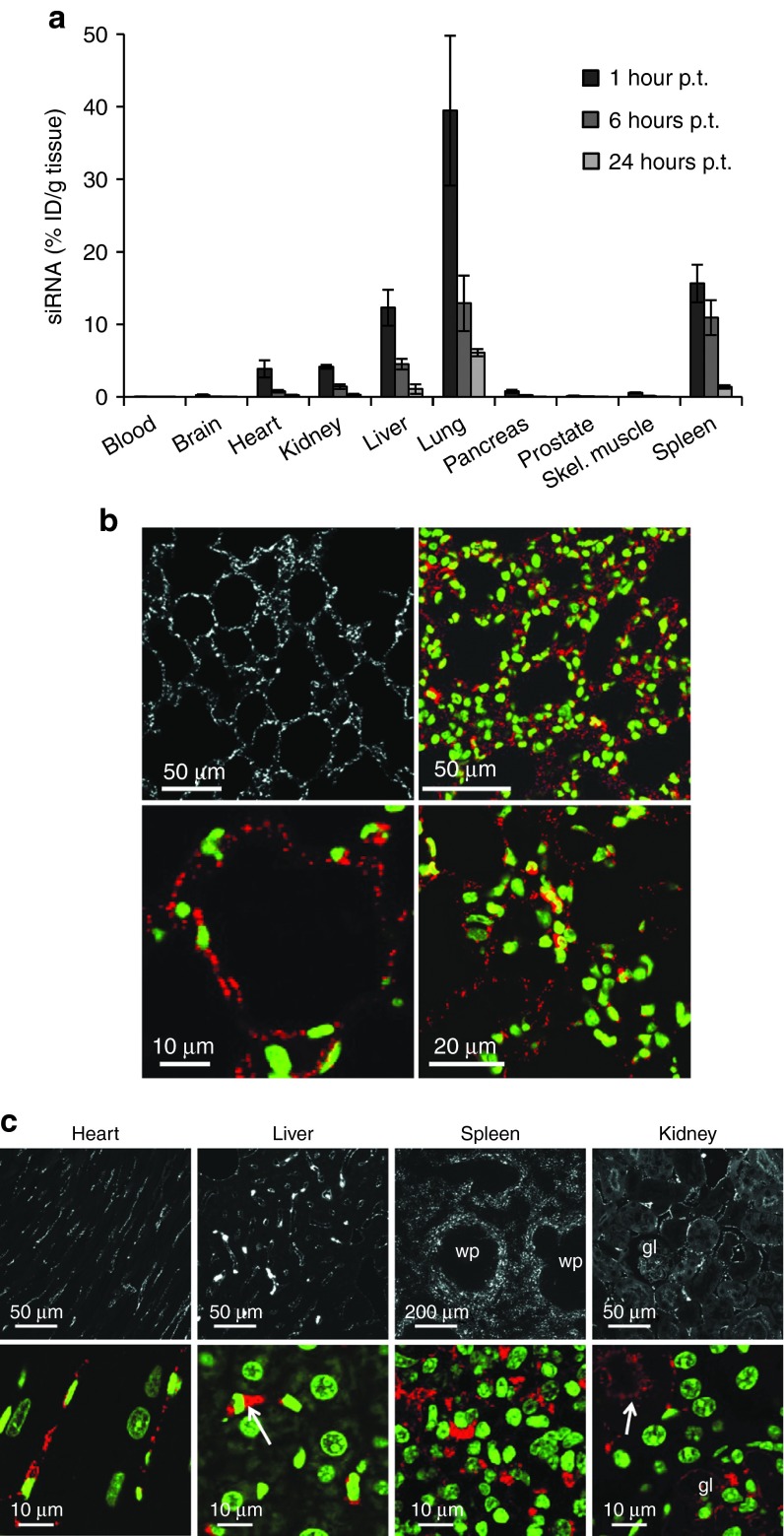
To characterize the tissue distribution and kinetics of siRNA delivery by systemic application of DACC lipoplexes in more detail, mice were treated with a single dose of DACC (2.8 mg siRNA/kg body weight). A number of tissue samples from different organs were collected 1, 6, and 24 hours after administration of the DACC lipoplex formulation to determine respective siRNA concentrations (Figure 3a). Highest siRNA concentrations (about 40% ID/g tissue) were found in lung tissue at the 1-hour time point, followed by spleen, liver and to lesser extent kidney and heart. siRNA concentrations in all tissues investigated diminished over time. siRNA levels in blood, brain, prostate, or skeletal muscle were below 1% of initial dose. In order to examine siRNA distribution within predominantly targeted tissues, mice were treated with DACC lipoplexes formulated with cyanine dye (Cy3) labeled siRNA. Cy3-labeled siRNAs in the tissues were then visualized by confocal microscopy of formalin-fixed paraffin embedded tissue sections. siRNA distribution patterns in lung, liver, kidney, and heart were reminiscent of vascular staining patterns (Figure 3b–c). From all organs investigated, lungs were most intensely stained by siRNA-Cy3. Upon close up-view, siRNA derived staining in the lungs was finely dotted and centered around cell nuclei, this observation being indicative for intracellular uptake of siRNAs (Figure 3b). In the heart, distinct Cy3 staining was found lining the capillaries, while muscle fibers were void of siRNA-Cy3 signal (Figure 3c). The sinusoidal endothelial cell layer in the liver showed weak siRNA-Cy3 staining pattern (Figure 3c), while individual cells within the liver sinusoids were strongly Cy3 stained. The oval shaped nucleus (Figure 3c, see arrow) marks Kupffer cells responsible for the removal of foreign particulate substances, hence, lipoplexes found here could also have been taken up by phagocytosis.15 Hepatocytes, discernible by their large, regular and round nucleus, were free of siRNA-Cy3 staining. siRNA-Cy3 staining in the spleen was pronounced in the marginal zones of the white pulp, while the center of the white pulp remained Cy3-staining free (Figure 3c). Since monocytes and macrophages known to be responsible for lipoplex clearance from the blood are sequestered into this zone, lipoplex clearance by respective macrophages could explain the enhanced siRNA-Cy3 staining pattern observed in this area. Distinct Cy3 signals were also detected in peritubular capillaries of the kidney (Figure 3c, arrow). Staining of tubular cells was diffuse, with a tendency for Cy3-siRNA accumulation towards the lumen of the tubuli, indicative for excretion of free siRNA by the kidney. Altogether, the quantitative and qualitative analysis of siRNA distribution by the DACC delivery system revealed siRNA uptake to occur mainly in the lungs, but distinct siRNA-Cy3 derived signals were also detected in the microvasculature of heart, liver, and kidney as well as in phagocytic cells of liver and spleen.
Figure 3.
Enhanced siRNA delivery to lungs using DACC lipoplexes, and cellular distribution of Cy3-labeled siRNA in heart, liver, spleen, and kidney 1 hour after systemic i.v. administration of DACC lipoplexes. (a) Mice received a single dose of DACC/siRNAPKN3 via tail-vein injection (2.8 mg siRNA/kg body weight). siRNA concentrations were determined at 1, 6, and 12 hours post treatment (p.t.) (n = 3) by capture probe ELISA assay. Mean values with standard deviation are depicted. Highest siRNA concentration (%ID/g tissue) was found in the lungs 1 hour p.t. (b) Cellular distribution of Cy3-labeled siRNA in the lungs 1 hour after systemic i.v. administration of DACC/siRNACy3. Confocal microscopic images of formaline fixed paraffin embedded lung tissue sections are shown. The upper left panel shows siRNA-Cy3 staining in white, the upper right panel and the lower panels show close up views of siRNA-Cy3 staining in red and nuclear staining in green. siRNA staining is evenly distributed in the lung vasculature. Scale bars are indicated. (c) siRNA-Cy3 staining of paraffin embedded sections of the heart, liver, spleen, and kidney is depicted in white at low magnification (upper panels), and in red in close up views (lower panels). Nuclei are counterstained with Sytox G (Molecular Probe) depicted in green in lower panels. Heart tissue shows siRNA-Cy3 labeling in capillaries. In the liver sinusoids are weakly, Kupffer cells are strongly Cy3 stained (arrow), but not hepatocytes. Distinct siRNA-Cy3 signal is visible in the spleen only in the marginal zones of the white pulp, (wp), upper panel and close up view lower panel. In the kidney, Cy3 signals are prominent in peritubular capillaries and in luminal side of tubules (see arrow). gl indicates glomerulus.
Inhibition of target gene expression by DACC in the lung vasculature
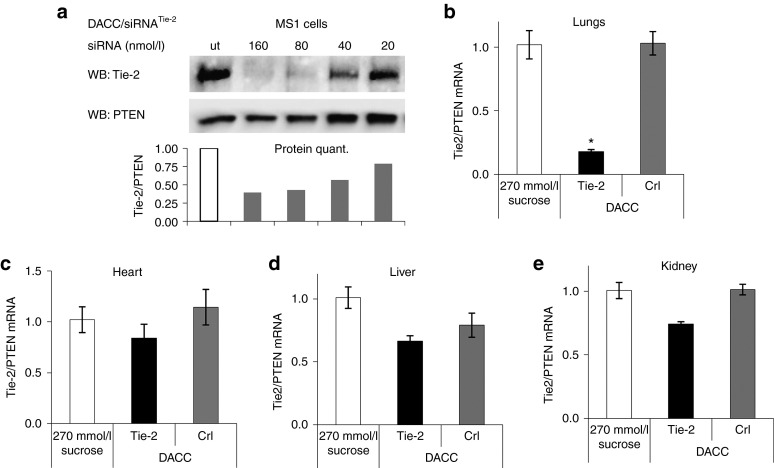
To test whether siRNA delivered by DACC is functionally active and can be used for inhibition of target gene expression in vascular beds, DACC formulations with siRNAs specific for the target gene Tie-2 were prepared. Tie-2 expression is highly specific for endothelial cells and it is a common marker for this cell type in many organs.16 RNAi activity of DACC/siRNATie-2 was first tested in vitro. The murine endothelial cell line MS1 was transfected with DACC/siRNATie-2, and 72 hours thereafter cells were lysed and Tie-2 protein expression was assessed by immunoblotting. Tie-2 levels were significantly reduced by DACC/siRNATie-2 at a dose of 160 and 80 nmol/l siRNA, and to a lesser extent 40 nmol/l siRNA, (Figure 4a), which confirmed that DACC can functionally deliver siRNA into cells and mediate RNAi. It should be noted that these concentrations are not reflective of the siRNAs IC50s, since DACC is not optimized for cell culture transfection experiments, and lower concentrations can be used in the latter. To investigate whether DACC is capable of gene silencing in the vascular endothelium in vivo, mice were given three doses of DACC lipoplexes on consecutive days by tail-vein injection (3 × 2.8 mg/kg). Lung, heart, liver, and kidney tissues were collected 24 hours after the last treatment. Total mRNA was prepared from whole tissue lysates, and target mRNA levels were assessed by quantitative RT-PCR (Figure 4b–e). Tie-2 mRNA levels were reduced by over 80% in lung tissue of mice treated systemically with DACC/siRNATie-2 as compared to control treatments with either DACC/siRNAcontrol or with sucrose solution (Figure 4b). No significant Tie-2 knock down was observed in liver, kidney, and heart tissue even after repeated dosing of DACC/siRNATie-2 formulation (Figure 4c–e), indicating that the lungs are the primary organ functionally targeted by DACC/siRNATie-2.
Figure 4.
Inhibition of Tie-2 target gene expression by DACC lipoplexes in endothelial cells and in mice. (a) Dose-dependent RNAi effect on Tie-2 protein expression in vitro after transfection of the mouse endothelial cell line MS1 with DACC/siRNA lipoplexes. Tie-2 protein levels were assessed 72 hours after transfection by western blot analysis. PTEN protein levels served as a loading control. The quantification of Tie-2 protein levels is shown below. DACC/siRNATie-2 inhibits Tie-2 protein expression at siRNA concentrations between 40 and 160 nmol/l. (b–e) Mice were treated with DACC lipoplexes with a daily dose of 2.8 mg siRNA/kg body weight on 3 consecutive days. Tie-2 target gene expression was measured in (b) lungs, (c) heart, (d) liver, and (e) kidney by quantitative reverse transcriptase–PCR analysis. Significant reduction (*P < 0.05) over 80% of Tie-2 mRNA levels, was seen in the lung tissue.
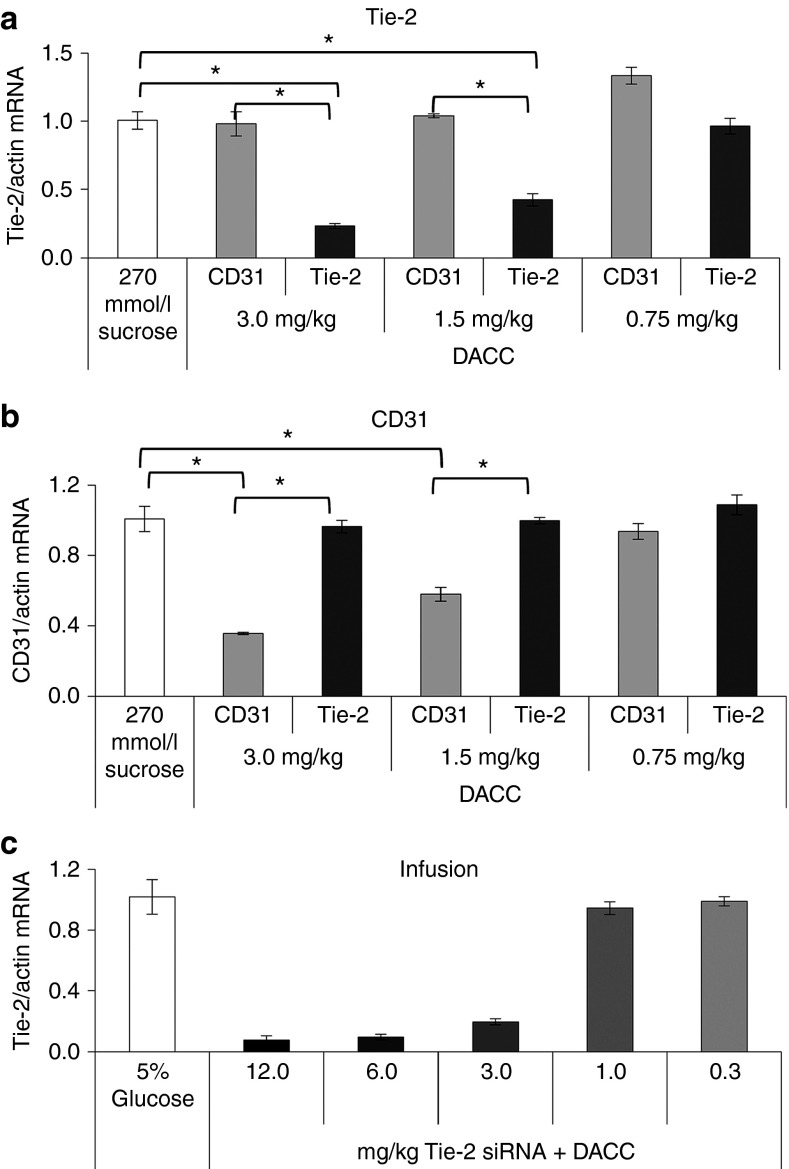
DACC/siRNA dose responsiveness of target gene expression
To further investigate the dose requirements for target gene inhibition by DACC lipoplexes in mice, single doses of 3.0, 1.5, and 0.75 mg siRNA/kg body weight of DACC/siRNATie-2 or DACC/siRNAcontrol were administered by intravenous tail-vein bolus injection. Lung tissue was collected 24 hours after injection for RNA analysis (Figure 5a). Remarkably, already a single dose of DACC/siRNATie-2 (3.0 mg siRNA/kg) sufficed to reduce Tie-2 mRNA levels similar to those obtained after repeated dosing as described for Figure 4b (3 × 2.8 mg siRNA/kg) (compare, Figures 5a and 4b). 1.5 mg siRNA/kg was also effective in reducing Tie-2 expression, but to a lesser extent, and 0.75 mg siRNA/kg did not affect Tie-2 levels significantly. A similar dose response for target gene inhibition was observed when DACC was prepared with siRNA for CD31 (Figure 5b). CD31 expression in lungs was reduced significantly with a dose of 3.0 and also 1.5 mg/kg, but not 0.75 mg/kg. Since all DACC applications described thus far were performed by bolus injection, but application via infusion is the preferred mode of lipoplex delivery in the clinical setting, we administered different doses of DACC/siRNATie-2 into the jugular vein of mice by 1-hour infusions (Figure 5c). Target gene silencing after DACC/siRNATie-2 infusion was comparable to that by bolus injection at 3 mg siRNA/kg body weight. Tie-2 mRNA levels in lung tissue were reduced by approximately 80%. Since this mode of application enabled us to use larger volumes of DACC lipoplexes as compared to bolus injection, doses administered were increased to 6 mg siRNA/kg and/or 12 mg siRNA/kg, respectively. This increase in lipoplex concentration was shown to reduce Tie-2 expression levels even further to over 95% (Figure 5c). Nonetheless, all animals tolerated infusion treatment of DACC/siRNATie-2 even at highest dosage (12 mg/kg) (Figure 5b).
Figure 5.
Dose-dependent target gene inhibition by DACC/siRNATie-2 after administration via bolus or infusion treatment. (a,b) Single doses of 3.0, 1.5, and 0.75 mg siRNA/kg body weight of DACC/siRNATie-2 and DACC/siRNACD31 (and sucrose control) were administered to mice by bolus treatment. 3.0 mg/kg single dose of DACC/siRNATie-2 decreased mRNA levels in the lungs by about 80% of control values (sucrose and CD31, respectively). 1.5 mg siRNA/kg reduced Tie-2 expression also significantly, but to a lesser extent. (b) CD31 expression was reduced in DACC/siRNACD31 treatment groups at doses of 3.0 and 1.5 mg/kg. * indicates P < 0.0001. (c) One-hour infusion treatments were performed on mice. A dose of 3 mg siRNA/kg body weight DACC/siRNATie-2 application by infusion was as effective at inhibiting Tie-2 target gene expression as bolus application (80% reduction). Dose increase of DACC/siRNATie-2 to 6 or 12 mg/kg reduced Tie-2 target gene expression in lungs by over 90%.
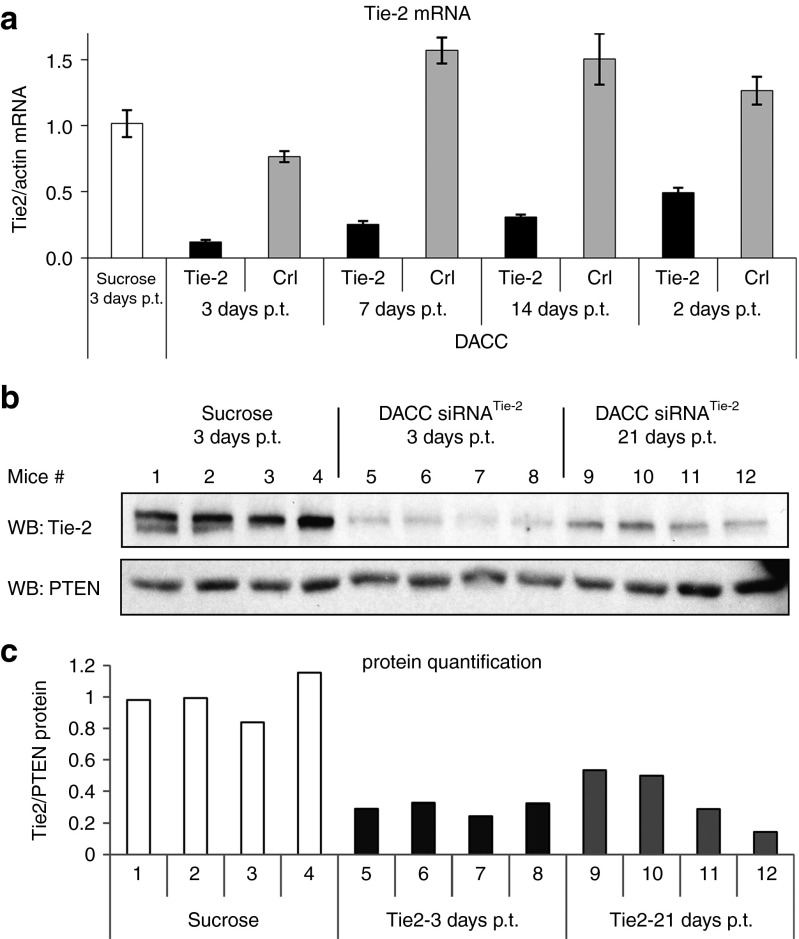
Duration of target inhibition and verification of RNAi-mediated knock down
To assess duration of target gene inhibition by DACC lipoplexes, mice were treated with a single dose (2.8 mg/kg) of DACC/siRNATie-2 or DACC/siRNAcontrol and cohorts were sacrificed 3, 7, 14, and 21 days after treatment. Tie-2 target mRNA levels in the respective lung tissue were quantified by quantitative RT-PCR (Figure 6a). Most prominent reduction of Tie-2 expression in the DACC/siRNATie-2 treatment group was observed 3 days after treatment with lipoplexes (over 80% Tie-2 reduction compared to DACC control groups). Nonetheless, Tie-2 mRNA levels were still reduced by over 50% after 21 days (Figure 6a), as compared to control groups treated either with sucrose or with control DACC lipoplex. To confirm that the reduction in Tie-2 mRNA leads to a corresponding reduction of Tie-2 protein, lung tissue homogenates were prepared from mice 3 and 21 days after treatment with DACC lipoplex or with sucrose as vehicle control, respectively. Tie-2 protein was detected by western blotting (Figure 6b). Prominent reductions of Tie-2 protein levels were seen in each individual mouse 3 days after treatment, corresponding to the respective decrease of Tie-2 mRNA levels and were shown to subside somewhat over time. However, about 70% reduction of Tie-2 protein expression was still observed 21 days after treatment (Figure 6b,c).
Figure 6.
Duration of target gene inhibition in lungs after single bolus treatment with DACC/siRNATie-2 lipoplexes. (a) Taqman analysis of Tie-2 expression in the lungs. Animals were treated with a single dose of DACC/siRNATie-2 or DACC/siRNAcontrol (2.8 mg/kg) and sacrificed 3, 7, 14, and 21 days post treatment (p.t.). DACC/siRNATie-2 treatment reduced Tie-2 mRNA level by over 80% at 3 days p.t. mRNA levels were also strongly reduced 7 and 14 days p.t., and remained reduced by over 70% even after 21 days. (b) Western blot of Tie-2 protein lung tissue homogenates prepared from mice 3 and 21 days after DACC/siRNATie-2 or DACC/siRNAcontrol application. Most prominent reduction of Tie-2 protein is seen 3 days p.t. Tie-2 mRNA levels remain reduced by over 70% after 21 days. (c) Quantification of Tie-2 protein levels by digital imaging of luminescent signal intensities of western blot (b).
Inhibition of additional target genes in the pulmonary endothelium by DACC/siRNA
In order to confirm that the DACC delivery system is in general capable of inhibiting target genes expressed in the pulmonary endothelium apart from Tie-2 and CD31, DACC lipoplexes were prepared with specific siRNAs for additional gene targets whose expression is highly restricted to endothelial cells, like the VEGF-R2 receptor, VE-Cadherin and the BMP receptor 2. For comparison, another DACC formulation was prepared with siRNA for Lamin B1, which is ubiquitously expressed.17 Treating mice with a single injection (2.8 mg siRNA/kg) of DACC/siRNAVEGFR2, DACC/siRNAVE-Cadherin or DACC/siRNABMPR-2 formulation, reduced mRNA levels of respective target genes by 60–90% (Figure 7). In contrast, moderate nonsignificant downregulation of Lamin B1 by DACC/siRNALaminB1, was observed (Figure 7) due to nontargeted Lamin B1 in lung parenchyme. The observed reduction of endothelial marker genes by specific DACC lipoplexes demonstrates that DACC formulations are capable of delivering siRNA functionally to the vascular endothelium, thereby enabling target-specific gene silencing in this tissue type. Furthermore, merely a single application of DACC lipoplexes sufficed to downregulate expression of the respective target genes in the lung vasculature.
Figure 7.
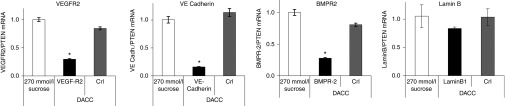
Target genes expressed in the pulmonary endothelium are inhibited by specific DACC lipoplexes. Mice received a single injection of DACC/siRNAVEGFR2, DACC/siRNAVE-Cadherin or DACC/siRNABMPR-2 or DACC/siRNALamin B (2.8 mg siRNA/kg). Expression of the target genes in lungs was assessed 24 hours after DACC/siRNA application by qRT-PCR. mRNA levels of the target genes VEGF-R2, VE-Cadherin, BMP-R2 and Lamin B were reduced by 70–90% in the respective DACC/siRNA treatment groups.
DACC/siRNACD31 treatment increases survival in experimental lung metastasis mouse model
CD31/Pecam1 is a cell surface protein required for homotypic as well as heterotypic cell interactions. It is involved in multiple processes of tumorigenesis, like angiogenesis, vascular permeability and metastases.18,19 We were able to demonstrate in previous studies that targeting CD31 by RNA interference leads to reduction of tumor growth in subcutaneous xenograft tumor models and in orthotopic prostate cancer model.20
Here, we investigated whether DACC/siRNACD31 could be employed therapeutically in the treatment of lung cancer. To this end, we tested whether treatment with DACC/siRNACD31 had a therapeutic benefit in an experimental lung metastasis mouse model.21 In this model, Lewis lung carcinoma cells are applied intravenously to syngeneic mice, thus leading to colonization of tumor cells in the lungs and subsequent outgrowths of pulmonary metastases. DACC/siRNACD31, control lipoplexes DACC/siRNAluciferase, and sucrose as vehicle control were prepared and injected into mice 5 days prior to i.v. Lewis lung tumor cell injection. Treatment was repeated on alternating days until day 15 (Figure 8a). Body weight was monitored continuously. During the treatment period, decrease in body weight due to DACC lipoplex application was not observed (Figure 8b). In the follow up phase, a decrease in body weight was first observed in the sucrose cohort starting on day 20 after cell challenge. Survival of all animals was monitored by defined end point criteria (including body weight) for up to 70 days after tumor cell challenge. Survival of animals which received DACC/siRNACD31 was significantly prolonged as compared to the control treatment groups that received sucrose or DACC/siRNAluciferase. Animals treated with isotonic sucrose solution or luciferase control lipoplexes showed poor survival: in the sucrose control group, none of the animals survived past 30 days, and only two animals of the DACC/siRNAluciferase control group survived up to day 70 (Figure 8c). In comparison, seven of eight animals receiving DACC/siRNACD31 survived up to day 70. CD31 expression was evaluated after completion of the treatment phase on day 16 after tumor cell challenge in separate cohorts (Figure 8a). Compared to animals treated with DACC/siRNAluciferase or sucrose as vehicle control, CD31 expression in lung tissue of animals treated with DACC/siRNACD31 was reduced by approximately 80% (Figure 8d) confirming that CD31 expression could be targeted by DACC lipoplexes in a therapeutic setting.
Figure 8.
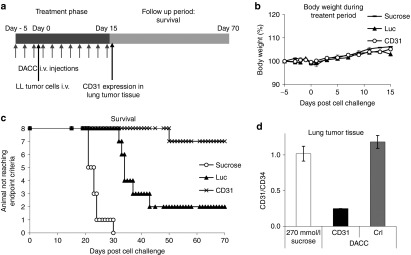
DACC/siRNACD31 treatment increases survival in experimental lung metastasis mouse model. (a) Experimental set up of lung metastasis model. Lewis lung carcinoma cells were administered intravenously into BDF1 mice, and formed metastases in the lungs. DACC lipoplexes (2.8 mg/kg) were given i.v. by bolus injection on alternating days during treatment phase. (b) Body weight development during DACC/siRNA treatment phase. (c) Survival of mice was monitored by defined end point criteria for a period of 70 days after cell challenge. Survival was significantly increased for those animals treated with DACC/siRNACD31 as compared to treatment with DACC/siRNALuciferase (P < 0.006, log-rank test) or compared to sucrose control treatment group (P < 0.001). (d) CD31 mRNA expression was assessed in lung tumor tissues of mice, day 16 after cell challenge and 24 hours after the last treatment with DACC lipoplexes.
Discussion
RNA-based therapeutics have broadened the scope of therapeutic targets for a variety of human diseases beyond the range of existing pharmacological drugs. Albeit a large number of RNA or RNA-derived therapeutics have already reached clinical testing,3 challenges remain with respect to delivery, stability, biodistribution, and immune activation, and have set off improvements in synthetic and natural nucleic acid carriers, as well as in the development of chemically modified oligonucleotides.3,13 Notwithstanding, the main and disease-relevant question in developing RNAi-based drugs is that of how to deliver small siRNAs to the cell in a therapeutically acceptable way. When injected intravenously, siRNAs are rapidly cleared by renal filtration and are susceptible to degradation by extracellular RNAases. Nucleic acid-lipid particles can be made by using cationic lipids which incorporate siRNAs. They can also contain a diffusible PEG-lipid conjugate coating which stabilizes the particle during formation and first contact with blood components, as well as provide a hydrophilic exterior, potentially preventing rapid systemic clearance. The lipid bilayer contains a mixture of cationic and colipids facilitating internalization of the lipoplex particle as well as endosomal escape of its siRNA load.13 In addition to changing colipids, much effort has also been devoted into the synthesis of new cationic lipids which are more serum resistant. In our experiments described above, we were able to confirm the inverse correlation between lipoplex formulation stability and functional organ uptake, i.e., the formulations most stable even with respect to their tendency to aggregate were also found to be least entrapped in the vascular beds of the pulmonary endothelium, but rather to accumulate directly in the liver and spleen for subsequent degradation22 (Figure 1). However, delivery of siRNA to the target tissue and cellular uptake are not the sole factors responsible for activity. This has been previously demonstrated by Heyes et al., suggesting that instead of endocytosis being rate-limiting, it is rather those events occurring once the siRNA has been internalized by the cell which have the greatest effect on the efficiency of gene silencing, such as endosomal release and RISC loading.23
Lipoplex formulation development
Our goal was to identify an siRNA delivery system suitable for functional gene silencing in the pulmonary endothelium by developing a formulation stable enough not to be degraded prior to reaching its target organ, but not too stable as to hinder functional uptake by the lung endothelium. We created and evaluated the novel DACC/siRNA lipoplex formulation which, upon intravenous administration, specifically targets and downregulates expression of genes of the pulmonary endothelium. It is composed of 70 mol% of the cationic lipid AtuFECT01, 29 mol% cholesterol and 1 mol% of mPEG2000-DSPE, as well as the functionally active AtuRNAi. Cholesterol was used at 29 mol% in the DACC/siRNA formulations to maintain lipoplex stability without losing fusogenicity, and thus affects lamellarity, plasma pharmacokinetics, and biodistribution of lipoplexes. Because intravenous administration of cationic delivery systems can result in systemic toxicity due to binding and rapid degradation of lipoplexes by blood serum opsonins (immunoglobulins, complement factor, fibronectins), PEG is conjugated to the surface of the lipoplex nanoparticles in order to form a hydrophilic protective layer around the nanoparticles able to repel the adsorption of opsonin proteins via steric repulsion forces. Cationic lipid AtuFECT01 was shown to allow for an active loading of negatively charged RNA into lipoplexes due to electrostatic interactions and entropic effects, respectively. It condenses oligonucleotides, protects them from degradation and facilitates uptake by cells and endosomal release. We observed siRNA accumulation in the lungs by DACC lipoplex. This lung distribution and gene silencing contrasts typical systemic behavior of cationic lipid nanocomplexes, which generally show prevalent gene knockdown in liver tissue with only transient accumulation of siRNA in the lungs.24,25,26
In addition, we were able to develop a lyophilization procedure ensuring effective long-term storage of our DACC formulations even at room temperature. We addressed this stability issue because lipoplex suspensions are known to be unstable in aqueous suspension for long-term storage, especially with respect to hydrolysis and size stability. DACC formulation stability during freezing, drying and rehydration steps was ensured by addition of sucrose as a cryo- and lyoprotectant. This addition also enabled adjustment of the suspension to correct osmolarity for in vivo applications (data not shown).
A single dose of DACC lipoplex (2.8 mg siRNA/kg) was shown to reduce mRNA levels of the target gene Tie-2 by 60–90% (Figure 6a), with this silencing effect persisting for up to 3 weeks. Gene silencing activity by DACC lipoplexes appeared to be restricted to the lung, since even repeated treatment regimens (3 × 2.8 mg/kg) did not affect target gene expression in heart, liver or kidney significantly (Figure 4c–e). We were able to demonstrate that DACC lipoplexes also maintained gene silencing activity when administered by means of slow (1 hour) infusion (Figure 5b), a prerequisite for its application in clinical settings.
Albeit lung retention due to cellular association has been reported previously for several types of cationic liposomes22,27,28,29,30 reduction of Tie2-mRNA levels by treatment with DACC/siRNATie-2 or other targets whose expression is restricted to the endothelial cell type, established functional tissue specific uptake and gene silencing by DACC in the pulmonary endothelium. We were unable to demonstrate inhibition of genes expressed in lung epithelial cells or alveolar macrophages, like Lamin B or L-GAL-S3, respectively (Figure 7 and data not shown). This is in contrast to liposomal formulations described by Mac Caskill et al.,29 which report gene silencing activity of a cationic formulation in endothelial and epithelial cells. We did not investigate the mechanism by which the DACC delivery system targets specifically the pulmonary endothelium. Because a targeting ligand was not added, we assume that targeting to the pulmonary vasculature occurs in a rather nonspecific fashion through electrostatic interaction of the positively charged lipoplex with negatively charged endothelium.28 Dose range finding experiments with DACC/siRNATie-2 established an EC50 in the mouse of about 1.5 mg siRNA/kg for inhibition of Tie-2 expression in the lungs using a single dose, but higher doses of 3 mg/kg (bolus) and up to 6 mg/kg (infusion) were also tolerated. However, higher single doses of siRNA lipoplexes had unfavorable effects on body weight development, showing a drop of over 10% at the 12 mg siRNA/kg dose levels (compared to negligible weight loss at a dose of 3 and 1 mg siRNA/kg (data not shown). From a clinical standpoint, the observed low and one-time dosing is favorable for subsequent toxicity profiling. As a proof-of-concept experiment to investigate whether DACC could have therapeutic efficacy in lung cancer settings, DACC/siRNACD31 was tested in an experimental mouse model for lung metastases. Repeated treatments by DACC/siRNACD31 (11 × 2.8 mg/kg) were very well tolerated and did not affect body weight development in the treatment phase (Figure 8b). At this dose, ALT/AST serum levels as parameter for liver toxicity were not elevated (Supplementary Figure S1), TNF-α or IFN-γ levels as markers for inflammatory response were also not increased by this formulation (Supplementary Figure S2), suggesting that DACC treatment was well tolerated. In the end, DACC/siRNACD31 led to an increase in survival of the DACC/siRNACD31 treatment group thus opening a new and promising therapeutic venue in the treatment of lung cancer as well as lung metastasis.31 In this experimental setting, treatment with DACC/siRNACD31 formulation was initiated before tumor cell challenge to reduce attachment, establishment and outgrowth of metastases in the lungs afterwards. This treatment could be beneficial for patient groups that undergo resection of the primary tumor, but have an enhanced risk of developing metastases in the lungs afterwards. Regarding further applications of the DACC/siRNA lipoplex system, the observed low and one-time dosing for prolonged target gene inhibition makes it an attractive candidate for treatment of acute diseases requiring hospital admission with intravenous drug-administration, such as acute respiratory distress syndrome, a life-threatening syndrome characterized by exaggerated inflammation and increased vascular permeability leading to oedema.16,32,33 Selective target gene inhibition by DACC employing synthetic nucleic acids such as siRNAs, antisense molecules, antagomers, and/or micro RNA mimics could therefore be a promising strategy to pursue the development of innovative therapeutic approaches for the management of acute life-threatening lung diseases.
Materials and Methods
Short interfering RNAs. The siRNA molecules (AtuRNAi) used in this study are listed in Supplementary Table S1. The siRNA molecules (AtuRNAi) used in this study are blunt, 19-mer double-stranded RNA oligonucleotides stabilized by alternating 2′-O-methyl modifications on both strands as previously described,5,34 and were synthesized by BioSpring (Frankfurt a.M., Germany).
Preparation and characterization of siRNA lipoplexes. Cationic liposomes comprised of cationic lipid AtuFECT01 (β-L-arginyl-2,3-L-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride; Silence Therapeutics GmbH, Berlin, Germany), cholesterol (Sigma Aldrich, Taufkirchen, Germany), and mPEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanol amine-N (methoxy (polyethylene glycol)-2000)); Avanti Polar Lipids, Alabaster, AL) at a molar ratio of 70:29:1 were prepared by lipid film rehydration5 with 270 mmol/l sterile sucrose solution. The resulting liposomal stock solutions had total lipid concentrations of 5 mg/ml or up to 9 mg/ml (i.e., for infusion studies), respectively. The formation of siRNA lipoplexes occurred by mixing equal volumes of liposomal dispersion and siRNA solution in 270 mmol/l sucrose. For this purpose, the concentration of both were adjusted in a way, that the final lipoplex formulation was characterized by a final lipid/siRNA ratio (m/m) of 6.8, which corresponded to a charge ratio between charged lipid nitrogen atoms to nucleic acid backbone phosphates (N/P ratio) of approximately 8.4. Particle sizes (Z-average size, intensity distribution) and zeta potentials of liposomes and lipoplexes were determined by Dynamic Light Scattering using a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK). Here, corresponding dispersant properties were adjusted to 270 mmol/l sucrose. Negative stain transmission electron microscopy was done by Vironova AB (Stockholm, Sweden).
siRNA distribution and RNAi in vivo. Eight-week-old C57Bl/6j (Harlan, Rossdorf, Germany) males and females were used for in vivo studies. All animal experiments in this study were performed according to approved protocols and in compliance with the guidelines of the Landesamt für Arbeits-, Gesundheitsschutz und technische Sicherheit Berlin, Germany. Isotonic siRNA-lipoplex formulations were administered intravenously through bolus tail-vein injection at the indicated doses. For siRNA-Cy3 biodistribution studies, mice received a single dose of DACCsiRNA-Cy3 (2.8 mg siRNA/kg body weight). One hour after injection, mice were sacrificed and tissues were processed for paraffin embedding as previously described.5 Sections of 4 µm were cut and deparaffinized with Roticlear (Carl Roth, Berlin, Germany, A538.5), rehydrated through graded ethanol washes and counterstained with Sytox Green dye (Life Technologies, Darmstadt, Germany). Fluorescence uptake was analyzed with a Zeiss LSM510 Meta confocal microscope. Images were recorded and processed by Axioversion software. For quantitative analysis of siRNA distribution in different organs, mice received a single dose of lipoplexes formulated with PKN3 siRNA. Concentrations of PKN3 siRNA in different tissues were determined by a modified capture probe sandwich hybridization assay.14 For target mRNA knock down analyses, tissues were dissected immediately after sacrifice of the mice and instantly snap-frozen in liquid nitrogen. Approximately 20 mg of tissue was homogenized in a Mixer Mill MM 301 (Retsch GmbH, Haan, Germany) using tungsten carbide beads (Qiagen, Hilden, Germany). Total RNA was isolated from the lysate with the Invisorb Spin Tissue RNA Mini Kit (Invitek, Berlin, Germany). Depending on the tissue, 25–100 ng total RNA was used for quantitative TaqMan RT-PCR with the amplicon sets (listed in Supplementary Table S2) obtained from BioTez GmBH, Berlin, Germany: The TaqMan RT-PCR reactions were carried out with an ABI PRISM 7700 Sequence Detector (Software: Sequence Detection System v1.6.3 (ABI Life Technologies)) or StepOnePlus Real Time PCR Sytem (ABI) using a standard protocol for RT-PCR as described previously5 with primers and probes at a concentration of 300 and 100 nmol/l respectively. TaqMan data were calculated by using the comparative Ct method. Target protein expression was assessed by western blotting of whole tissue lysates as described previously.5 Snap frozen tissues were homogenized as described above in a Mixer Mill MM 301 (Retsch GmbH, Haan, Germany), and proteins were extracted in RIPA lysis buffer. Equal amounts of protein were loaded for immunoblot analysis using the following antibodies: rabbit anti-PTEN (Ab-2, Neomarkers, Fremont, CA) and mouse anti-Tie2 (clone Ab33, Upstate Biotechnology, Lake Placid, NY, 05-584).
Infusion studies. For infusion studies, jugular vein catheterized mice (Harlan) received a single 1-hour infusion of DACC/lipoplexes of the highest dose (12 mg siRNA/kg body weight; 40 ml/kg body weight). For dose titrations, DACC lipoplex stock solution was diluted in 5% glucose solution to keep the administration volume constant.
Transfection of mouse endothelial cell line MS1. The mouse endothelial cell line MS1 (ATCC CRL-2279) was obtained from the American type cell culture collection and cultivated according to the supplier's recommendations. Cells were seeded in six-well plates and transfected with DACC/siRNATie-2 as described previously.5 Briefly, about 12 hours after cell seeding different amounts of DACC/siRNA formulations diluted in 10% serum-containing medium were added to the cells to achieve transfection concentrations in a range of 10–160 nmol/l siRNA. Three days after transfection cells were lysed. Proteins were separated by SDS-PAGE and subjected to immune blotting as previously described.5 Immunoblots were analyzed by luminescence imaging using the Stella camera system and AIDA image analyzer software 4.25 from Raytest (Mannheim, Germany).
Experimental lung metastasis mouse model. Lewis lung carcinoma cells were cultured in RPMI medium supplemented with 10% fetal calf serum and 4 mmol/l glutamine. Cells were dissociated with trypsin and subsequently washed in cell culture medium and in phosphate-buffered saline. A total of 250,000 cells in 200 µl phosphate-buffered saline were injected into the tail vein of 8-week-old male BDF1 mice (Harlan). Mice (12 animals/group) were treated 11 times with isotonic sucrose or with DACC lipoplexes on alternating days beginning 5 days before tumor cell challenge. Twenty-four hours after the last treatment (day 16 after cell challenge), four mice/group were sacrificed to collect lung tissue for analysis of CD31 target gene expression. The remaining eight mice per group were considered for the survival study. Mice were monitored daily for body weight development and signs of suffering. When reaching defined endpoint criteria (score ≥ 4) animals were sacrificed.
Statistical analysis. Data are expressed as means ± SEM. Statistical significance of differences was determined by analysis of variance followed by Dunnett's multiple comparisons test log-rank test using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA). the P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. ALT/AST enzyme levels of mice treated with DACC. Figure S2. TNF-α and IFN-γ levels are not elevated after treatment with DACC. Table S1. siRNA sequences. Table S2. Primer sets for Taqman PCR.
Acknowledgments
We thank Hüseyin Aygün (Biospring, Frankfurt a.M.) for providing high-quality siRNA molecules. All authors are employees of and have stock options from Silence Therapeutics plc. The authors declare competing financial interests.
Supplementary Material
ALT/AST enzyme levels of mice treated with DACC.
TNF-α and IFN-γ levels are not elevated after treatment with DACC.
siRNA sequences.
Primer sets for Taqman PCR.
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shum KT, Burnett JC, Rossi JJ. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals (Basel) 2013;6:85–107. doi: 10.3390/ph6010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KA, Sahay G, Li GZ, Love KT, Alabi CA, Ma M, et al. Synergistic silencing: combinations of lipid-like materials for efficacious siRNA delivery. Mol Ther. 2011;19:1688–1694. doi: 10.1038/mt.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, Barenholz Y, Behr JP, Cheng SH, Cullis P, Huang L, et al. Nomenclature for synthetic gene delivery systems. Hum Gene Ther. 1997;8:511–512. doi: 10.1089/hum.1997.8.5-511. [DOI] [PubMed] [Google Scholar]
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang R, Wilcox D, Sarthy A, Lin X, Huang X, et al. Developing lipid nanoparticle-based siRNA therapeutics for hepatocellular carcinoma using an integrated approach. Mol Cancer Ther. 2013;12:2308–2318. doi: 10.1158/1535-7163.MCT-12-0983-T. [DOI] [PubMed] [Google Scholar]
- Peer D, Lieberman J. Special delivery: targeted therapy with small RNAs. Gene Ther. 2011;18:1127–1133. doi: 10.1038/gt.2011.56. [DOI] [PubMed] [Google Scholar]
- Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M, van Nieuw Amerongen GP, Chedamni S, van Hinsbergh VW, Johan Groeneveld AB. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets. 2009;13:39–53. doi: 10.1517/14728220802626256. [DOI] [PubMed] [Google Scholar]
- Adam SA, Goldman RD. Insights into the differences between the A- and B-type nuclear lamins. Adv Biol Regul. 2012;52:108–113. doi: 10.1016/j.advenzreg.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, Delisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–915. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser H, Liu Y, Desprez PY, Thor A, Briasouli P, Handumrongkul C, et al. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced metastatic progression. Proc Natl Acad Sci USA. 2010;107:18616–18621. doi: 10.1073/pnas.1004654107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, et al. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006;13:1360–1370. doi: 10.1038/sj.gt.3302778. [DOI] [PubMed] [Google Scholar]
- Santel A, Aleku M, Röder N, Möpert K, Durieux B, Janke O, et al. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res. 2010;16:5469–5480. doi: 10.1158/1078-0432.CCR-10-1994. [DOI] [PubMed] [Google Scholar]
- Simberg D, Weisman S, Talmon Y, Faerman A, Shoshani T, Barenholz Y. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003;278:39858–39865. doi: 10.1074/jbc.M302232200. [DOI] [PubMed] [Google Scholar]
- Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Polach KJ, Matar M, Rice J, Slobodkin G, Sparks J, Congo R, et al. Delivery of siRNA to the mouse lung via a functionalized lipopolyamine. Mol Ther. 2012;20:91–100. doi: 10.1038/mt.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Davide JP, Cai M, Zhang GJ, South VJ, Matter A, et al. Noninvasive imaging of lipid nanoparticle-mediated systemic delivery of small-interfering RNA to the liver. Mol Ther. 2010;18:1657–1666. doi: 10.1038/mt.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahato RI, Anwer K, Tagliaferri F, Meaney C, Leonard P, Wadhwa MS, et al. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration. Hum Gene Ther. 1998;9:2083–2099. doi: 10.1089/hum.1998.9.14-2083. [DOI] [PubMed] [Google Scholar]
- Kuruba R, Wilson A, Gao X, Li S. Targeted delivery of nucleic-acid-based therapeutics to the pulmonary circulation. AAPS J. 2009;11:23–30. doi: 10.1208/s12248-008-9073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill J, Singhania R, Burgess M, Allavena R, Wu S, Blumenthal A, et al. Efficient Biodistribution and Gene Silencing in the Lung epithelium via Intravenous Liposomal Delivery of siRNA. Mol Ther Nucleic Acids. 2013;2:e96. doi: 10.1038/mtna.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardita C, Predescu D, Predescu S. Long-term silencing of intersectin-1s in mouse lungs by repeated delivery of a specific siRNA via cationic liposomes. Evaluation of knockdown effects by electron microscopy. J Vis Exp. 2013;76:e50316. doi: 10.3791/50316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, et al. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit Care Med. 2012;40:3034–3041. doi: 10.1097/CCM.0b013e31825fdc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ, et al. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31:2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ALT/AST enzyme levels of mice treated with DACC.
TNF-α and IFN-γ levels are not elevated after treatment with DACC.
siRNA sequences.
Primer sets for Taqman PCR.