Abstract
Using stem cell–conditioned medium (CM) might be a viable alternative to stem cell transplantation, which is often hampered by low grafting efficiency and potential tumorigenesis, but the concentrations of angiogenic growth factors in CM are too low for therapeutic use and some components of the medium are not for human use. We used three-dimensional (3D) spheroid culture of human adipose-derived stem cells (ADSCs) with clinically relevant medium composed of amino acids, vitamins, glucose, and human serum to produce clinically relevant CM containing angiogenic and/or antiapoptotic factors such as vascular endothelial cell growth factor, fibroblast growth factor 2, hepatocyte growth factor, and chemokine (C-X-C motif) ligand 12. The concentrations of these factors were 23- to 27-fold higher than that in CM produced by conventional monolayer culture. Compared with injection of either monolayer culture CM or human ADSC, injection of spheroid culture CM to an ischemic region in mice significantly enhanced endothelial cell growth, CD34+/PTPRC− (endothelial progenitor) cell mobilization from bone marrow, and bone marrow cell homing to the ischemic region, resulting in improved blood vessel density, limb salvage, and blood perfusion in a mouse hindlimb ischemia model. The stem cell CM developed in this study will likely be an effective alternative to conventional stem cell transplantation therapy.
Introduction
Cell-based therapy using stem cells such as bone marrow–derived stem cells, which contain subsets of cells such as hematopoietic stem cells, mesenchymal stem cells, and endothelial progenitor cells, is a promising option for treating ischemic diseases, including ischemic heart diseases and chronic limb ischemia.1,2 However, poor engraftment of the transplanted cells undermines the therapeutic efficacy of the treatment and remains a major limitation of cell-based therapy.3,4 Additionally, studies have shown a potential risk of developing cancer after hematopoietic stem cell transplantation,5,6 raising safety concerns over the therapeutic use of stem cells.
One potential approach to overcome such limitations of current cell-based therapy could be the use of stem cell–conditioned medium (CM). For example, neovascularization, one of the most well-known features of stem cell–based therapy, can be mediated by the physical incorporation of transplanted cells into newly formed vessels;7 however, accumulating evidence indicates that the paracrine signaling initiated by stem cells, which involves secretion of various angiogenic growth factors and cytokines, is also responsible for stem cell therapy–induced angiogenesis.8,9 Thus, injection of stem cell CM, containing various angiogenic factors secreted by stem cells, is a promising alternative that can overcome the poor engraftment of the transplanted stem cells and the potential risk of cancer development. An additional benefit is that stem cell CM would be an off-the-shelf material that could be used to treat patients promptly without stem cell isolation from the patients and subsequent culture.
Although the benefits of stem cell CM therapy are clear, several issues must be addressed before its clinical application. One problem concerns the components of the culture medium. Currently available culture media for stem cells contain components that are not intended for human use, such as 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), phenol red, and bovine serum, necessitating the development of an alternative medium that is safe for human clinical use.
A second problem is the low concentration of angiogenic factors in the CM. For example, a recent study showed that the concentration of vascular endothelial cell growth factor (VEGF) in mesenchymal stem cell CM was 217 ± 97 pg/ml,10 whereas the reported minimum effective VEGF concentration to induce effective in vivo angiogenesis is ~5 ng/ml.11,12 As such, the low concentration of angiogenic factors in CM might undermine the efficacy of the CM injection therapy. One potential solution to this problem was found in a previous study showing that human adipose-derived stem cells (ADSCs) produced angiogenic factors and chemokines.13 In addition, we recently reported that when human ADSCs were implanted as spheroids into mouse ischemic hindlimbs, the secretion of angiogenic and antiapoptotic factors in vivo was enhanced, thereby improving the therapeutic potential of human ADSCs.14 Herein, we showed that a novel clinically relevant medium (CRM), composed of amino acids, vitamins, glucose, and human serum, can be used for human ADSC culture, and that human ADSC spheroid culture in a three-dimensional (3D) bioreactor using CRM can produce CM that contains high concentrations of angiogenic and antiapoptotic factors. In the present study, we evaluated the ability of human ADSCs to secrete VEGF, fibroblast growth factor 2 (FGF2), hepatocyte growth factor (HGF), and chemokine (C-X-C motif) ligand 12 (CXCL12), which are known to be critical cytokines for angiogenesis.15,16,17,18,19 We used a mouse hindlimb ischemia model to examine the mechanism by which the CRM-based human ADSC spheroid culture CM induces angiogenesis and compared its efficacy with that of either human ADSC monolayer culture CM or human ADSC implantation.
Results
3D spheroid culture system supports high-cell-density culture
With serum supplementation, the 3D spheroid culture system was able to support the growth of cells at a density approximately four times higher than the density of the matching monolayer culture (×105 cells/ml; 12.0 ± 0.5 versus 3.1 ± 0.3 in α-minimum essential medium (αMEM) and 11.9 ± 0.7 versus 3.1 ± 0.5 in CRM; Figure 1a). This increase in supported density did not depend on the type of medium or serum used (Figure 1a). Even the spheroid culture in CRM without serum supported culture at a significantly higher maximal cell density compared with the monolayer culture supplemented with serum (×105 cells/ml; 7.0 ± 0.8 versus 3.1 ± 0.5).
Figure 1.
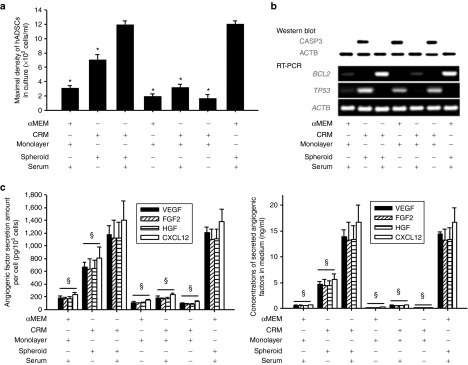
Three-dimensional (3D) spheroid culture supports high-density growth of human adipose-derived stem cells (ADSCs) with increased antiapoptotic signals and enhanced angiogenic factor production. Human ADSCs were cultured in a conventional monolayer culture or a 3D spheroid culture for 4 days by using two different media (α-minimum essential medium (αMEM) and clinically relevant medium (CRM)) with or without supplementary serum. (a) Maximal density of human ADSCs in culture. (b) Expression of apoptosis-related factors in human ADSCs cultured in monolayer or spheroids with various media, as evaluated by western blot using anti-CASP3 antibodies (upper panel) or by reverse transcription–polymerase chain reaction (lower panel, BCL2 and TP53). (c) The amount of angiogenic factors (vascular endothelial cell growth factor (VEGF), fibroblast growth factor 2 (FGF2), hepatocyte growth factor (HGF), and chemokine (C-X-C motif) ligand 12 (CXCL12)) expressed as the amount produced by same number of cells (left) or ng/ml of conditioned medium (right). Quantitative data are mean ± SD of at least three independent cultures per group. *P < 0.05 compared with the CRM (serum+), spheroid group. §P < 0.05 compared with the CRM (serum+), spheroid group for all four angiogenic factors. RT–PCR, reverse transcription–polymerase chain reaction.
Antiapoptotic effect of 3D spheroid culture system
Only the spheroid culture groups supplemented with serum showed increased BCL2 messenger RNA (mRNA) expression, whereas the matching monolayer culture groups did not (Figure 1b). Serum deprivation caused CASP3 pathway activation and increased TP53 mRNA expression, regardless of the type of medium or culture system used. The results of CD177 and CASP3 staining on day 3 after injection of human ADSCs as single cells into mouse ischemic hindlimbs indicate that the activation of CASP3 was obvious on day 3 (Supplementary Figure S1, upper panel). When human ADSCs were transplanted into normal muscle tissue without any treatment, the survival of human ADSCs was superior to that of human ADSCs injected into an ischemic region (Supplementary Figure S1, bottom panel).
Enhanced angiogenic factor production in 3D spheroid culture system
The amount of angiogenic factors (VEGF, FGF2, HGF, and CXCL12) produced by the same number of cells (1 × 105 cells) after 2 days in culture was significantly increased by 4- to 6-fold in both the αMEM- and the CRM-based spheroid culture systems (Figure 1c, left). Regardless of the type of medium used, the spheroid culture CM had a significantly higher concentration of angiogenic factors than the monolayer culture CM did (Figure 1c, right). The concentration of VEGF, FGF2, HGF, and CXCL12 in the αMEM (serum+) spheroid culture CM was 14.4 ± 0.4, 13.2 ± 2.2, 13.3 ± 2.3, and 16.6 ± 2.9 ng/ml, respectively. Although we found no significant difference in the concentration of angiogenic factors between the CRM (serum+) spheroid culture CM and the αMEM (serum+) spheroid culture CM, the values from the spheroid culture CM are 23−27 times higher than those from the αMEM (serum+) monolayer culture CM.
Support of human endothelial cell growth by CRM-based spheroid culture CM
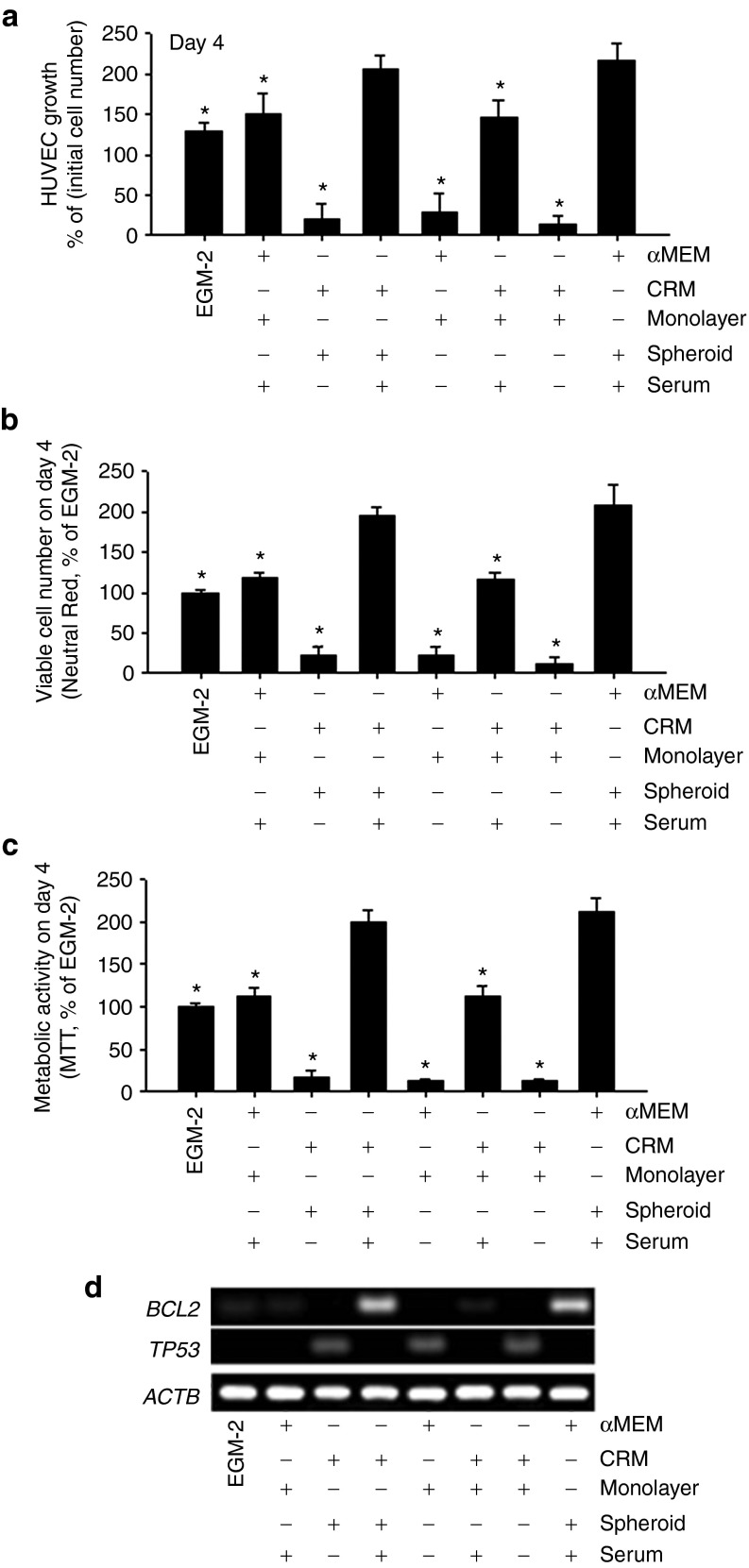
Human umbilical vein endothelial cell (HUVEC) proliferation was significantly increased in the spheroid culture CM groups compared with the monolayer culture CM groups, and no significant medium type–dependent differences were observed (Figure 2a). Regardless of the type of medium, HUVEC proliferation was significantly inhibited by depletion of serum. Neutral Red assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay indicated that the viability and the metabolic activity of HUVECs were significantly increased in the spheroid culture CM groups compared with the matching monolayer culture CM groups, as well as a commercially available endothelial cell culture medium with serum (endothelial growth medium (EGM2; Figure 2b,c). Additionally, mRNA expression of BCL2, a key factor of the antiapoptotic signal pathway, increased in the spheroid culture CM groups, whereas mRNA expression of the proapoptotic signaling molecule TP53 increased in those groups without serum (Figure 2d).
Figure 2.
Clinically relevant medium (CRM)–based spheroid culture conditioned medium (CM) increases proliferation and antiapoptotic signaling of human umbilical vein endothelial cells (HUVECs) in vitro. (a) Growth of HUVECs cultured in different CM. The same number of HUVECs was cultured in different CM for 4 days, and the HUVECs grew fastest in the CM (CRM [serum+], spheroid) group (*P < 0.05 compared withto the growth in the CM (CRM [serum+], spheroid) group. (b) Viable cell number as evaluated by Neutral Red assay on day 4. *P < 0.05 compared with the CM (CRM [serum+], spheroid) group. Quantitative data are mean ± SD of at least three independent cultures per group. (c) Metabolic activity evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on day 4. (d) Messenger RNA expression of apoptosis-related factors BCL2 and TP53, analyzed by reverse transcription polymerase chain reaction on day 4.
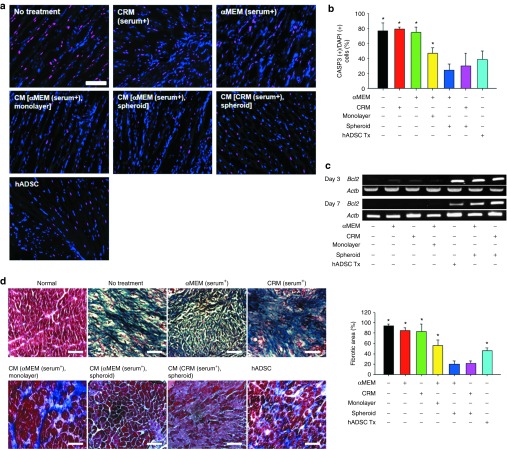
In vivo antiapoptotic effect of CRM-based 3D spheroid culture CM
To confirm the in vitro antiapoptotic effect of the spheroid culture CM, the animals with ischemic hindlimbs were treated by either a single injection of human ADSC suspension (200 μl, 2 × 106 cells) or daily injection of 40 μl of the assigned media for 7 days into the gracilis muscle following the induction of the ischemic hindlimb. Theoretically, the total amount of CM used to treat each animal (40 μl × 7 days = 280 μl) would contain various factors secreted from 8.7 × 104 to 3.4 × 105 cells depending on the culture type (monolayer or spheroid; from Figure 1a), and these cell numbers are much smaller than the number of human ADSCs (2 × 106 cells) in the human ADSC implantation group. Seven (Figure 3a,b) and 28 days (Supplementary Figure S3) after the initiation of treatment, the ratio of CASP3-positive cells to 4,6-diamidino-2-phenylindole (DAPI)–positive cells in the ischemic region was calculated. The ratios in both the CRM (serum+)-only and the αMEM (serum+)-only groups did not differ significantly from the ratio in the no-treatment group. However, the ratio was significantly decreased in the spheroid culture CM groups and the human ADSC implantation group. In those groups with a decreased CASP3 ratio, the expression of BCL2 was increased, whereas TP53 expression was decreased at early time points (days 3 and 7, Figure 3c). In agreement with the result in a previous study,14 human ADSCs transplanted into ischemic area survived and remained active until 28 days after transplantation based on the secretion of human paracrine factors from the cells (Supplementary Figure S2). Masson's trichrome staining and fibrotic area quantification based on color performed on the specimens retrieved 28 days after treatment indicated that the fibrosis in the ischemic region was less severe in the spheroid culture CM groups (Figure 3d).
Figure 3.
Treatment of human adipose-derived stem cells (ADSCs) with conditioned medium (CM) from clinically relevant medium (CRM)–based three-dimensional (3D) spheroid culture attenuated apoptosis and fibrosis in the ischemic region. (a) Apoptosis in the ischemic region treated with various methods. α-Minimum essential medium (αMEM), CRM, αMEM-based CM, CRM-based CM (40 μl/day for 7 days), or human ADSC suspension (1 × 107 cells/ml, 200 μl per mouse, single injection on day 0) was injected intramuscularly into the ischemic hindlimb. After 7 days, ischemic tissue samples were stained with 4,6-diamidino-2-phenylindole (DAPI; nucleus: blue) and anti-CASP3 antibodies (purple). Bar = 100 μm. (b) Quantification of both CASP3- and DAPI-positive cells in the ischemic regions (*P < 0.05 compared with the CM (CRM [serum+], spheroid) group). Quantitative data are mean ± SD of at least three animals per group). (c) Messenger RNA expression pattern of Bcl2 after treatment. Animals from each group were sacrificed on days 3 and 7, and reverse transcription–polymerase chain reaction was performed using the total RNA extracted from the ischemic region. (d) Fibrosis in ischemic region and quantification. Tissues in the ischemic region were stained and quantified with Masson's trichrome staining 28 days after surgery (Bar = 25 μm, *P < 0.05 compared with the CM (CRM [serum+], spheroid) group).
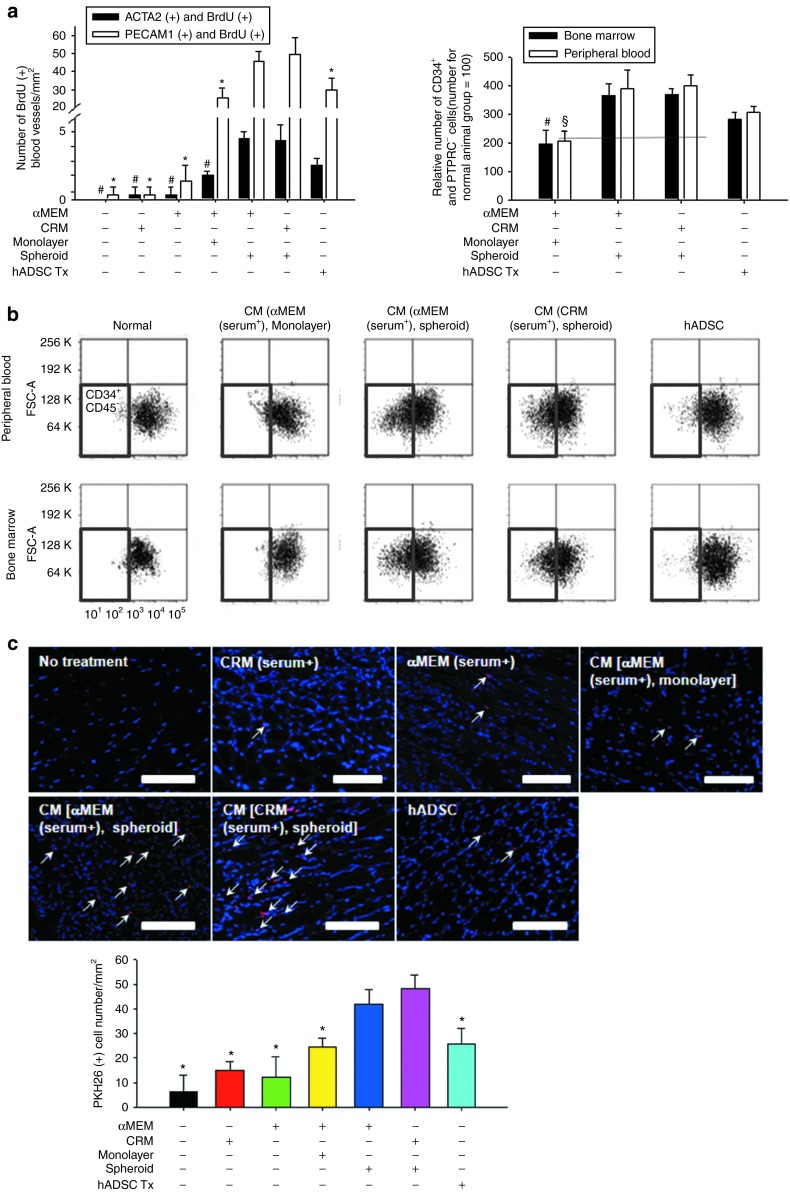
Enhancement of endothelial cell growth, CD34+/PTPRC− cell mobilization, and homing of BMMNC by spheroid culture CM
The number of proliferating vascular cells present in the ischemic region, which was judged by bromodeoxyuridine (BrdU) incorporation, was increased in the spheroid culture CM groups (Figure 4a). The CRM-based spheroid culture CM group showed a significantly higher number of CD31 and BrdU double-positive cells than did the other groups, except the αMEM-based spheroid culture CM group. The CRM-based spheroid culture CM group also showed a significantly higher number of SM α-actin and BrdU double-positive cells than did the other groups, except the αMEM-based spheroid culture CM group and the human ADSC implantation group. Flow cytometric analysis of peripheral blood and bone marrow cells collected 7 days after treatment indicated that the number of CD34+/PTPRC− cells was increased ~2.5- to 3.5-fold in the spheroid culture CM injection groups (both the αMEM- and the CRM-based) and the human ADSC implantation group compared with the normal control (Figure 4b). PKH26-labeled cell counting data showed that the number of cells recruited to the ischemic region after the intracardiac injection of PKH26-labeled bone marrow mononuclear cells (BMMNCs) was significantly increased in animals treated with spheroid culture CM (both αMEM- and CRM-based) for 7 days before the injection of PKH26-labeled BMMNCs compared with the rest of the groups (Figure 4c).
Figure 4.
Injection of spheroid culture conditioned medium (CM) into ischemic hindlimbs enhanced endothelial cell growth in the ischemic region, endothelial progenitor cell mobilization from bone marrow, and bone marrow cell homing to the ischemic region. (a) The density of CD31 and BrdU double-positive cells and SM α-actin and BrdU double-positive cells in the ischemic region on day 28. BrdU was injected for the last 3 days of the treatment period. #P < 0.05 compared with the CM (clinically relevant medium (CRM) [serum+], spheroid) group for CD31/BrdU double-positive cells. *P < 0.05 compared with the CM (CRM [serum+], spheroid) group for SM α-actin and BrdU double-positive cells. (b) Flow cytometry of peripheral blood and bone marrow on day 7 for CD34+/PTPRC− (endothelial progenitor) cells (quadrant enclosed with red line). #P < 0.05 compared with the CM (CRM [serum+], spheroid) group for bone marrow. §P < 0.05 compared with the CM (CRM [serum+], spheroid) group for peripheral blood. (c) Bone marrow cell homing to ischemic region. PKH26-labeled mouse bone marrow mononuclear cells (indicated in red) were introduced into the treated mice via intracardiac injection on day 7. One day later, animals were sacrificed, and the number of PKH26-positive cells (white arrows) in the ischemic hindlimb tissues was determined. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI: blue). Scale bars = 100 μm. *P < 0.05 compared with the CM (CRM [serum+], spheroid) group. Quantitative data are mean ± SD of at least three independent animals per group. αMEM, α-Minimum essential medium; ADSC, adipose-derived stem cell.
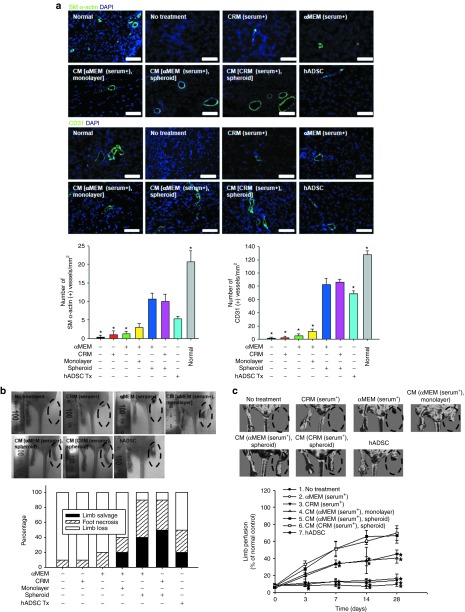
In vivo angiogenic effect of CRM-based spheroid CM
The density of SM α-actin–stained microvessels significantly increased in the spheroid culture CM groups (both αMEM- and CRM-based) and the human ADSC implantation group compared with all the other groups (Figure 5a, upper panel) in the ischemic region after 28 days of treatment. The spheroid culture CM groups (both αMEM- and CRM-based) showed a significantly higher number of CD31-positive capillaries than did all the other groups (Figure 5a, bottom panel). Additionally, reverse transcription–polymerase chain reaction analysis of the ischemic tissue samples showed that the mRNA expression of SM α-actin and CD31 was significantly increased in the spheroid culture CM groups (Supplementary Figure S4).
Figure 5.
Injection of spheroid culture conditioned medium (CM) enhanced angiogenesis, limb salvage, and blood perfusion in a mouse hindlimb ischemia model. (a) SM α-actin-positive microvessels (upper left panel) and CD31-positive microvessels (lower left panel) in the ischemic region after 28 days of treatment were stained with mouse SM α-actin antibody (light green) and CD31 antibody (light green), respectively. Bar = 100 μm. Blue indicates nuclei stained with 4,6-diamidino-2-phenylindole (DAPI). Right panel: the density of SM α-actin–stained microvessels and CD31-stained microvessels in the ischemic region was determined. *P < 0.05 compared with the CM (clinically relevant medium (CRM) [serum+], spheroid) group. (b) Representative pictures of ischemic limbs 28 days after treatment. Red eclipse indicates the ischemic limb. Limb salvage, limb loss, and foot necrosis of each group were evaluated (n = 10 animals per group). (c) Representative laser Doppler images showing blood perfusion in the ischemic limb on day 28. Perforated eclipse indicates the ischemic limb. Blood perfusion of the ischemic limb was monitored for 28 days. Group 6 (CM [CRM (serum+), spheroid]) was significantly different from groups 1–4 on day 3. From day 7 and on, group 6 was significantly different from groups 1–4 and group 7. Quantitative data are mean ± SD of 10 independent animals per group. *P < 0.05 compared with the CM (CRM [serum+], spheroid) group. αMEM, α-Minimum essential medium; ADSC, adipose-derived stem cell.
Improved ischemic limb salvage by spheroid culture CM
The therapeutic potential of the spheroid culture CM was evaluated by monitoring the ischemic limbs for 28 days (Figure 5b and see Supplementary Figure S5 for a full set of follow-up images). In the no-treatment, the CRM (serum+), and the αMEM (serum+) groups, no limb salvage was observed with 10–20% of foot necrosis and 80–90% limb loss, whereas the spheroid culture CM groups and the human ADSC implantation group showed 20–50% of limb salvage with 30–50% of foot necrosis and 10–50% limb loss. Among the groups showing limb salvage, the CRM-based spheroid culture CM group had the highest limb salvage rate of 50%, and the αMEM-based monolayer culture CM group and the human ADSC implantation group had the lowest limb salvage rate of 20%. Fisher's exact test indicated that there was a correlation between the type of treatment and the status of hindlimb salvage (P = 0.0002827).
Improvement of blood perfusion in the ischemic limbs by spheroid culture CM
Laser Doppler perfusion imaging analysis (Figure 5c and see Supplementary Figure S6 for a full set of images) indicated that blood perfusion in the ischemic limbs was significantly improved in the CRM-based spheroid culture CM group (69.7 ± 8.6% of normal healthy control) and the αMEM-based spheroid culture CM group (66.7 ± 8.6%) compared with the other groups. Although the perfusion rate of the human ADSC implantation group (45.3 ± 4.7%) was also significantly higher than that of the no-treatment group (9.7 ± 2.1%), the CRM (serum+) group (14.3 ± 2.5%), or the αMEM (serum+) group (16.7% ± 5.1%), it was still significantly lower than the perfusion rate of the spheroid culture CM groups.
Discussion
The self-renewal and differentiation potential of stem cells is the driving force behind their use for cell therapy in regenerative medicine. However, these same characteristics can pose a risk of developing into cancer. Although no clear and direct evidence has been reported, observations such as the outgrowth of transformed cells in human mesenchymal stem cell culture and the similarities between stem cells and cancer stem cells suggest that stem cells within normal tissues could be involved in tumorigenesis.20,21,22 A further hindrance to the therapeutic efficacy of stem cell transplantation therapy is the low grafting efficiency of implanted stem cells.3,4 Consequently, development of an alternative to the direct transplantation of stem cells might be necessary to ensure the safety and therapeutic efficacy of stem cell–based regenerative therapies. In the current study, we cultured human ADSCs in spheroids in 3D bioreactors (Table 1) using a mixture of amino acid solution, vitamin solution, glucose solution, and human serum to demonstrate the development of therapeutically efficacious and clinically relevant stem cell CM that contains a high concentration of angiogenic factors and does not contain components inappropriate for human use. Because we aimed to develop CM for a clinical application and focused on enhancing the therapeutic effect of CM derived directly from stem cells, comparing with the control groups in the present study would be adequate to show the therapeutic effect of our new CM.
Table 1. Comparisons of stem cell–conditioned media prepared by conventional monolayer culture versus 3D spheroid culture.
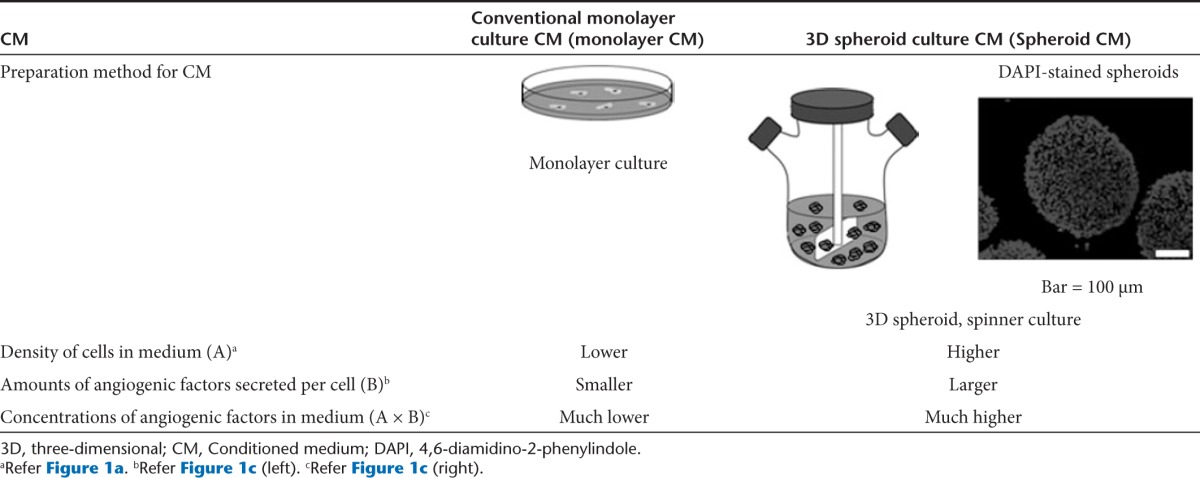
We were able to produce a CM containing a high concentration (>10 ng/ml) of angiogenic factors, sufficient to induce in vivo angiogenesis.11,12 The enhanced angiogenic factor secretion in the spheroid culture (Figure 1c) seems to be due to a mild hypoxic condition established in the core of the spheroids.23 We observed upregulated hypoxia-induced factor 1, alpha subunit (HIF-1A) mRNA expression in the spheroid culture regardless of the type of medium used (Supplementary Figure S7). Hypoxia-induced expression of angiogenic factors such as VEGF24,25 and FGF226 has been reported, explaining the enhanced angiogenic factor production in the spheroid culture observed in the present study. Another factor that increased the concentration of angiogenic factors in the spheroid culture CM is the use of 3D bioreactors. These vessels supported maximal cell density four times higher than the conventional monolayer culture (Figure 1a), partially contributing to the 23- to 27-fold increase in the concentration of angiogenic factors in spheroid CM.
The CRM did not show any difference in its ability to support cell growth compared with the conventional αMEM (Figure 1a), suggesting that our in-house-prepared CRM is as compatible with human cell culture as αMEM. Because all the components of the CRM are being used clinically and are eligible for use in humans, we do not expect any significant adverse effects from the CRM in a clinical setting; however, clinical studies are necessary to validate this hypothesis.
Injection of the CM from spheroid culture (both αMEM-and CRM-based) significantly increased angiogenesis compared with injection of the CM from monolayer culture (Figure 5). This enhanced angiogenesis may be attributed to endothelial cell proliferation, CD34+/PTPRC− (endothelial progenitor) cell mobilization from bone marrow, and bone marrow cell homing to the ischemic region, all of which were enhanced by injection of the spheroid culture CM. A previous study intramuscularly injected recombinant angiopoietin-1, which resulted in recruitment of bone marrow–derived progenitor cells in the muscle.27 Our result has shown that the amount of angiopoietin-1 produced from spheroids was similar to that in a previous study (Supplementary Figure S8). Thus, we used intramuscular CM injection for progenitor cell mobilization. Our data showed increased CD31-positive cell proliferation in the ischemic region (Figure 4a) and mobilization of CD34+/PTPRC− cells28 (Figure 4b) by the injection of the spheroid culture CM compared with the results of injection of the monolayer culture CM. Furthermore, the spheroid culture CM induced homing of a higher number of the injected PKH26-labeled BMMNCs to the ischemic region (Figure 4c). The enhanced mobilization of endothelial progenitor cells and the homing of BMMNCs to the ischemic region were probably facilitated by the higher concentration of CXCL12 contained in the spheroid CM, which has been reported to play an important role in the recruitment and homing of various stem/progenitor cells.29 Although the results, especially the limb salvage ratio, in this manuscript may not be nearly as good as that in our previous study,14 the results were significantly enhanced as compared with the conventional CM injection method. Because we did not use any additional treatment to human ADSCs, such as genes or cytokines, to enhance the angiogenic efficacy, we expect that better results might be possible in future studies using CM based on our method.
In addition to the observed angiogenic effect, the spheroid culture CM upregulated antiapoptotic signals, while suppressing proapoptotic signals (Figure 3 and Supplementary Figure S3). Considering that the majority of human ADSCs transplanted into the ischemic region undergo massive apoptosis a few days after transplantation (Supplementary Figure S1), the use of CM seems to be more effective in preventing apoptosis in the ischemic region than is direct injection of stem cells. The antiapoptotic effect of the spheroid culture CM could be attributed to the increased amount of VEGF because evidence indicates the presence of cross talk between VEGF and BCL2.30,31 Additionally, the spheroid CM contains an increased amount of HGF, which has been reported to inhibit fibrosis in myocardial ischemia,32,33 and this may also be responsible for the decreased fibrosis.
In the present study, we demonstrated that human ADSC spheroid culture CM enhanced therapeutic angiogenesis in a mouse model of ischemic hindlimb. Such improved angiogenesis could have contributed to increased production of angiogenic and/or antiapoptotic factors. Furthermore, increased mobilization and homing of bone marrow–derived angiogenic cells to the ischemic region is likely a major mechanism underlying the improved angiogenesis. However, further study is necessary to investigate whether a simple combination of these four factors is sufficient to produce the angiogenic effects observed with CM, or if there are other unknown factors in the CM that are required. Furthermore, additional animal studies using large animals to detect any possible adverse effects are warranted before the CRM-based stem cell CM for therapeutic angiogenesis can be tested for human use.
In conclusion, in conjunction with a 3D spheroid culture system, the CRM-based stem cell CM system may represent a therapeutically efficacious and clinically relevant stem cell CM.
Materials and Methods
Preparation of CRM. Amino acid solution (Youvasol, Choongwae Pharmaceutical Company, Seoul, Korea), vitamin solution (M.V.I. Injection, Samsung Pharmaceutical Industrial Company, Seoul, Korea), and glucose solution (Dextrose, Choongwae Pharmaceutical Company), which are used clinically, were mixed at the volume ratio of 69:1:20, and ascorbic acid (Sigma, St Louis, MO) was added to the mixture. Detailed components of the CRM are listed in Supplementary Table S1. For human serum supplement, human blood was collected from healthy donors in an ethylene diamine tetraacetic acid (EDTA)–coated container (Vacutainer, BD, Franklin Lakes, NJ). The donors did not take any medications for at least 14 days. The blood was centrifuged at 2,400 rpm for 10 minutes at room temperature, and the upper layer was collected as serum.
Isolation and culture of human ADSCs. Human ADSCs were isolated from lipoaspirates collected from patients giving informed consent and these ADSCs were cultured as previously described.14 Human ADSCs were maintained in αMEM (Gibco BRL, Gaithersburg, MD) supplemented with 10% (v/v) fetal bovine serum (Gibco BRL), 100 units/ml of penicillin (Gibco BRL), and 100 μg/ml of streptomycin (Gibco BRL). Up to five passages were used for the experiments. For monolayer cultures, human ADSCs (2.5 × 105 cells/ml) were cultured in a 150-cm tissue culture plate (Corning, Corning, NY) containing 24 ml of αMEM or CRM with or without supplementary serum (fetal bovine serum for αMEM and human serum for CRM). To generate hypoxic culture conditions, cells were cultured in hypoxic chamber (MCO-18 M, Sanyo, Japan) containing 1% oxygen and 5% CO2 at 37 °C.
Culture of human ADSC spheroids. To generate spheroids, 30 μl drops of human ADSC suspension (1 × 106 cells/ml) were applied onto the inside of the lid of a Petri dish containing phosphate-buffered saline to prevent dry out. After 24 hours of incubation in a 37 °C incubator, spheroids were retrieved using a Pasteur pipette. For 3D bioreactor culture, human ADSC spheroids (6 × 105 cells/ml) were cultured in a spinner flask (Bellco, Vineland, NJ) containing 70 ml of either αMEM or CRM with stirring at 45 rpm.
Preparation of CM. Human ADSCs were cultured in monolayer or as spheroids in 3D bioreactors in αMEM or CRM with or without serum supplement for 2 days, and then, the CM was collected and centrifuged. The maximal cell densities were 3 × 105 cells/ml for monolayer culture and 1.2 × 106 cells/ml for spheroid culture (Figure 1A). We used three different batches to collect the CM for our experiments. At least three independent cell cultures were performed to collect the CM.
Reverse transcription polymerase chain reaction. Cells or tissue samples were homogenized and lysed in TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using 5 μg of pure total RNA and SuperScriptTM II reverse transcriptase (Invitrogen), followed by polymerase chain reaction using primers listed in Supplementary Table S2. Target mRNA expressions were normalized to Actb (mouse) or ACTB (human) expression.
Enzyme-linked immunosorbent assay. The level of angiogenic factors in the CM was determined using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN) specific for human VEGF, HGF, FGF2, CXCL12, and angiopoietin 1 according to the manufacturer's instructions. Moreover, the level of angiogenic factors in the ischemic hindlimbs after cell transplantation was determined using enzyme-linked immunosorbent assay kits (R&D Systems) specific for human VEGF, HGF, and FGF2. The amount of growth factors was calculated as mass per cell, mass per milliliter of medium, or mass per milligram of tissue.
HUVEC culture with CMs. HUVECs were plated at a density of 5 × 104 cells per well in a six-well plate containing αMEM with 5% (v/v) fetal bovine serum. After 24 hours, the medium was replaced with either EGM2 (Lonza, Allendale, NJ), αMEM-based CM, or CRM-based CM. Cells were grown for an additional 4 days for determination of cell growth, viability, and metabolic activity.
Assays for cell viability and metabolic activity. The viability of cells was evaluated using a colorimetric Neutral Red (3-amino-7-dimethylamino-2-methylphenazine hydrochloride, Sigma) assay. The viability of HUVECs cultured in different CMs was expressed as a percentage of the viability of cells cultured in EGM2, a commercial medium for endothelial cell culture. The mitochondrial metabolic activity of the cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) assay. Metabolic activity was expressed as a percentage of the metabolic activity of cells cultured in EGM2.
Mouse hindlimb ischemia model. Four-week-old female athymic mice (20–25 g body weight, Orient, Seoul, Korea) were anesthetized with xylazine (10 mg/kg) and ketamine (100 mg/kg). The femoral artery and its branches were ligated using a 6-0 silk suture (Ethicon, Somerville, NJ). The external iliac artery and all of the upstream arteries were then ligated. The femoral artery was excised from its proximal origin as a branch of the external iliac artery to the distal point from where it bifurcates into the saphenous and popliteal arteries. All animal treatments and experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University (No. SNU-100203-3).
Treatment of hindlimb ischemia. Immediately after arterial dissection, the mice were randomly divided into seven groups (n = 14 per group): no-treatment, CRM (serum+), αMEM (serum+), CM (αMEM [serum+], monolayer), CM (αMEM [serum+], spheroid), CM (CRM [serum+], spheroid), and human ADSC. Untreated mice (no-treatment group) served as negative controls. The human ADSC group received a single injection of human ADSC suspension (200 μl, 2 × 106 cells) into the gracilis muscle in the medial thigh on day 0. The other groups received a daily injection of 40 μl of the assigned medium for 7 days. The site of injection for all types of media and human ADSCs was the same (gracilis muscle). A previous study intramuscularly injected recombinant angiopoietin-1, which resulted in recruitment of bone marrow–derived progenitor cells in the muscle.27 Thus, we adopted intramuscular CM injection for progenitor cell mobilization.
Immunohistochemistry. Ischemic limb muscles were embedded in optimal cutting temperature compound (O.C.T. compound, Tissue-Tek 4583, Sakura Finetek USA, Torrance, CA), frozen, and cut into 10-μm-thick sections at −22 °C. For the quantification of apoptotic activity, samples were collected on days 7 and 28. The sectioned samples were stained with anti-CASP3 antibodies (Abcam, Cambridge, UK) and DAPI (Vector Laboratories, Burlingame, CA). For the quantification of microvessels in ischemic regions, sections were subjected to immunofluorescent staining with anti-CD31 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-SM α-actin antibodies (Abcam). Fluorescein isothiocyanate–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used to visualize the signals. The sections were counterstained with DAPI and examined using a fluorescence microscope (Nikon TE2000, Tokyo, Japan). BrdU antibodies were used to detect actively forming microvessels. For the evaluation of human ADSC apoptosis after transplantation, slides were stained with anti-CASP3 antibodies, anti-CD177 antibodies (Abcam), and DAPI. Total number of vessels in the hindlimb was investigated with CD31 and anti-SM α-actin antibodies from thigh muscle region. We observed the angiogenesis in the thigh muscle because mice in some groups, including the no-treatment group, lost their hindlimb at 28 days after treatment. Therefore, we were not able to compare the angiogenesis or arteriogenesis results from the hindlimbs in each group.
Histological examination. Samples were collected from thigh muscles of the mice because mice in some groups, such as in the no-treatment group, lost their feet and calves at 28 days after treatment. Ischemic limb muscle samples were fixed in formaldehyde, dehydrated with a graded ethanol series, and embedded in paraffin. Specimens were sliced into 4-μm-thick sections and stained with Masson's trichrome staining method to assess tissue fibrosis in the ischemic regions. All samples (n = 5 per group) were completely sectioned, and six slides were selected from the beginning, middle, and end part of each sample for fibrosis quantification. The fibrotic area quantification was performed by separating for the blue color with image analysis software (e.g., Image Pro Plus, Photoshop).
Flow cytometry. To evaluate endothelial progenitor cell (EPC) mobilization, we used flow cytometry to determine the portion of CD34+/PTPRC− cells in the bone marrow and the peripheral blood collected 7 days after surgery (n = 8). Peripheral blood was obtained by heart puncture from mice under deep anesthesia using xylazine (10 mg/kg) and ketamine (100 mg/kg) and was collected in heparin-treated tubes. Mice were also sacrificed to collect bone marrow cells. Immediately following isolation, mononuclear cells from bone marrow and peripheral blood were processed for flow cytometry analysis. The expressions of surface markers CD34 (Santa Cruz Biotechnology) and PTPRC (Abcam) were analyzed with a fluorescence-activated cell sorter (BD Biosciences, San Jose, CA).
Western blot analysis. The cell and mice tissue samples were lysed using a Dounce homogenizer (50 strokes, 4 °C) in ice-cold lysis buffer (15 mmol/l Tris–HCl (pH 8.0), 0.25 mol/l sucrose, 15 mmol/l NaCl, 1.5 mmol/l MgCl2, 2.5 mmol/l ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol tetraacetic acid, 1 mmol/l dithiothreitol, 2 mmol/l sodium pyrophosphate, 1 μg/ml pepstatin A, 2.5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.5 mmol/l phenylmethylsulfonyl fluoride, 0.125 mmol/l Na3VO4, 25 mmol/l NaF, and 10 μmol/l lactacystin). Protein concentration was determined using a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Equal protein concentrations from each sample were mixed with sample buffer, loaded, and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% (v/v) resolving gel. Proteins were separated by SDS-PAGE and were transferred to an Immobilon-P membrane (Millipore, Billerica, MA) and then probed with antibody against human nucleus antigen (Chemicon, Temecula, CA), human-specific caspase-3, VEGF, FGF2, and HGF (all from Abcam) for 1 hour at room temperature. The membranes were incubated with horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology) for 1 hour at room temperature. The blots were developed using an enhanced chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ). Luminescence was recorded on X-ray film (Fuji super RX, Fujifilm Medical Systems, Tokyo, Japan), and bands were imaged and quantified with an Imaging Densitometer (Bio-Rad, Hercules, CA).
Transplantation of PKH26-labeled BMMNCs. To evaluate the effect of various CM treatments on cell homing, BMMNCs from mice obtained from the Institute of Cancer Research were labeled with PKH26 (Sigma), and the labeled cells (1 × 106 cells in 100 μl of phosphate-buffered saline per animal) were injected into mice by intracardiac injection 7 days after the various CM treatments, following the induction of hindlimb ischemia. The animals were sacrificed on the next day, and the PKH26-positive cells in hindlimb muscle were directly counted to quantify the homing of bone marrow cells to the ischemic area.
Laser Doppler imaging analysis. Laser Doppler imaging analysis was performed with a laser Doppler perfusion imager (Moor Instruments, Devon, UK) for serial noninvasive physiological evaluation of neovascularization. Mice were monitored by serial scanning of surface blood flow in hindlimbs on days 0, 3, 7, 14, 21, and 28 after treatment. Digital color-coded images were analyzed to quantify blood flow in the region from the knee joint to the toe, and the mean values of perfusion were subsequently calculated (n = 10 per group).
Statistical analysis. Quantitative data were expressed as mean ± SD. For the statistical analysis, the one-way analysis of variance test with Bonferroni correction was performed using OriginPro 8 SR4 software (version 8.0951; OriginLab, Northampton, MA). For the hindlimb salvage, nonparametric Fisher's exact test was performed using SAS/STAT software (SAS Institute, Cary, NC). A P value less than 0.05 was considered to be statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Apoptosis of hADSCs following injection into the ischemic hindlimb or normal hindlimb. Figure S2. hADSCs survival and activity in the ischemic tissues 28 days after transplantation. Figure S3. Treatment of hADSCs with CM from CRM-based 3D spheroid culture attenuated apoptosis and fibrosis in the ischemic region. Figure S4. Estimation of SM α-actin- and CD31-positive microvessels in the ischemic tissues after 28 days by quantifying the mRNA expression of SM α-actin and CD31, respectively. Figure S5. Morphological changes of the ischemic right limb were monitored for 28 days after various treatments as denoted. Figure S6. Laser Doppler images of the ischemic limb treated with various therapies. Figure S7. mRNA expression of HIF1A in human ADSCs increased under hypoxic culture condition as well as in the 3D spheroid culture. Figure S8. 3D spheroid culture supports high-density growth of hADSCs with increased angiopoietin 1 (ANGP 1) production. Table S1. Composition of clinically relevant medium (CRM). Table S2. Primers used for RT–PCR.
Acknowledgments
This study was supported by a grant (2010-0020352) from the National Research Foundation of Korea; a grant (sc3220) from the Stem cell Research Center of the 21st Century Frontier Program, Ministry of Education, Science, and Technology; and a grant (A050082) from the Korea Health 21 R&D Project, Ministry of Health, Welfare, and Family Affairs, Republic of Korea.
Supplementary Material
Apoptosis of hADSCs following injection into the ischemic hindlimb or normal hindlimb.
hADSCs survival and activity in the ischemic tissues 28 days after transplantation.
Treatment of hADSCs with CM from CRM-based 3D spheroid culture attenuated apoptosis and fibrosis in the ischemic region.
Estimation of SM α-actin- and CD31-positive microvessels in the ischemic tissues after 28 days by quantifying the mRNA expression of SM α-actin and CD31, respectively.
Morphological changes of the ischemic right limb were monitored for 28 days after various treatments as denoted.
Laser Doppler images of the ischemic limb treated with various therapies.
mRNA expression of HIF1A in human ADSCs increased under hypoxic culture condition as well as in the 3D spheroid culture.
3D spheroid culture supports high-density growth of hADSCs with increased angiopoietin 1 (ANGP 1) production.
Composition of clinically relevant medium (CRM).
Primers used for RT–PCR.
References
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up of the BOOST trial. Circulation. 2008;118:S764–S765. [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Menasché P. Stem cells for clinical use in cardiovascular medicine: current limitations and future perspectives. Thromb Haemost. 2005;94:697–701. doi: 10.1160/TH05-03-0218. [DOI] [PubMed] [Google Scholar]
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109:84–92. doi: 10.1002/cncr.22375. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Leisenring W, Schwartz JL, Deeg HJ. Second malignant neoplasms following hematopoietic stem cell transplantation. Int J Hematol. 2004;79:229–234. doi: 10.1532/ijh97.03178. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- Barcelos LS, Duplaa C, Kränkel N, Graiani G, Invernici G, Katare R, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2011;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- Yuen WW, Du NR, Chan CH, Silva EA, Mooney DJ. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci U S A. 2010;107:17933–17938. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Horii M, Yokoyama A, Shoji T, Mifune Y, Kawamoto A, et al. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest. 2011;91:539–552. doi: 10.1038/labinvest.2010.191. [DOI] [PubMed] [Google Scholar]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Villaschi S, Nicosia RF. Angiogenic role of endogenous basic fibroblast growth factor released by rat aorta after injury. Am J Pathol. 1993;143:181–190. [PMC free article] [PubMed] [Google Scholar]
- Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;10:590–594. doi: 10.1016/j.autrev.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Marx J. Cancer research. Mutant stem cells may seed cancer. Science. 2003;301:1308–1310. doi: 10.1126/science.301.5638.1308. [DOI] [PubMed] [Google Scholar]
- Stagg J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4:119–124. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
- Sell S. On the stem cell origin of cancer. Am J Pathol. 2010;176:2584–2494. doi: 10.2353/ajpath.2010.091064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SC, Rae C. iNOS initiates and sustains metabolic arrest in hypoxic lung adenocarcinoma cells: mechanism of cell survival in solid tumor core. Am J Physiol Cell Physiol. 2005;289:C918–C933. doi: 10.1152/ajpcell.00476.2004. [DOI] [PubMed] [Google Scholar]
- Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Yamada K, Otsuki H, Yuguchi T, Kohmura E, Hayakawa T. Brief exposure to hypoxia induces bFGF mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress. Neurosci Res. 1995;23:289–296. doi: 10.1016/0168-0102(95)00954-x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee CS, Hur J, Cho HJ, Jun SI, Kim TY, et al. Priming with angiopoietin-1 augments the vasculogenic potential of the peripheral blood stem cells mobilized with granulocyte colony-stimulating factor through a novel Tie2/Ets-1 pathway. Circulation. 2009;120:2240–2250. doi: 10.1161/CIRCULATIONAHA.109.856815. [DOI] [PubMed] [Google Scholar]
- Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D'Amario D, et al. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890–899. doi: 10.1093/eurheartj/ehp078. [DOI] [PubMed] [Google Scholar]
- Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189–197. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Minatoguchi S, Kosai K, Yuge K, Takahashi T, Arai M, et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J Card Fail. 2007;13:874–883. doi: 10.1016/j.cardfail.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131–H2139. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apoptosis of hADSCs following injection into the ischemic hindlimb or normal hindlimb.
hADSCs survival and activity in the ischemic tissues 28 days after transplantation.
Treatment of hADSCs with CM from CRM-based 3D spheroid culture attenuated apoptosis and fibrosis in the ischemic region.
Estimation of SM α-actin- and CD31-positive microvessels in the ischemic tissues after 28 days by quantifying the mRNA expression of SM α-actin and CD31, respectively.
Morphological changes of the ischemic right limb were monitored for 28 days after various treatments as denoted.
Laser Doppler images of the ischemic limb treated with various therapies.
mRNA expression of HIF1A in human ADSCs increased under hypoxic culture condition as well as in the 3D spheroid culture.
3D spheroid culture supports high-density growth of hADSCs with increased angiopoietin 1 (ANGP 1) production.
Composition of clinically relevant medium (CRM).
Primers used for RT–PCR.