Abstract
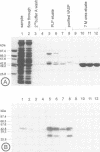
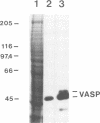
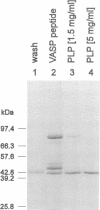
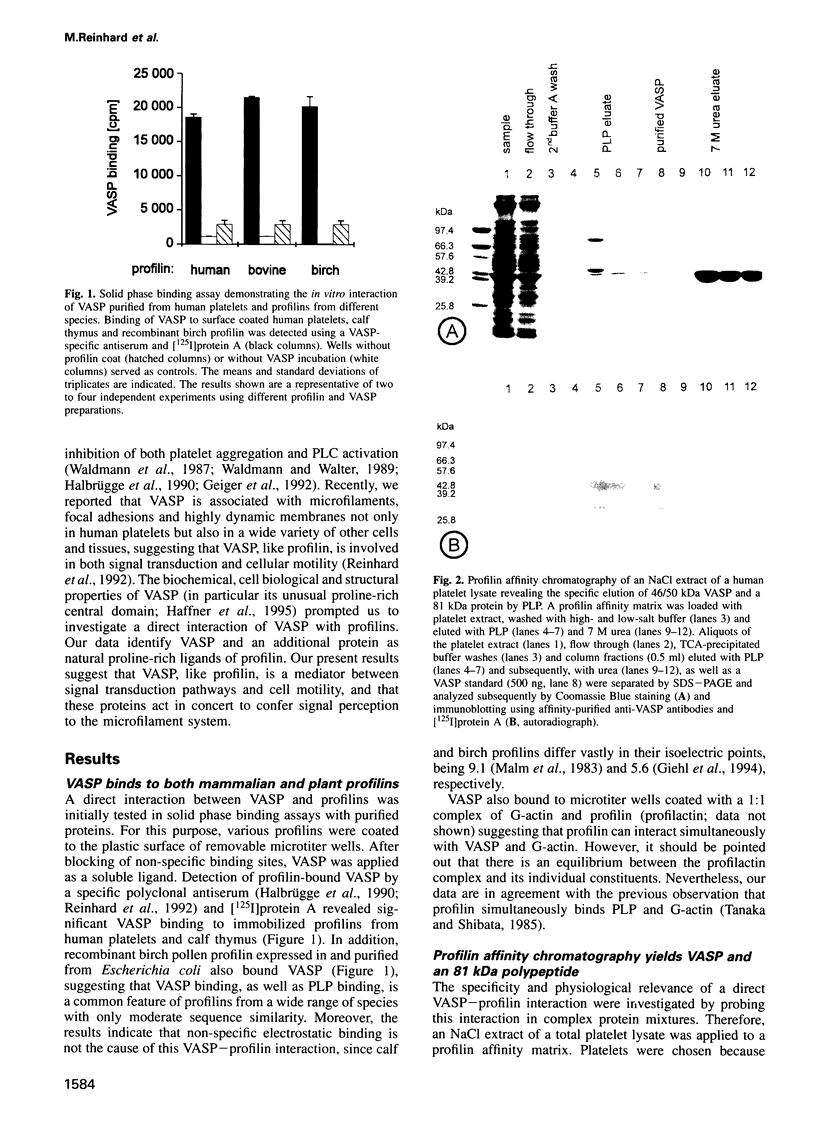
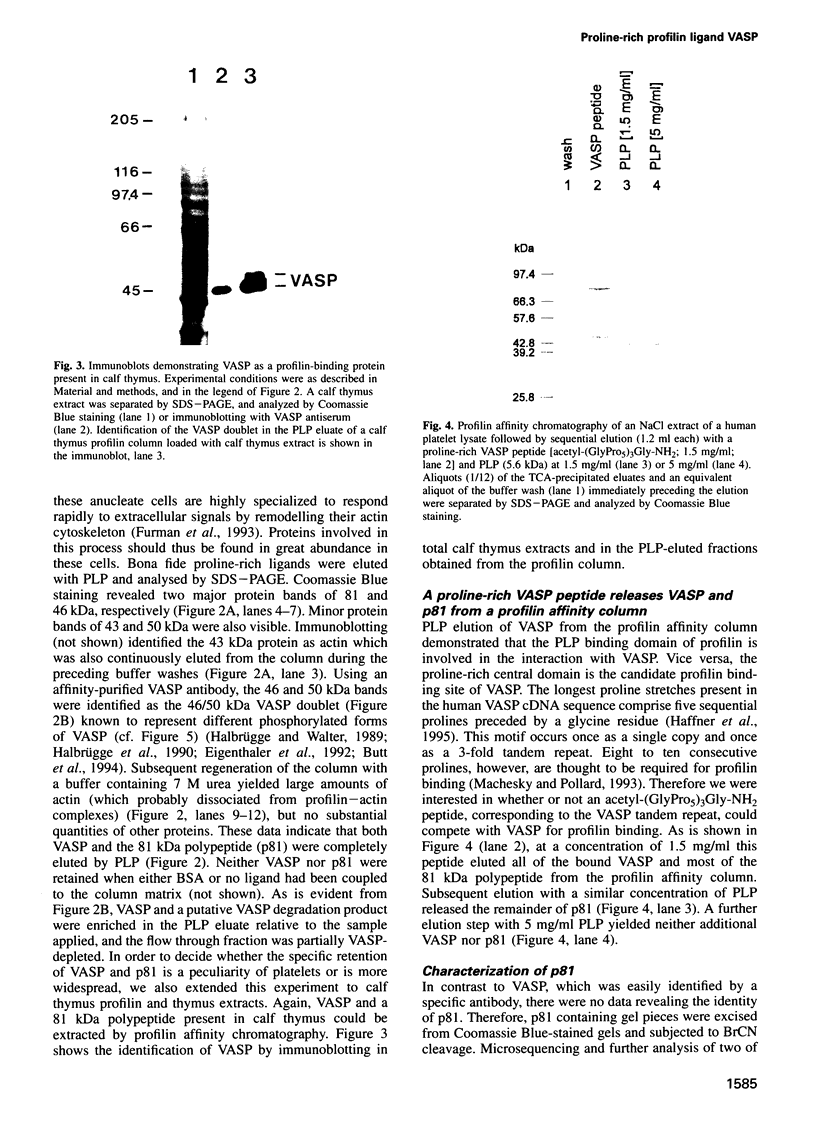
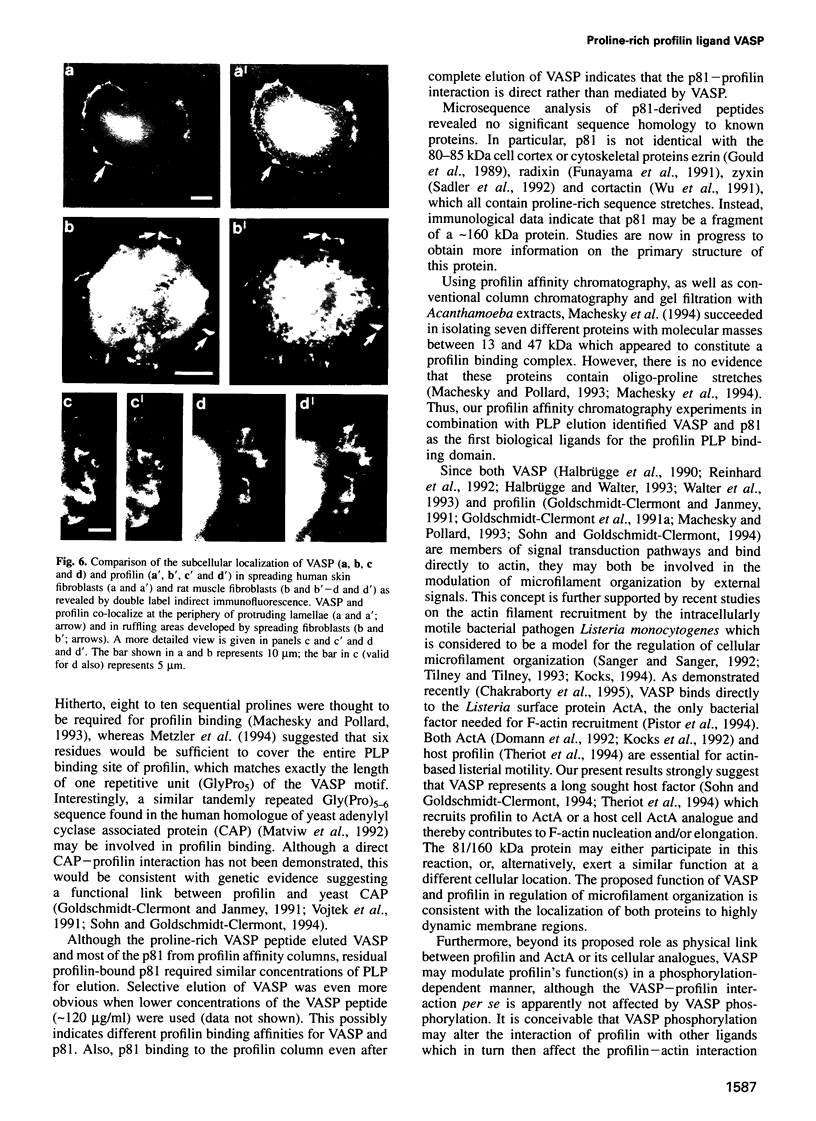
Profilins are small proteins that form complexes with G-actin and phosphoinositides and are therefore considered to link the microfilament system to signal transduction pathways. In addition, they bind to poly-L-proline, but the biological significance of this interaction is not yet known. The recent molecular cloning of the vasodilator-stimulated phosphoprotein (VASP), an established in vivo substrate of cAMP- and cGMP-dependent protein kinases, revealed the presence of a proline-rich domain which prompted us to investigate a possible interaction with profilins. VASP is a microfilament and focal adhesion associated protein which is also concentrated in highly dynamic regions of the cell cortex. Here, we demonstrate that VASP is a natural proline-rich profilin ligand. Human platelet VASP bound directly to purified profilins from human platelets, calf thymus and birch pollen. Moreover, VASP and a novel protein were specifically extracted from total cell lysates by profilin affinity chromatography and subsequently eluted either with poly-L-proline or a peptide corresponding to a proline-rich VASP motif. Finally, the subcellular distributions of VASP and profilin suggest that both proteins also interact within living cells. Our data support the hypothesis that profilin and VASP act in concert to convey signal transduction to actin filament formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. J., Vinson V. K., Pollard T. D., Torchia D. A. Elucidation of the poly-L-proline binding site in Acanthamoeba profilin I by NMR spectroscopy. FEBS Lett. 1994 Jan 10;337(2):145–151. doi: 10.1016/0014-5793(94)80262-9. [DOI] [PubMed] [Google Scholar]
- Björkegren C., Rozycki M., Schutt C. E., Lindberg U., Karlsson R. Mutagenesis of human profilin locates its poly(L-proline)-binding site to a hydrophobic patch of aromatic amino acids. FEBS Lett. 1993 Oct 25;333(1-2):123–126. doi: 10.1016/0014-5793(93)80388-b. [DOI] [PubMed] [Google Scholar]
- Buss F., Temm-Grove C., Henning S., Jockusch B. M. Distribution of profilin in fibroblasts correlates with the presence of highly dynamic actin filaments. Cell Motil Cytoskeleton. 1992;22(1):51–61. doi: 10.1002/cm.970220106. [DOI] [PubMed] [Google Scholar]
- Butt E., Abel K., Krieger M., Palm D., Hoppe V., Hoppe J., Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994 May 20;269(20):14509–14517. [PubMed] [Google Scholar]
- Chakraborty T., Ebel F., Domann E., Niebuhr K., Gerstel B., Pistor S., Temm-Grove C. J., Jockusch B. M., Reinhard M., Walter U. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995 Apr 3;14(7):1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenthaler M., Nolte C., Halbrügge M., Walter U. Concentration and regulation of cyclic nucleotides, cyclic-nucleotide-dependent protein kinases and one of their major substrates in human platelets. Estimating the rate of cAMP-regulated and cGMP-regulated protein phosphorylation in intact cells. Eur J Biochem. 1992 Apr 15;205(2):471–481. doi: 10.1111/j.1432-1033.1992.tb16803.x. [DOI] [PubMed] [Google Scholar]
- Fox J. E. Regulation of platelet function by the cytoskeleton. Adv Exp Med Biol. 1993;344:175–185. doi: 10.1007/978-1-4615-2994-1_13. [DOI] [PubMed] [Google Scholar]
- Funayama N., Nagafuchi A., Sato N., Tsukita S., Tsukita S. Radixin is a novel member of the band 4.1 family. J Cell Biol. 1991 Nov;115(4):1039–1048. doi: 10.1083/jcb.115.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman M. I., Gardner T. M., Goldschmidt-Clermont P. J. Mechanisms of cytoskeletal reorganization during platelet activation. Thromb Haemost. 1993 Jul 1;70(1):229–232. [PubMed] [Google Scholar]
- Geiger J., Nolte C., Butt E., Sage S. O., Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl K., Valenta R., Rothkegel M., Ronsiek M., Mannherz H. G., Jockusch B. M. Interaction of plant profilin with mammalian actin. Eur J Biochem. 1994 Dec 1;226(2):681–689. doi: 10.1111/j.1432-1033.1994.tb20096.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Janmey P. A. Profilin, a weak CAP for actin and RAS. Cell. 1991 Aug 9;66(3):419–421. doi: 10.1016/0092-8674(81)90002-7. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991 Jun;113(5):1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B. K., Petzold A. S., Brown S. S. Mutational analysis of yeast profilin. Mol Cell Biol. 1993 Dec;13(12):7864–7873. doi: 10.1128/mcb.13.12.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C., Jarchau T., Reinhard M., Hoppe J., Lohmann S. M., Walter U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. EMBO J. 1995 Jan 3;14(1):19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbrügge M., Friedrich C., Eigenthaler M., Schanzenbächer P., Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990 Feb 25;265(6):3088–3093. [PubMed] [Google Scholar]
- Halbrügge M., Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989 Oct 20;185(1):41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- Horstrup K., Jablonka B., Hönig-Liedl P., Just M., Kochsiek K., Walter U. Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur J Biochem. 1994 Oct 1;225(1):21–27. doi: 10.1111/j.1432-1033.1994.00021.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. The impact of molecular biology on models for cell adhesion. Bioessays. 1994 Sep;16(9):663–669. doi: 10.1002/bies.950160912. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. Polyproline affinity method for purification of platelet profilin and modification with pyrene-maleimide. Methods Enzymol. 1991;196:92–99. doi: 10.1016/0076-6879(91)96011-f. [DOI] [PubMed] [Google Scholar]
- Kaiser D. A., Sato M., Ebert R. F., Pollard T. D. Purification and characterization of two isoforms of Acanthamoeba profilin. J Cell Biol. 1986 Jan;102(1):221–226. doi: 10.1083/jcb.102.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kocks C. Intracellular motility. Profilin puts pathogens on the actin drive. Curr Biol. 1994 May 1;4(5):465–468. doi: 10.1016/s0960-9822(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Larsson H., Lindberg U. The effect of divalent cations on the interaction between calf spleen profilin and different actins. Biochim Biophys Acta. 1988 Mar 2;953(1):95–105. doi: 10.1016/0167-4838(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Atkinson S. J., Ampe C., Vandekerckhove J., Pollard T. D. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol. 1994 Oct;127(1):107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Poland T. D. Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 1993 Nov;3(11):381–385. doi: 10.1016/0962-8924(93)90087-h. [DOI] [PubMed] [Google Scholar]
- Malm B., Larsson H., Lindberg U. The profilin--actin complex: further characterization of profilin and studies on the stability of the complex. J Muscle Res Cell Motil. 1983 Oct;4(5):569–588. doi: 10.1007/BF00712116. [DOI] [PubMed] [Google Scholar]
- Matviw H., Yu G., Young D. Identification of a human cDNA encoding a protein that is structurally and functionally related to the yeast adenylyl cyclase-associated CAP proteins. Mol Cell Biol. 1992 Nov;12(11):5033–5040. doi: 10.1128/mcb.12.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler W. J., Bell A. J., Ernst E., Lavoie T. B., Mueller L. Identification of the poly-L-proline-binding site on human profilin. J Biol Chem. 1994 Feb 11;269(6):4620–4625. [PubMed] [Google Scholar]
- Nolte C., Eigenthaler M., Schanzenbächer P., Walter U. Endothelial cell-dependent phosphorylation of a platelet protein mediated by cAMP- and cGMP-elevating factors. J Biol Chem. 1991 Aug 5;266(22):14808–14812. [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F. How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell. 1993 Dec 3;75(5):1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Pistor S., Chakraborty T., Niebuhr K., Domann E., Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994 Feb 15;13(4):758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry. 1984 Dec 18;23(26):6631–6641. doi: 10.1021/bi00321a054. [DOI] [PubMed] [Google Scholar]
- Pring M., Weber A., Bubb M. R. Profilin-actin complexes directly elongate actin filaments at the barbed end. Biochemistry. 1992 Feb 18;31(6):1827–1836. doi: 10.1021/bi00121a035. [DOI] [PubMed] [Google Scholar]
- Reinhard M., Halbrügge M., Scheer U., Wiegand C., Jockusch B. M., Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992 Jun;11(6):2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Crawford A. W., Michelsen J. W., Beckerle M. C. Zyxin and cCRP: two interactive LIM domain proteins associated with the cytoskeleton. J Cell Biol. 1992 Dec;119(6):1573–1587. doi: 10.1083/jcb.119.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M. Cell motility. Beads, bacteria and actin. Nature. 1992 Jun 11;357(6378):442–442. doi: 10.1038/357442a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sohn R. H., Goldschmidt-Clermont P. J. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. Bioessays. 1994 Jul;16(7):465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Staiger C. J., Goodbody K. C., Hussey P. J., Valenta R., Drøbak B. K., Lloyd C. W. The profilin multigene family of maize: differential expression of three isoforms. Plant J. 1993 Oct;4(4):631–641. doi: 10.1046/j.1365-313x.1993.04040631.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Shibata H. Poly(L-proline)-binding proteins from chick embryos are a profilin and a profilactin. Eur J Biochem. 1985 Sep 2;151(2):291–297. doi: 10.1111/j.1432-1033.1985.tb09099.x. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. The three faces of profilin. Cell. 1993 Dec 3;75(5):835–838. doi: 10.1016/0092-8674(93)90527-w. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Rosenblatt J., Portnoy D. A., Goldschmidt-Clermont P. J., Mitchison T. J. Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell. 1994 Feb 11;76(3):505–517. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S. The wily ways of a parasite: induction of actin assembly by Listeria. Trends Microbiol. 1993 Apr;1(1):25–31. doi: 10.1016/0966-842x(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Vojtek A., Haarer B., Field J., Gerst J., Pollard T. D., Brown S., Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991 Aug 9;66(3):497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Nieberding M., Walter U. Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur J Biochem. 1987 Sep 15;167(3):441–448. doi: 10.1111/j.1432-1033.1987.tb13357.x. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Walter U. Cyclic nucleotide elevating vasodilators inhibit platelet aggregation at an early step of the activation cascade. Eur J Pharmacol. 1989 Jan 17;159(3):317–320. doi: 10.1016/0014-2999(89)90165-9. [DOI] [PubMed] [Google Scholar]
- Walter U., Eigenthaler M., Geiger J., Reinhard M. Role of cyclic nucleotide-dependent protein kinases and their common substrate VASP in the regulation of human platelets. Adv Exp Med Biol. 1993;344:237–249. doi: 10.1007/978-1-4615-2994-1_19. [DOI] [PubMed] [Google Scholar]
- Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991 Oct;11(10):5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]