Abstract
Fragile skin, susceptible to decubitus ulcers and incidental trauma, is a problem particularly for the elderly and for those with spinal cord injury. Here, we present a simple approach to strengthen the skin by the topical delivery of keratinocyte growth factor-1 (KGF-1) DNA. In initial feasibility studies with the novel minimalized, antibiotic-free DNA expression vector, NTC8385-VA1, the reporter genes luciferase and enhanced green fluorescent protein were delivered. Transfection was documented when luciferase expression significantly increased after transfection. Microscopic imaging of enhanced green fluorescent protein–transfected skin showed green fluorescence in hair follicles, hair shafts, and dermal and superficial epithelial cells. With KGF-1 transfection, KGF-1 mRNA level and protein production were documented with quantitative reverse transcriptase–polymerase chain reaction and immunohistochemistry, respectively. Epithelial thickness of the transfected skin in the KGF group was significantly increased compared with the control vector group (26 ± 2 versus 16 ± 4 µm) at 48 hours (P = 0.045). Dermal thickness tended to be increased in the KGF group (255 ± 36 versus 162 ± 16 µm) at 120 hours (P = 0.057). Biomechanical assessment showed that the KGF-1–treated skin was significantly stronger than control vector–transfected skin. These findings indicate that topically delivered KGF-1 DNA plasmid can increase epithelial thickness and strength, demonstrating the potential of this approach to restore compromised skin.
Introduction
Fragile skin, susceptible to decubitus ulcers and incidental trauma, is a problem particularly for the elderly and for those with spinal cord injury.1,2,3,4 A simple treatment approach to strengthen the skin and protect individuals at risk for skin breakdown with decubitus ulcers and other conditions would be very useful.
We know from our prior work, and that of others, that keratinocyte growth factor-1 (KGF-1) has the capacity to promote keratinocyte proliferation and mobilization,5,6 promote angiogenesis,7,8 and improve impaired dermal wound healing.9 It is massively expressed during the early stages of wound healing, attesting to its important role.10 These features make it an appealing candidate for the task of restoring atrophic skin.
Topical application of peptide growth factors has not been effective because of poor absorption and because the peptides are rapidly destroyed by peptidases in the dermis. Delivery of DNA to constantly express the peptides is an attractive solution to this problem. A needle-free topical transfection would be particularly suitable for this treatment. The hindrance for topical gene delivery is its inability to penetrate across the entire epidermis, including the superficial hydrophobic stratum corneum, the outermost layer composed of dead epidermal cells and lipids.11 Topical transfection of the dermis has been shown to transfect hair follicles and to have potential for hair restoration.12 Our goal was to strengthen the skin by transfecting epidermal and dermal cells, as well as hair follicles. Techniques reported thus far to extend the transfection beyond the hair follicles have required chemical alteration of the skin barrier13,14 or transportation of supportive agents such as cationic polymers15,16 and lipoplexes.17,18,19,20 All these options raise safety concerns, especially for treatment of fragile skin.
The alternative we are exploring is the topical application of DNA plasmid expression vectors. The NTC plasmids with which we have been working are not only minimalized and have high expression levels but are also antibiotic free and easy to manufacture in a good manufacturing practices facility, making them safe and clinically feasible. Furthermore, in contrast to viral vectors, plasmids have the capacity to be repeatedly administered due to the low immune response elicited.21 Currently manufactured plasmids have limitations that preclude initiating gene expression in vivo after topical application. However, NTC8385-VA1, a plasmid carrier with both human T-lymphotropic virus type I (HTLV-I) R region and adenovirus serotype 5 virus-associated (VA) RNA interference (RNAI; termed VA1) expression enhancers, is a safer and more potent alternative to improve transgene expression.22
To improve transfection efficiency of topically delivered DNA plasmid, we explored microdermabrasion, a minimally invasive, clinically practiced cosmetic procedure used for skin care. Microdermabrasion has substantial patient satisfaction.23 Unlike dermabrasion, which completely removes the epidermis and penetrates to the level of the papillary or reticular dermis, microdermabrasion removes only the uppermost layer of the epidermis, accelerating the natural process of exfoliation.24 Microdermabrasion has been used to increase skin permeability for drug delivery25,26,27,28 and has the potential to promote topical DNA plasmid delivery by overcoming the obstacle of protective skin without damaging the structural integrity or skin components.
We propose that transdermal gene delivery can be accomplished by using the NTC8385-VA1 plasmid vector enhanced with microdermabrasion. Previous methods for topical transfection have used relatively harsh agents that could potentially damage skin. Skin that is structurally fragile, such as atrophic skin in paraplegic patients or healed chronic wounds from diabetic patients, would benefit from less aggressive methods.
Results
NTC8385-VA1-Luc plasmid topical application alone was insufficient in transfecting reporter gene luciferase
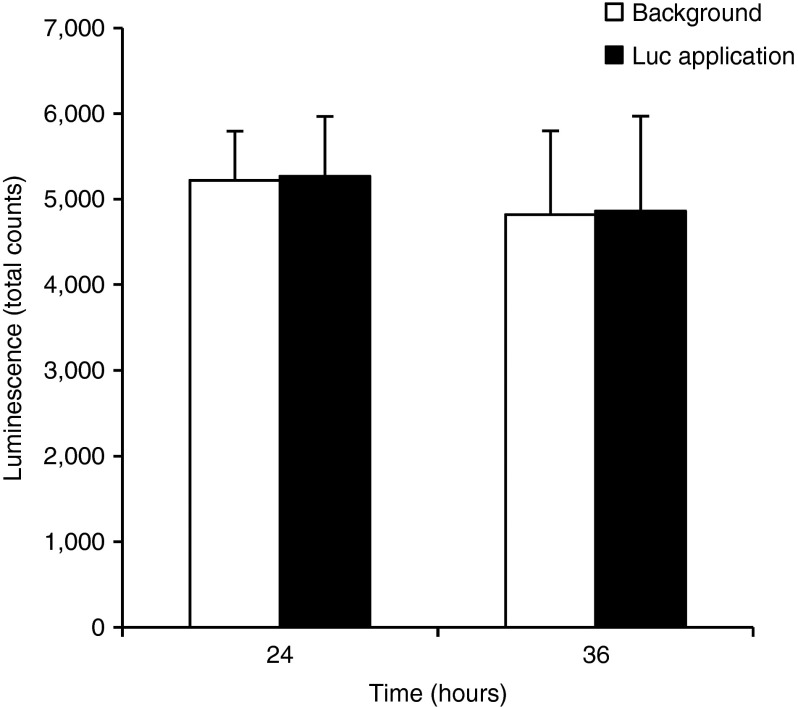
The initial experiment was performed to explore whether topical application of NTC8385-VA1-Luc plasmid alone would be sufficient to transfect mouse skin with the reporter gene luciferase. Four SV129 mice were shaved and depilated. NTC8385-VA1-Luc (50 μg) was directly applied to a 15 × 15 mm caudal area and then covered with DuoDERM (20 × 20 mm) to keep the plasmid localized to the region of interest. The cephalic area was covered with DuoDERM (20 × 20 mm) as background control. IVIS Xenogen Imaging system was used to acquire luminescence at 24 and 36 hours after the plasmid application. There was no evidence of luciferase expression in the area where NTC8385-VA1-Luc plasmid was applied as compared with the background (Figure 1).
Figure 1.
Topical delivery of the luciferase plasmid without microdermabrasion. Time course of luciferase expression in area wherein NTC8385-VA1-Luc plasmid was topically applied versus untransfected background is shown. Luminescent images were acquired 24 and 36 hours after plasmid application. The luciferase expression is represented as total counts (mean ± SEM; n = 4). Luc, luciferase; VA1, adenovirus serotype 5 virus-associated (VA) RNA interference.
Multiple topical applications of NTC8385-VA1-Luc plasmid following microdermabrasion successfully transfected mouse skin with reporter gene luciferase.
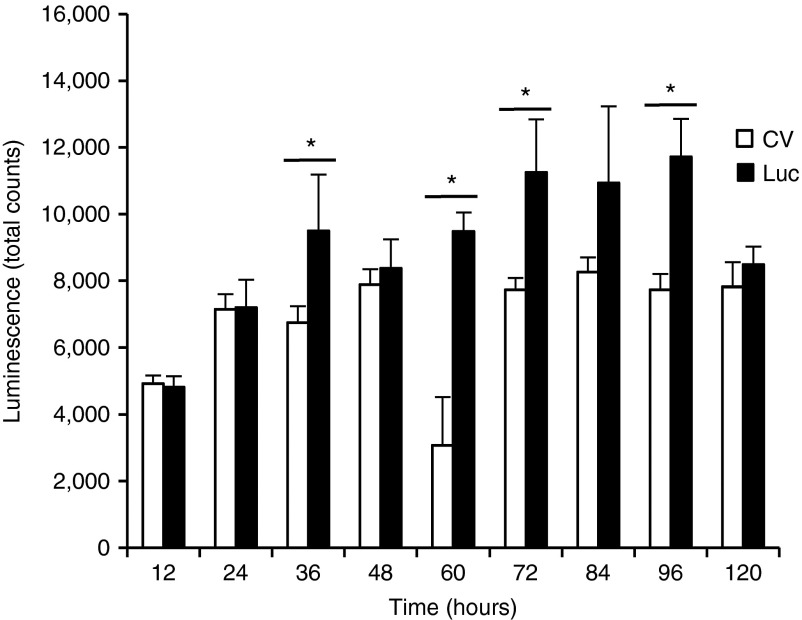
Because the initial experiment showed that topical plasmid application alone was insufficient for transfecting mouse skin, another experiment was conducted to determine whether microdermabrasion could enhance transfection of the topically applied NTC8385-VA1-Luc plasmid. NTC8385-VA1-KGF1 plasmid was used as the control vector. Twenty SV129 mice were shaved, depilated, and microdermabraded following the standardized protocol and then randomly and evenly assigned to the luciferase and control vector groups. Based on the grouping, each mouse received multiple applications of 50 μg NTC8385-VA1-Luc or control vector topically applied on the microdermabraded skin and covered with DuoDERM every 12 hours for a 4-day period (Supplementary Figure S1). Luminescent images were acquired at 12, 24, 36, 48, 60, 72, 84, 96, and 120 hours after the initial plasmid application. The luciferase group had significantly greater luminescence at 36, 60, 72, and 96 hours compared with the control vector group (P < 0.05; Figure 2).
Figure 2.
Time course of luciferase expression for NTC8385-VA1-Luc and NTC8385-VA1 control vector for the multiple-application group. Luminescent images were acquired 12, 24, 36, 48, 60, 72, 84, 96, and 120 hours after the initial application. The luciferase expression is represented as total counts (mean ± SEM; n = 10; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis; *P < 0.05). CV, control vector; Luc, luciferase; VA1, adenovirus serotype 5 virus-associated (VA) RNA interference.
Multiple topical applications of NTC8385-VA1-Luc plasmid were more effective than single topical application for achieving transfection
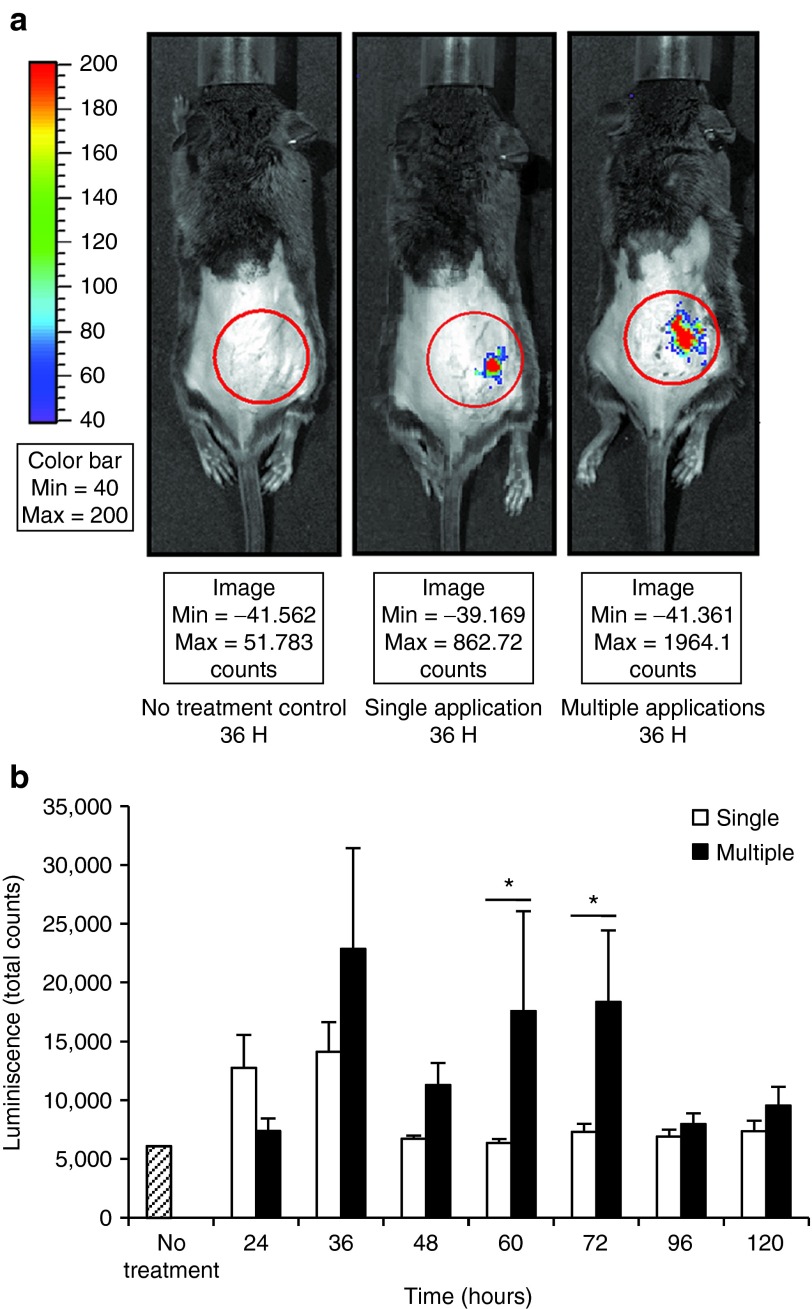
This experiment was designed to compare the transfection efficiency and expression duration of multiple topical applications and those of a single topical application. Eight SV129 mice were shaved, depilated, and microdermabraded following the standardized protocol and then randomly and evenly assigned to the single-application and multiple-application groups. For single application, 50 μg NTC8385-VA1-Luc was topically applied immediately after microdermabrasion and covered with DuoDERM. For multiple applications, 50 μg NTC8385-VA1-Luc was topically applied on the microdermabraded skin and covered with DuoDERM every 12 hours for a 4-day period (Supplementary Figure S1). Vehicle gel was applied every 12 hours in the single-application group. Luminescent images were acquired at 24, 36, 48, 60, 72, 96, and 120 hours. At 36 hours, there was obvious luminescence in both the single-application and multiple-application groups, but none in the untreated animals (Figure 3a). In the single-application group, there was increased luminescence at 24 and 36 hours. In the multiple-application group, increased luminescence persisted through 72 hours (Figure 3b).
Figure 3.
Time course of luciferase expression after NTC8385-VA1-Luc single application and multiple applications. (a) Example of luminescent images for no-treatment control, single-application, and multiple-application groups at 36 hours. (b) Comparison of luminescence between the single-application group and multiple-application group. Luminescent images were acquired 24, 36, 48, 60, 72, 96, and 120 hours after the initial application. The luciferase expression is represented as total counts (mean ± SEM, n = 4; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis; *P < 0.05). Luc, luciferase; VA1, adenovirus serotype 5 virus-associated (VA) RNA interference.
Transfection imaging with microscopy
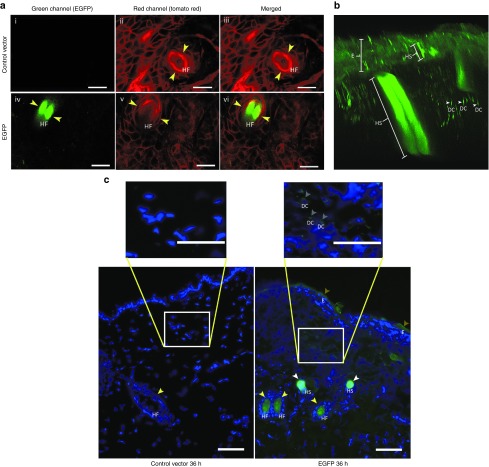
Two imaging systems were used to identify the cell types transfected with enhanced green fluorescent protein (EGFP) by topical application of the NTC8385-VA1 DNA plasmid. Multiphoton imaging of intact skin and fluorescent imaging of frozen sections were carried out. When intact tissue from transgenic ROSA mice expressing membrane-tagged tdTomato in all cells was imaged with a multiphoton laser-scanning microscope, the red fluorescent background was visible in all images as expected (Figure 4a ii,iii,v,vi). However, only EGFP-transfected tissue exhibited green fluorescence. The green fluorescence appeared in hair follicles (Figure 4a iv,vi), as well as in hair shafts, epidermis, and dermal cells (Figure 4b).
Figure 4.
Visual confirmation of plasmid transfection using microscopy. Images were taken at 36 hours after microdermabrasion with multiple topical applications. (a) Multiphoton images showing fluorescent signals acquired from the green (EGFP-transfected cells) channel, red (tomato red showing all cell membranes) channel, and the combination. (i–iii) Topical application of the control vector. (iv–vi) Topical application of the EGFP plasmid. Yellow arrows indicate the hair follicles. Bars = 100 μm. (b) An image reconstructed from multiphoton Z-stacks showing the hair shafts with EGFP green fluorescence penetrating the dermis and epidermis. (c) Fluorescent images of frozen sections of the skin transfected with either EGFP or control vector. Cell nuclei are stained blue with DAPI. EGFP-transfected cells are stained green. Green staining is identified by arrows seen in hair follicles, hair shafts, the superficial epidermis, and more weakly, in the dermis. DAPI, 4',6-diamidino-2-phenylindole ; DC, dermal cell; E, epidermis; EGFP, enhanced green fluorescent protein; HF, hair follicle; HS, hair shaft. Bars = 100 μm.
In a second approach, fluorescent microscopy was used. In the control vector specimens, 4',6-diamidino-2-phenylindole–stained cell nuclei were seen with blue fluorescence, and as expected, there was no green staining (Figure 4c). As with the multiphoton imaging, fluorescent microscopy showed EGFP transfection resulting in green fluorescence within the superficial epidermis, hair follicles, hair shafts, and weakly in fibroblasts in the dermis (Figure 4c).
Multiple topical applications of NTC8385-VA1-KGF1 plasmid following microdermabrasion resulted in successful transfection with KGF-1
Because the reporter gene luciferase was transfected more efficiently by multiple topical applications of NTC8385-VA1-Luc plasmid with microdermabrasion, these experiments were carried out to explore whether functional gene KGF-1 could be transfected into the mouse skin using a similar approach. First, the feasibility of transfection was explored at the mRNA and peptide level. Fifty SV129 mice were shaved, depilated, and microdermabraded following the standardized protocol and then randomly and evenly assigned to the NTC8385-VA1-KGF1 and NTC8385-VA1-Luc (used as the control vector in this round) multiple topical applications groups. Both the control vector treatment and KGF-1 plasmid treatment followed the same procedure (50 µg every 12 hours over a 4-day period) on each mouse. Treatment locations were covered with DuoDERM (Supplementary Figure S1).
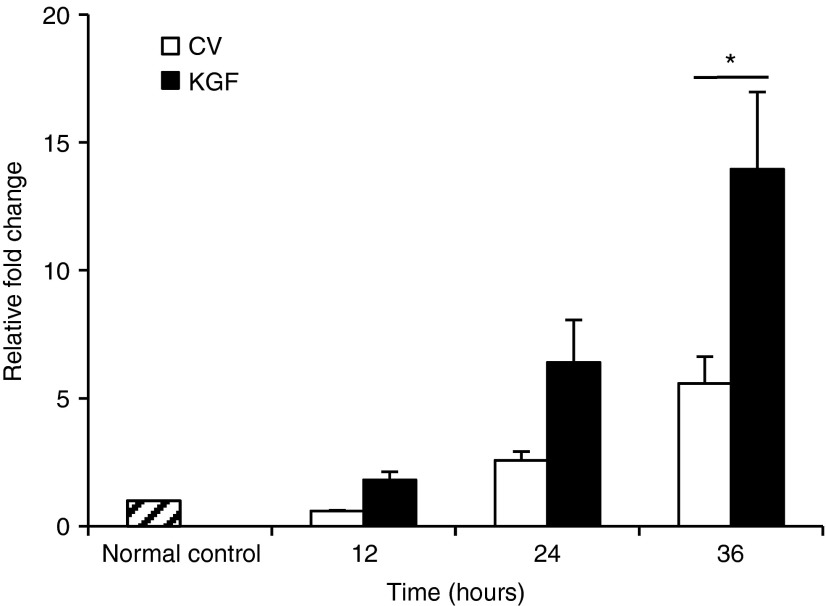
KGF-1 mRNA content assessed by quantitative reverse transcriptase–polymerase chain reaction. Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) was executed to quantitatively analyze KGF-1 transcription in the transfected mouse skin. Skin samples were harvested from five mice from each group, which were sacrificed at 12, 24, and 36 hours. To perform qRT-PCR, total RNA was extracted from the plasmid-treated (caudal dorsum) and normal control skins (cephalic dorsum) of each mouse with Trizol and then treated with DNase I according to the manufacturer's protocol. Compared with the control vector group, NTC8385-VA1-KGF1 plasmid–transfected areas had significantly increased KGF-1 mRNA expression 36 hours after the initial application (Figure 5). As expected, there were small increases in KGF-1 mRNA in the group treated with the control vector due to the inflammatory response elicited by the microdermabrasion procedure and the DNA plasmid application.
Figure 5.
Evaluation of KGF-1 gene transcription by quantitative reverse transcriptase–polymerase chain reaction. The normal skin on the cephalic dorsum was used as normal control (hatched bar). The mRNA content is represented as relative fold change versus normal control. KGF-1 mRNA content was measured at 12, 24, and 36 hours after initial application (mean ± SEM, n = 5; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis, *P < 0.05). CV, control vector; KGF-1, keratinocyte growth factor-1.
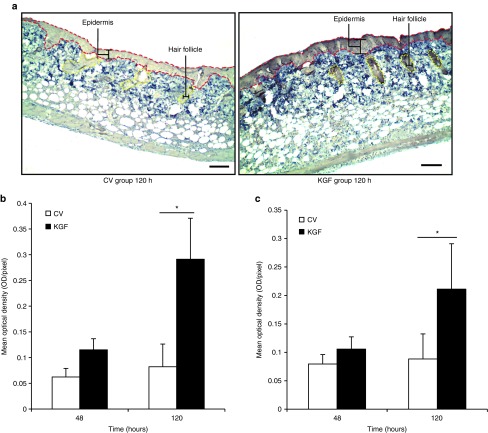
KGF-1 peptide content assessed by immunohistochemistry. Immunohistochemistry was performed to evaluate KGF-1 translation in the transfected mouse skin. Skin samples in the transfected area were harvested from five mice from each group sacrificed at 48 and 120 hours. The samples were embedded in paraffin blocks. The KGF-1 content was quantified as mean optical density (average optical density per unit area) by Image-Pro (Media Cybernetics, Silver Spring, MD)Plus analysis. The unit for optical density was OD for the region of interest. Prominent KGF-1 expression was localized to the epidermis and hair follicles (Figure 6a). Both the epidermis and hair follicles showed evidence of higher KGF-1 peptide levels in the KGF-1 plasmid–treated group compared with the control vector group (Figure 6b,c). If KGF-1 was being expressed in the dermis, it was not concentrated enough to produce an image with this method of immunohistochemistry.
Figure 6.
Evaluation of KGF-1 gene expression by immunohistochemical staining. (a) Immunohistochemical images comparing KGF-1 protein between the KGF group and control vector group mice at 120 hours. The epidermis is outlined in red and hair follicles in yellow. Bars = 100 μm. (b) Comparison of KGF-1 staining optical density in the epidermis between the KGF and control vector groups of mice at 48 and 120 hours (mean ± SEM, n = 5; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis, *P < 0.05). (c) Comparison of KGF-1 staining optical density in hair follicles between the KGF and control vector groups of mice at 48 and 120 hours (mean ± SEM, n = 5; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis, *P < 0.05). CV, control vector; KGF-1, keratinocyte growth factor-1.
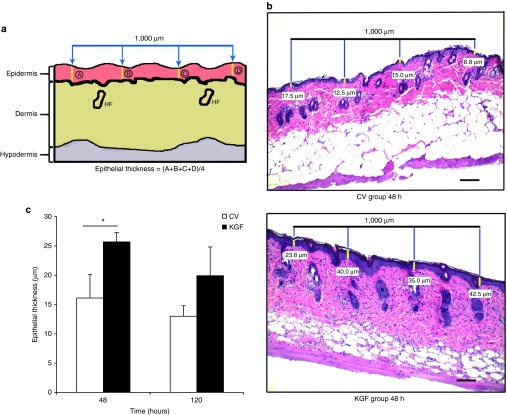
Epithelial and dermal thickness assessment in KGF-1 and control vector mice. To assess epithelial thickness, hematoxylin and eosin staining was performed on 5-μm-thick sections prepared from the previously embedded paraffin blocks of the transfected skin. Four equidistant measurements in the epidermis were taken in the transfected location spanning a distance of 1,000 μm. These four measurements were averaged to obtain the epithelial thickness values (Figure 7a,b). Because the hair follicles can overlap with the epidermis, care was taken to measure only the thickness of the epidermis and not the depths of the hair follicles. Assessment by two-way analysis of variance and Holm-Sidak post hoc analysis demonstrated that the KGF group had a thicker epidermis than the control vector group 48 hours after the initial plasmid application (26 ± 2 versus 16 ± 4 µm; P = 0.045; Figure 7b,c). Dermal thickness measurement was quantified through a similar method (Supplementary Figure S2a,b). The analysis of variance and post hoc analysis demonstrated a tendency for increased dermal thickness in the KGF-1 plasmid–applied group (255 ± 36 µm) when compared with the control vector group mice (162 ± 16 µm) at 120 hours (P = 0.057; Supplementary Figure S2b,c).
Figure 7.
Assessment of epithelial thickness with hematoxylin-and-eosin staining procedure. (a) Schematic diagram showing the method for epithelial thickness measurement. A 1,000-μm line was placed at the center of each microdermabrasion location. Four equidistant measurements were taken in the epidermis, and the mean of these values was computed to give epithelial thickness value. (b) Hematoxylin-and-eosin images comparing epithelial thickness in typical KGF-1 and control vector animals at 48 hours. Yellow hashes measure the epithelial thickness, with the values underneath corresponding to the thickness. Bars = 100 μm. (c) Comparison of epithelial thickness between the KGF-1 and control vector groups at 48 and 120 hours (mean ± SEM, n = 5; P < 0.05 by two-way analysis of variance; Holm-Sidak post hoc analysis, *P < 0.05). CV, control vector; HF, hair follicle; KGF-1, keratinocyte growth factor-1.
KGF-1 plasmid–treated microdermabraded skin resulted in better biomechanical properties
Twenty SV129 mice were randomly and evenly assigned to the NTC8385-VA1-KGF1 and NTC8382-VA1 (used as the control vector in this round) multiple topical applications groups. Mice were subsequently shaved, depilated, and microdermabraded in the caudal area. Both the control vector treatment and KGF-1 plasmid treatment followed the same procedure (50 μg every 12 hours over a 4-day period) on each mouse. Treatment locations were covered with DuoDERM (Supplementary Figure S1). Seven days after the initial application, the transfected areas were harvested and tested with the tensiometry procedure (Supplementary Figure S3a,b). Figure 8a shows the tension–elongation curve. Total work was calculated from the integral of the curve. All biomechanical properties (tension at break, work at break, and elongation at break) were found to be significantly greater in the KGF-1–treated group (P < 0.05; Figure 8b–d).
Figure 8.
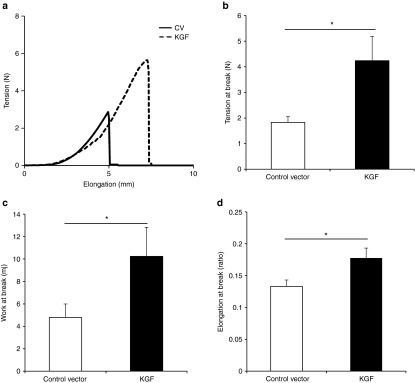
Biomechanical assessment of KGF-1–treated skin. (a) Sample representation of tension–elongation curve comparing KGF-1– to control vector–treated tissue 7 days after the initial treatment. (b) Comparison of the average tension at the breaking point of the KGF-1 and control groups (mean ± SEM, n = 10; *P < 0.05, Mann–Whitney analysis). (c) Average work at the breaking point of both groups (calculated from the integral of the curve; *P < 0.05, Mann–Whitney analysis). (d) Average elongation at the breaking point of both groups (*P < 0.05, t-test). KGF-1, keratinocyte growth factor-1.
Discussion
Skin fragility is an unfortunate consequence of aging.3 It is also seen after dermal denervation, which occurs in cerebrovascular accidents or spinal cord injury.4 When fragile skin is present, minor trauma results in laceration and ulceration; extensive dermal breakdown can result. Ideally, a simple and effective remedy to promote proliferation of epidermal and dermal components would reconstitute atrophic skin as a healthy, durable protective barrier to trauma. Here, we present a simple approach to strengthen the skin by the topical delivery of a DNA plasmid expressing KGF-1. The treatment approach we developed in the current experiments significantly improved biodynamic parameters of skin strength in the mouse model. Unlike previous studies using topical transfection of the dermis, we found that the transfection using this approach extended beyond the hair follicles to include dermal and epidermal cells, resulting in strengthening of the skin.12,29
Skin is an ideal organ for the development of therapeutic and prophylactic gene delivery due to its accessibility for treatment and monitoring.30 DNA delivery to dermis has been extensively studied at the cellular and molecular levels, providing a better understanding of DNA transport, absorption, and gene expression.31,32 A multiplicity of in vivo approaches, including gene gun transfer,33,34 microseeding,35 magnetofection,36 ultrasonography,37 jet injection,38 direct injection,39 and electroporation9,40 have been developed to achieve transdermal delivery of target genes. However, the common themes among these methods include significant invasiveness and pain, which is an insurmountable obstacle for their clinical use, especially for fragile skins. Furthermore, they are not suited to the treatment of relatively large areas, as is required for this application.
Topical cutaneous gene delivery would be expected to have high patient acceptance because it is simple and noninvasive. The safety of this approach is substantially enhanced through the use of a nonviral plasmid vector. However, the low transfection efficiency of many common DNA expression plasmids is a severe obstacle for implementation of this method. In this study, an improved plasmid with high transfection efficiency was used. NTC8385-VA1 is a potent, minimalized, antibiotic-free plasmid vector with VA1 and HTLV-I R expression enhancers.22 But even with this plasmid, we met with initial failure. Topical application of the NTC8385-VA1-Luc plasmid to shaved and depilated mouse skin failed to elicit a luciferase signal. This experience demonstrated that delivering this plasmid alone was not sufficient to achieve gene transfection in mouse skin.
The stratum corneum represents the principal barrier for the penetration of macromolecules. Microdermabrasion, a noninvasive cosmetic technique, has been used to increase skin permeability for drug delivery (i.e., insulin,41 5-aminolevulinic acid,42 and vitamin C)43 by the calibrated removal of the stratum corneum. Based on this application, the subsequent experiment was conducted to determine the effect of microdermabrasion on topical cutaneous gene delivery. With microdermabrasion, obvious luciferase expression was observed in both the single-application and multiple-application groups. Greater luminescence levels were obtained with multiple applications when compared with those after single application. This result demonstrated that with microdermabrasion, NTC8385-VA1 could efficiently transfect the luciferase gene into mouse skin.
Next, microscopic imaging was used to determine the cell types that were transfected using this procedure. Transfection was performed with an EGFP-loaded DNA plasmid. First, multiphoton microscopy of fresh tissue from tomato red transgenic mice was performed. Green fluorescent signals were seen in hair follicles, hair shafts, and dermal and epidermal cells. To get more detailed imaging, a fluorescent microscope was used to image frozen sections of EGFP-transfected tissue nuclei stained with 4',6-diamidino-2-phenylindole. Again, strong green fluorescence appeared in hair follicles and hair shafts. Others have noted that the GFP fluorescence initially seen in hair follicles later migrated into growing keratinized hair shafts.29 Transfection was also evident in the superficial epidermis and more weakly in the dermis in cells that appeared to be fibroblasts. Hair follicles may enhance topically applied DNA uptake and expression by retaining the plasmid DNA and functioning as a reservoir.12,17
Having demonstrated that topical application of the NTC8385-VA1 plasmid, combined with microdermabrasion, could achieve successful transfection, we turned our attention to strategies to strengthen the skin using this delivery system. We chose to work with KGF-1 for a number of reasons. KGF-1, also called FGF-7, is one of the fibroblast growth factor group of proteins synthesized primarily by mesenchymal cells such as fibroblasts and endothelial cells.44 Acting predominantly on epithelial cells and cells involved with vascularization, KGF-1 has the capacity to promote keratinocyte proliferation and mobilization,5,6 stimulate angiogenesis,7,8,45 and strengthen layers of the skin, including increasing collagen deposition; when viewed by electron microscopy, the skin has been shown to have better developed hemidesmosomes and thicker tonofilaments, suggesting an increase in durability.46,47 Importantly, transgenic mice expressing a dominant-negative KGF receptor were characterized by epidermal atrophy and abnormalities in the hair follicles.48 Previous studies in our laboratory concluded that delivery of human KGF-1 DNA by intradermal injection plus electroporation improved wound healing in a diabetic mouse model and a septic rat model.9,40,49
In the current study, we explored the biophysical and biomechanical effects of KGF-1 on mouse skin after topical gene delivery. KGF-1 mRNA and protein levels were significantly increased with multiple plasmid applications. Expressed KGF-1 peptide was primarily localized in the epidermis and hair follicles, wherein EGFP transfection was confirmed using multiphoton and fluorescent microscopy. This was consistent with previous reports stating that epidermal cells and hair follicles would be the targets of this delivery.29,50 Transfection with human KGF-1 significantly improved three biodynamic parameters of skin strength, including tension at break (ultimate tensile strength), elongation at break (ductility), and work at break (toughness). At this point, we cannot determine from these findings whether strengthening of the mouse skin resulted from the epidermal and dermal transfection or the transfection of stem cells in the hair follicles that migrated into the dermis and epidermis.
From these studies, we conclude that the topical delivery of KGF-1 using a minimalized, high-expression DNA plasmid after microdermabrasion is a particularly attractive strategy for translational research, leading to clinical applications. Moreover, the topical delivery approach is not limited to KGF-1. Rather this novel topical approach can provide the future framework for safely and efficiently transfecting a variety of peptide therapeutics into the skin.
Materials and Methods
Mice. SV129 (12 week old, male) and B6.129 (Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (12 week old, male) mice from The Jackson Laboratory (Bar Harbor, ME) were used for all procedures. All procedures involving mice were approved by The Johns Hopkins University Animal Care and Use Committee.
Microdermabrasion procedure. Mice were anesthetized with 3% isoflurane (Baxter Healthcare, Deerfield, IL) using an anesthesia machine (VetEquip, Pleasanton, CA). The dorsum of each mouse was shaved and depilated with Nair cream (Church & Dwight, Princeton, NJ). The caudal 15-mm-diameter microdermabrasion zone was brushed with the yellow tip (coarse) of the DermaSweep-Mini machine (DermaSweep Inc., Rocklin, CA) at pressure of 20 mm Hg. Six perpendicular strokes were performed (three horizontal and three vertical).
Plasmid application. The plasmids NTC8385-VA1-Luc, NTC8385-VA1-KGF1, NTC8385-VA1-EGFP, and NTC8382-VA1 were provided by Nature Technology Corporation (Lincoln, NE). They were diluted in vehicle (1% hydroxypropyl methylcellulose, 5% glycerin, and 94% Tris–ethylenediaminetetraacetic acid buffer), provided by GenArmor (Winston Salem, NC), to a final concentration of 2 μg/μl. The gel was topically applied on the 15-mm-diameter microdermabraded skin with a pipette, gently distributed without rubbing, and then covered with 20 × 20 mm DuoDERM (ConvaTec, Skillman, NJ).
In vivo luciferase imaging. IVIS Xenogen imaging system (Caliper Life Science, Alameda, CA) was used to take whole-body bioluminescent and conventional black-and-white photographs of the mice. Luminescent images were taken 30 minutes following intraperitoneal injection of 100 μl (15 µg/µl) luciferin. Bioluminescent images were overlaid onto the conventional black-and-white images. Luminescence was calculated by the Living Image 2.5 software and expressed as total counts for equal-sized regions of interest at each transfected area.
Imaging of intact skin and frozen tissue sections. For these experiments, EGFP plasmid or control vector was topically applied after microdermabrasion once every 12 hours for 36 hours. Imaging was performed at 36 hours comparing tissues transfected with EGFP with tissues subjected to control vector transfection.
Multiphoton imaging of intact skin. An Olympus FV1000 MPE multiphoton laser-scanning microscope (Olympus, Center Valley, PA) was used to visualize EGFP transfection in three B6.129 (Cg)-Gt (ROSA) transgenic mice that possessed a membrane-tagged tdTomato (mT) cassette and expressed strong red fluorescence in all tissues. The mice were maintained under ketamine (76 mg/kg) and xylazine (5 mg/kg) anesthesia during the imaging session. The transfected skin was scanned at different depths (known as Z-stacks) in the green channel (750 nm excitation wavelength and 495–540 nm emission wavelength) and the red channel (750 nm excitation wavelength and 575–630 nm emission wavelength) separately. Images were processed using Fluoview software (Ver. 4.0; Olympus, Tokyo, Japan).
Fluorescent imaging of frozen sections. A Zeiss Axio Imager fluorescent microscope (Carl Zeiss Meditec, Dublin, CA) was used to visualize detailed EGFP transfection locations in three SV129 mice. Thirty-six hours after initial application, a 5-mm-diameter biopsy specimen of the treated tissue was harvested. Tissue was fixed in Optimal Cutting Temperature medium (Tissue-Tek, Torrance, CA) and then cut into 15-µm slices. Nuclei were then stained with 4',6-diamidino-2-phenylindole blue (Invitrogen, Eugene, OR). The transfected skin was observed in the green channel (450–490 nm excitation wavelength and 515–565 nm emission wavelength) and the blue channel (350–365 nm excitation wavelength and 445–450 nm emission wavelength).
RNA isolation and qRT-PCR assay for KGF-1. Total RNA was extracted from transfected (caudal dorsum) and normal control (cephalic dorsum) skin of each mouse with Trizol (Invitrogen, Frederick, MD) and treated with DNase I (Ambion, Austin, TX) according to the manufacturer's protocol. Total RNA (10 μg) was used for first-strand synthesis with the iScript cDNA Synthesis System (BioRad, Hercules, CA). qRT-PCR was performed using iQ SYBR Green Supermix and the iCycler Real-Time PCR Detection System (BioRad). The fold change in expression of human KGF-1 mRNA (primer sequence: Fwd: 5′-CCGAGCGACACACCAGAAGT-3′; Rev: 5′-AACTGCCACGGTCCTGATTT-3′) relative to hypoxanthine phosphoribosyltransferase 1 (HPRT1) mRNA was calculated based on the threshold cycle (CT) for amplification as 2−(ΔCT), where ΔCT = CT,KGF − CT,HPRT1.
Hematoxylin-and-eosin and immunohistochemical staining. Skin was harvested and fixed with 10% formalin for 24 hours, and 25-μm-thick paraffin sections were prepared. The sections were stained using hematoxylin and eosin. For immunohistochemical staining, high-temperature antigen retrieval was performed by immersing the slides in Trilogy (Cell Marque, Hot Springs, AR). Slides were then incubated for 30 minutes with AB9598 rabbit polyclonal antibody to human KGF-1 (Abcam, Cambridge, MA), followed by 10-minute incubation with a rabbit horseradish peroxidase polymer conjugate (SuperPicture; Invitrogen, Camarillo, CA). The slides were then stained with ImmPACT DAB (Vector Labs, Burlingame, CA) and counterstained with hematoxylin. KGF-1 protein content was determined by the Image-Pro plus 6.0 (Media Cybernetics, Silver Spring, MD). All slides were analyzed by two examiners who were blinded to the identity of each sample.
Biomechanical testing of transfected skin. Tensiometry was carried out on transfected tissues, which were harvested and cut into 35 × 4 mm sections (Supplementary Figure S3a). Immediately after harvesting (<1 minute), sections were placed between the two clamps of the motorized horizontal test stand so that the center of the treated area was directly in the center of the clamps with the tissue being pulled lengthwise (Check Line FGS-100PXH, Cedarhurst, NY). Attached to the clamps on the test stand was the force gauge (Shimpo FGV-10XY) used to measure the tension of the tissue as it was being pulled apart (Supplementary Figure S3b). Data were automatically recorded into Microsoft Excel 2003 (Microsoft, Redmond, WA). All testing was done in a climate-controlled room (70 °F, 60% humidity) to account for the effects that varying climate has on skin conditions. The rate of displacement was held constant at 20 mm/minute. The procedure was terminated once the force gauge displayed that the tension decreased to less than 0.1 N, indicating full tissue breakage.
Statistical analysis. Data were presented as mean ± SEM. Differences in means between groups were analyzed for significance using two-way analysis of variance, followed by Holm-Sidak post hoc analysis when appropriate. Student's t-test and Mann–Whitney test were also used to analyze the biomechanical tensiometry data. A probability value of <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Schematic diagram of the topical application procedure. Figure S2. Assessment of dermal thickness with hematoxylin and eosin staining. Figure S3. Schematic diagram of tensiometry procedure.
Acknowledgments
This work was supported by the Hendrix Burn Fund, Johns Hopkins University (JHU), as well as by a National Institutes of Health Small Business Innovation Research (SBIR) award issued to Nature Technology Corporation/JHU Subcontract from SBIR R44 GM080768-02 phase 2. The following authors reported conflict of interest: A.T.T., President, GenArmor, Winston Salem, NC 27101, USA; J.W.H., Equity holder, GenArmor, Winston Salem, NC 27101, USA; G.P.M., Equity holder, GenArmor, Winston Salem, NC 27101, USA; J.A.W., Chief Scientific Officer, Nature Technology Corporation, Lincoln, Nebraska 68521, USA. The authors acknowledge the support of the JHSOM Ross confocal facility and John Gibas.
Supplementary Material
References
- Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M, Tarnovskaya A, Ehrlich HP, Baskin-Bey E, Gill K, et al. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. Surg Technol Int. 2003;11:161–167. [PubMed] [Google Scholar]
- Kaya G, Saurat JH. Dermatoporosis: a chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology (Basel) 2007;215:284–294. doi: 10.1159/000107621. [DOI] [PubMed] [Google Scholar]
- Blissitt PA. Nutrition in acute spinal cord injury. Crit Care Nurs Clin North Am. 1990;2:375–384. [PubMed] [Google Scholar]
- Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Yanagihara D, Klopchin K, Danilenko DM, Hsu E, Kenney WC, et al. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994;179:831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis P, Savla U, Volpert OV, Jimenez B, Waters CM, Panos RJ, et al. Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. J Cell Sci. 1999;112 (Pt 12):2049–2057. doi: 10.1242/jcs.112.12.2049. [DOI] [PubMed] [Google Scholar]
- Peng C, He Q, Luo C. Lack of keratinocyte growth factor retards angiogenesis in cutaneous wounds. J Int Med Res. 2011;39:416–423. doi: 10.1177/147323001103900209. [DOI] [PubMed] [Google Scholar]
- Marti G, Ferguson M, Wang J, Byrnes C, Dieb R, Qaiser R, et al. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther. 2004;11:1780–1785. doi: 10.1038/sj.gt.3302383. [DOI] [PubMed] [Google Scholar]
- Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA. 1992;89:6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm Res. 2013;30:1099–1109. doi: 10.1007/s11095-012-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hoffman RM. The feasibility of targeted selective gene therapy of the hair follicle. Nat Med. 1995;1:705–706. doi: 10.1038/nm0795-705. [DOI] [PubMed] [Google Scholar]
- Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J Control Release. 2007;122:375–383. doi: 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci USA. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SF, Yang MJ, Su CJ, Chen HL, Lee PW, Wei MC, et al. Effects of incorporation of poly(gamma-glutamic acid) in chitosan/DNA complex nanoparticles on cellular uptake and transfection efficiency. Biomaterials. 2009;30:1797–1808. doi: 10.1016/j.biomaterials.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Özbas-Turan S, Akbuga J. Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Deliv. 2011;18:215–222. doi: 10.3109/10717544.2010.544688. [DOI] [PubMed] [Google Scholar]
- Domashenko A, Gupta S, Cotsarelis G. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol. 2000;18:420–423. doi: 10.1038/74480. [DOI] [PubMed] [Google Scholar]
- Meykadeh N, Mirmohammadsadegh A, Wang Z, Basner-Tschakarjan E, Hengge UR. Topical application of plasmid DNA to mouse and human skin. J Mol Med. 2005;83:897–903. doi: 10.1007/s00109-005-0669-x. [DOI] [PubMed] [Google Scholar]
- Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53:341–358. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- Tam P, Monck M, Lee D, Ludkovski O, Leng EC, Clow K, et al. Stabilized plasmid-lipid particles for systemic gene therapy. Gene Ther. 2000;7:1867–1874. doi: 10.1038/sj.gt.3301308. [DOI] [PubMed] [Google Scholar]
- Vogel JC. Nonviral skin gene therapy. Hum Gene Ther. 2000;11:2253–2259. doi: 10.1089/104303400750035780. [DOI] [PubMed] [Google Scholar]
- Luke JM, Vincent JM, Du SX, Gerdemann U, Leen AM, Whalen RG, et al. Improved antibiotic-free plasmid vector design by incorporation of transient expression enhancers. Gene Ther. 2011;18:334–343. doi: 10.1038/gt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR. The use of microdermabrasion for acne: a pilot study. Dermatol Surg. 2001;27:329–331. doi: 10.1046/j.1524-4725.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- Alkhawam L, Alam M. Dermabrasion and microdermabrasion. Facial Plast Surg. 2009;25:301–310. doi: 10.1055/s-0029-1243078. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Shirakami K, Tojo K. Effect of microdermabrasion on barrier capacity of stratum corneum. Chem Pharm Bull. 2005;53:1014–1016. doi: 10.1248/cpb.53.1014. [DOI] [PubMed] [Google Scholar]
- Andrews SN, Zarnitsyn V, Bondy B, Prausnitz MR. Optimization of microdermabrasion for controlled removal of stratum corneum. Int J Pharm. 2011;407:95–104. doi: 10.1016/j.ijpharm.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S, Lee JW, Prausnitz M. Recovery of skin barrier after stratum corneum removal by microdermabrasion. AAPS PharmSciTech. 2011;12:1393–1400. doi: 10.1208/s12249-011-9715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. Dermatol Surg. 2006;32:1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- Saito N, Zhao M, Li L, Baranov E, Yang M, Ohta Y, et al. High efficiency genetic modification of hair follicles and growing hair shafts. Proc Natl Acad Sci USA. 2002;99:13120–13124. doi: 10.1073/pnas.192453799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branski LK, Pereira CT, Herndon DN, Jeschke MG. Gene therapy in wound healing: present status and future directions. Gene Ther. 2007;14:1–10. doi: 10.1038/sj.gt.3302837. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Dexling B, Mirmohammadsadegh A. Safety and pharmacokinetics of naked plasmid DNA in the skin: studies on dissemination and ectopic expression. J Invest Dermatol. 2001;116:979–982. doi: 10.1046/j.1523-1747.2001.01341.x. [DOI] [PubMed] [Google Scholar]
- Basner-Tschakarjan E, Mirmohammadsadegh A, Baer A, Hengge UR. Uptake and trafficking of DNA in keratinocytes: evidence for DNA-binding proteins. Gene Ther. 2004;11:765–774. doi: 10.1038/sj.gt.3302221. [DOI] [PubMed] [Google Scholar]
- Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol. 1999;112:297–302. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Dileo J, Miller TE, Jr, Chesnoy S, Huang L. Gene transfer to subdermal tissues via a new gene gun design. Hum Gene Ther. 2003;14:79–87. doi: 10.1089/10430340360464732. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Yao F, Svensjö T, Winkler T, Slama J, Macklin MD, et al. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152:330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura D, Ina S, Itai K, Meng X, Kon A, Tamai K, et al. In vivo gene introduction into keratinocytes using jet injection. Gene Ther. 1999;6:1785–1787. doi: 10.1038/sj.gt.3301002. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Chan EF, Foster RA, Walker PS, Vogel JC. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet. 1995;10:161–166. doi: 10.1038/ng0695-161. [DOI] [PubMed] [Google Scholar]
- Lin MP, Marti GP, Dieb R, Wang J, Ferguson M, Qaiser R, et al. Delivery of plasmid DNA expression vector for keratinocyte growth factor-1 using electroporation to improve cutaneous wound healing in a septic rat model. Wound Repair Regen. 2006;14:618–624. doi: 10.1111/j.1743-6109.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Andrews S, Lee JW, Choi SO, Prausnitz MR. Transdermal insulin delivery using microdermabrasion. Pharm Res. 2011;28:2110–2118. doi: 10.1007/s11095-011-0435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151:132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- Lee WR, Shen SC, Kuo-Hsien W, Hu CH, Fang JY. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol. 2003;121:1118–1125. doi: 10.1046/j.1523-1747.2003.12537.x. [DOI] [PubMed] [Google Scholar]
- Smola H, Thiekötter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- Staiano-Coico L, Krueger JG, Rubin JS, D'limi S, Vallat VP, Valentino L, et al. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993;178:865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, Morawiecki A, et al. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am J Pathol. 1995;147:1261–1277. [PMC free article] [PubMed] [Google Scholar]
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Marti GP, Mohebi P, Liu L, Wang J, Miyashita T, Harmon JW. KGF-1 for wound healing in animal models. Methods Mol Biol. 2008;423:383–391. doi: 10.1007/978-1-59745-194-9_30. [DOI] [PubMed] [Google Scholar]
- Fan H, Lin Q, Morrissey GR, Khavari PA. Immunization via hair follicles by topical application of naked DNA to normal skin. Nat Biotechnol. 1999;17:870–872. doi: 10.1038/12856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.