Abstract
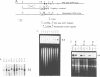
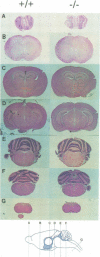
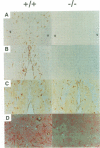
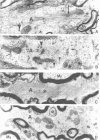
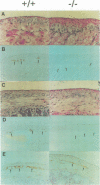
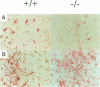
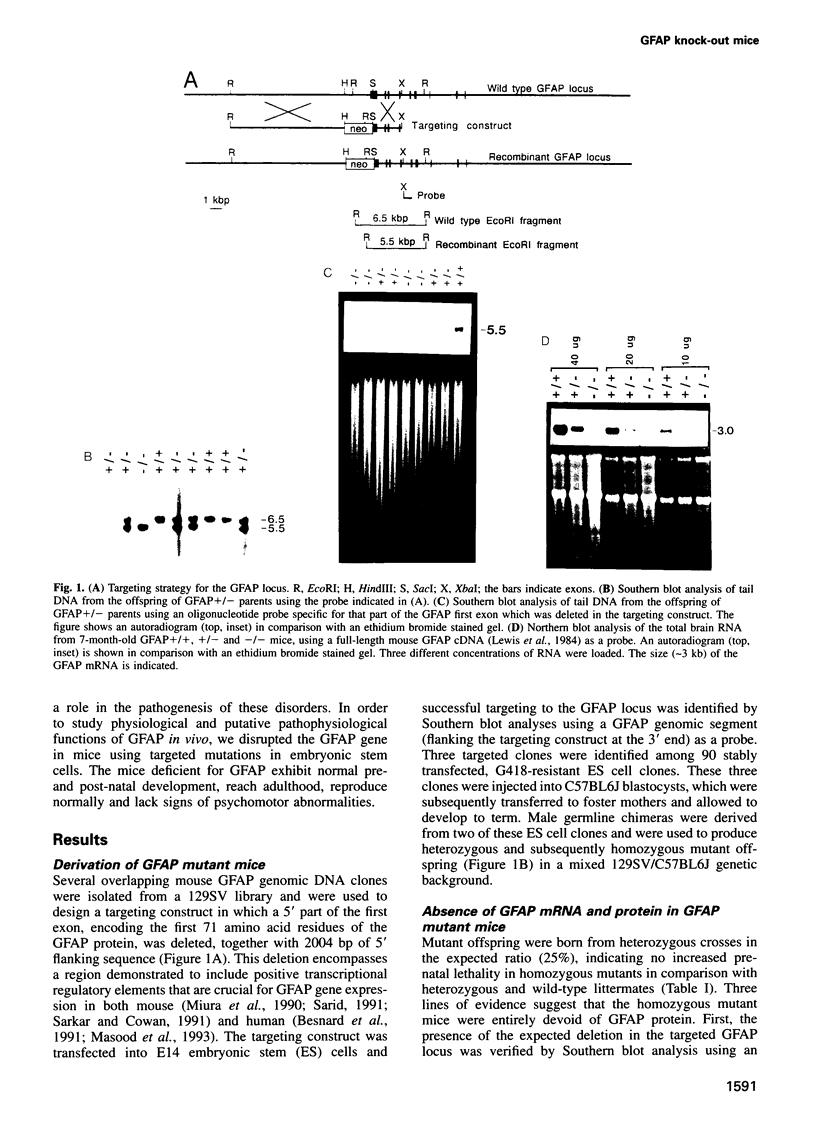
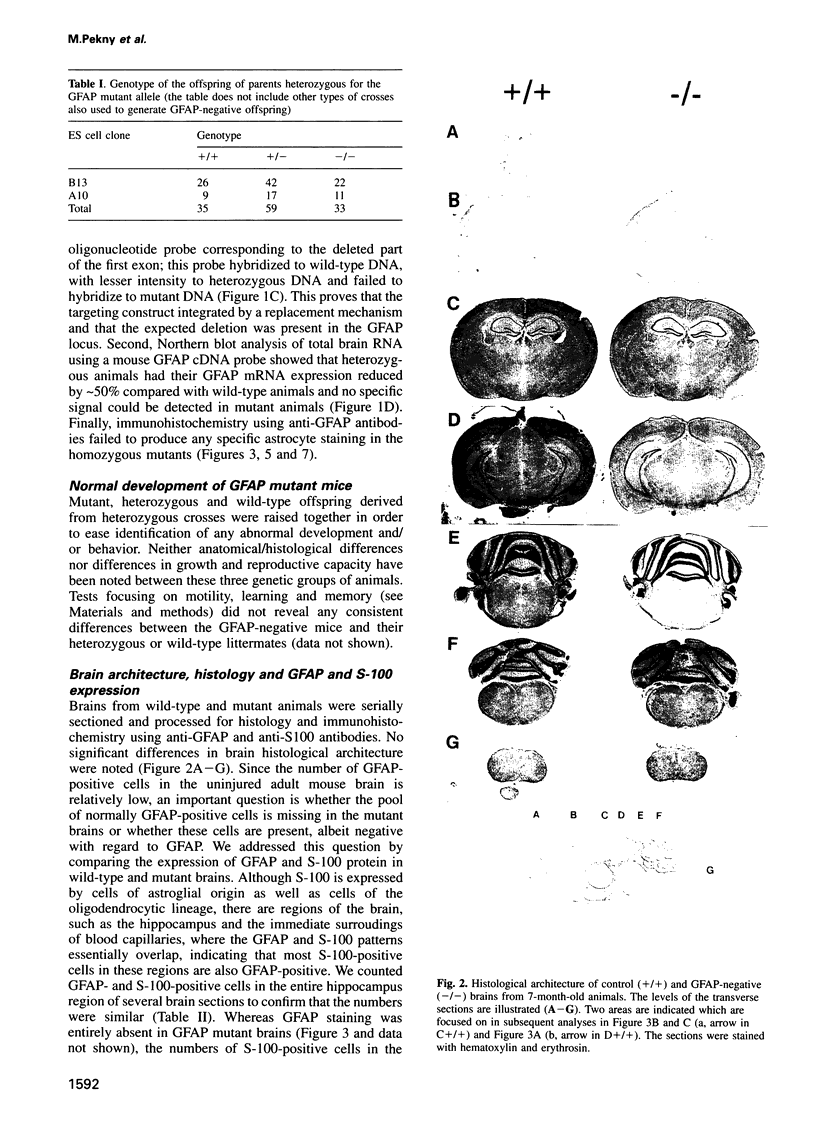
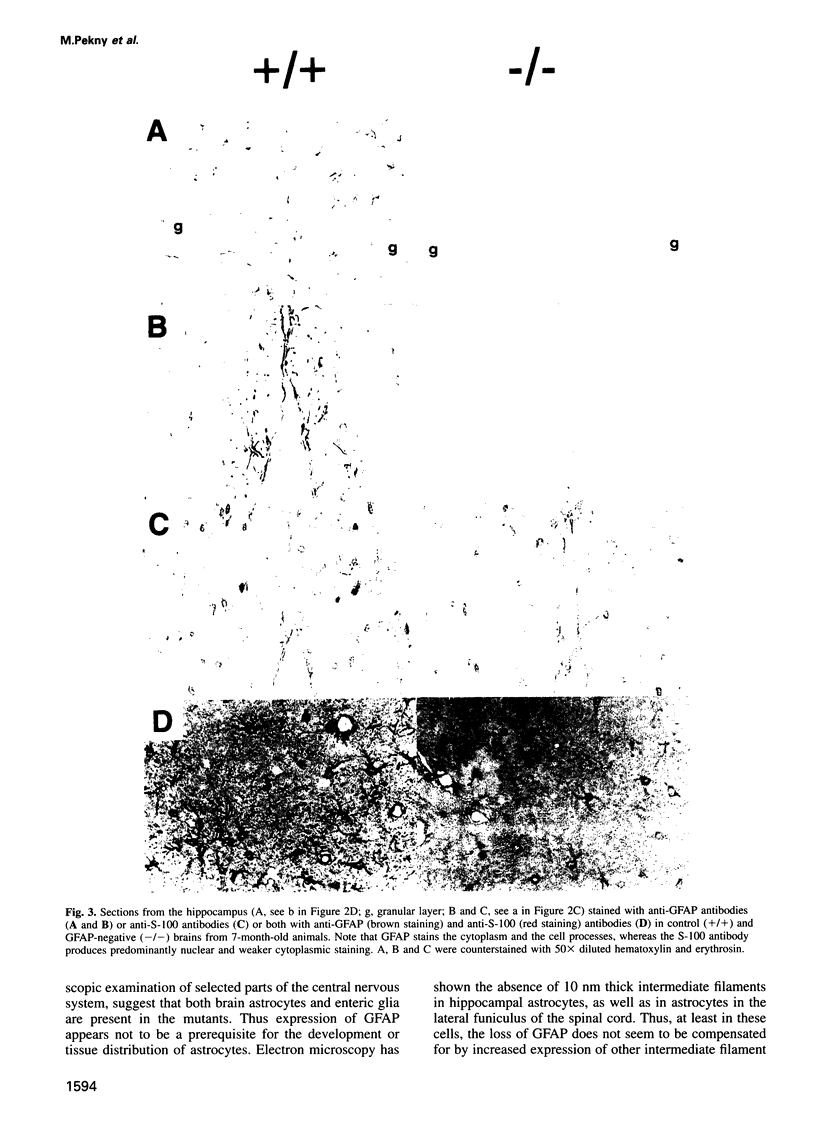
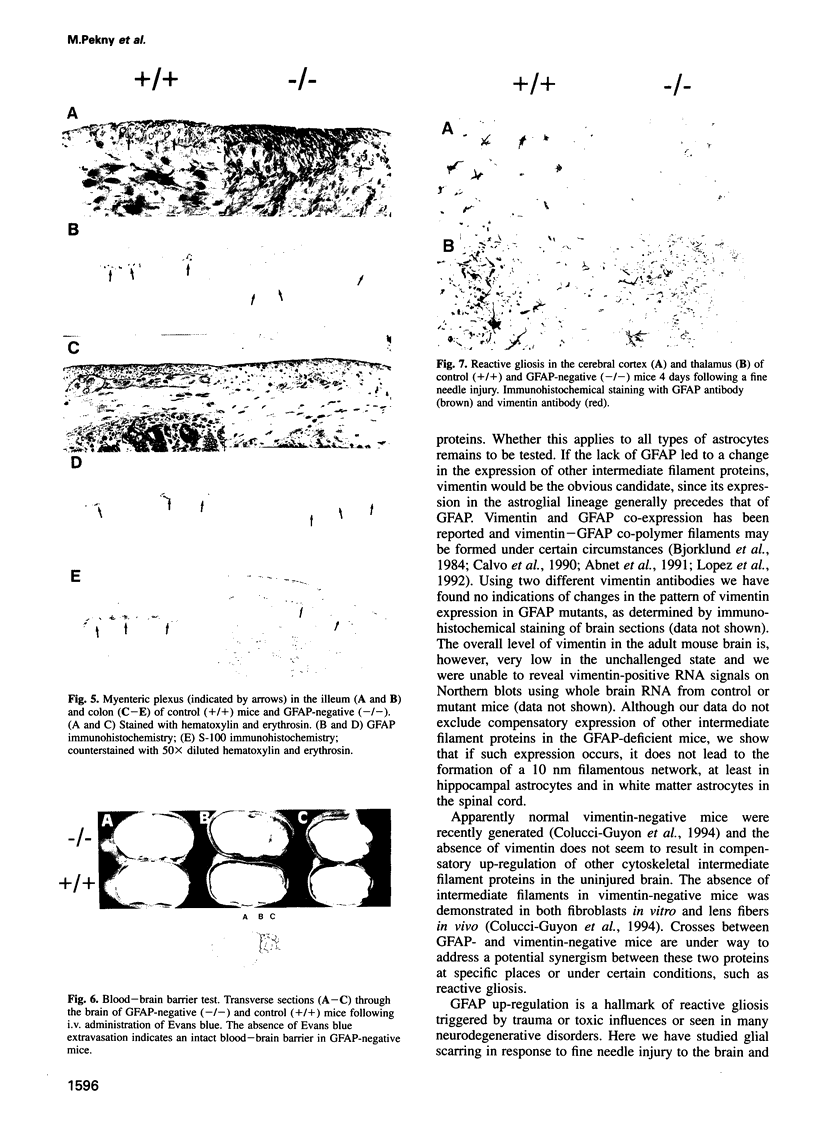
Glial fibrillary acidic protein (GFAP) is the main component of the intermediate filaments in cells of astroglial lineage, including astrocytes in the CNS, nonmyelin forming Schwann cells and enteric glia. To address the function of GFAP in vivo, we have disrupted the GFAP gene in mice via targeted mutation in embryonic stem cells. Mice lacking GFAP developed normally, reached adulthood and reproduced. We did not find any abnormalities in the histological architecture of the CNS, in their behavior, motility, memory, blood-brain barrier function, myenteric plexi histology or intestinal peristaltic movement. Comparisons between GFAP and S-100 immunohistochemical staining patterns in the hippocampus of wild-type and mutant mice suggested a normal abundance of astrocytes in GFAP-negative mice, however, in contrast to wild-types, GFAP-negative astrocytes of the hippocampus and in the white matter of the spinal cord were completely lacking intermediate filaments. This shows that the loss of GFAP intermediate filaments is not compensated for by the up-regulation of other intermediate filament proteins, such as vimentin. The GFAP-negative mice displayed post-traumatic reactive gliosis, which suggests that GFAP up-regulation, a hallmark of reactive gliosis, is not an obligatory requirement for this process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abnet K., Fawcett J. W., Dunnett S. B. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res Dev Brain Res. 1991 Apr 24;59(2):187–196. doi: 10.1016/0165-3806(91)90099-5. [DOI] [PubMed] [Google Scholar]
- Baribault H., Price J., Miyai K., Oshima R. G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993 Jul;7(7A):1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- Besnard F., Brenner M., Nakatani Y., Chao R., Purohit H. J., Freese E. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J Biol Chem. 1991 Oct 5;266(28):18877–18883. [PubMed] [Google Scholar]
- Björklund H., Eriksdotter-Nilsson M., Dahl D., Olson L. Astrocytes in smears of CNS tissues as visualized by GFA and vimentin immunofluorescence. Med Biol. 1984;62(1):38–48. [PubMed] [Google Scholar]
- Calvo J. L., Carbonell A. L., Boya J. Coexpression of vimentin and glial fibrillary acidic protein in astrocytes of the adult rat optic nerve. Brain Res. 1990 Nov 5;532(1-2):355–357. doi: 10.1016/0006-8993(90)91784-e. [DOI] [PubMed] [Google Scholar]
- Chan Y., Anton-Lamprecht I., Yu Q. C., Jäckel A., Zabel B., Ernst J. P., Fuchs E. A human keratin 14 "knockout": the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev. 1994 Nov 1;8(21):2574–2587. doi: 10.1101/gad.8.21.2574. [DOI] [PubMed] [Google Scholar]
- Cheng J., Syder A. J., Yu Q. C., Letai A., Paller A. S., Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992 Sep 4;70(5):811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- Chipev C. C., Korge B. P., Markova N., Bale S. J., DiGiovanna J. J., Compton J. G., Steinert P. M. A leucine----proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992 Sep 4;70(5):821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T., Fields K. L. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J Neurochem. 1981 Jul;37(1):147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E., Portier M. M., Dunia I., Paulin D., Pournin S., Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994 Nov 18;79(4):679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Julien J. P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993 Apr 9;73(1):35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- Dupouey P., Benjelloun S., Gomes D. Immunohistochemical demonstration of an organized cytoarchitecture of the radial glia in the CNS of the embryonic mouse. Dev Neurosci. 1985;7(2):81–93. doi: 10.1159/000112279. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr Molecular genetics of epidermolysis bullosa. Science. 1992 May 8;256(5058):799–804. doi: 10.1126/science.1375393. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Chan Y. M., Paller A. S., Yu Q. C. Cracks in the foundation: keratin filaments and genetic disease. Trends Cell Biol. 1994 Sep;4(9):321–326. doi: 10.1016/0962-8924(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Goebel H. H., Schlie M., Eng L. F. Immunohistopathologic spectrum of the glial fibrillary acidic protein in neurooncology. Acta Histochem Suppl. 1987;34:81–93. [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983 Nov;3(11):2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Morgan L., Stewart H. J., Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990 May;109(1):91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992 Mar 19;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Levéen P., Pekny M., Gebre-Medhin S., Swolin B., Larsson E., Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994 Aug 15;8(16):1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Muñoz F., Calvo J. L., Boya J., Carbonell A. L. Coexpression of vimentin and glial fibrillary acidic protein in glial cells of the adult rat pineal gland. J Pineal Res. 1992 May;12(4):145–148. doi: 10.1111/j.1600-079x.1992.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Masood K., Besnard F., Su Y., Brenner M. Analysis of a segment of the human glial fibrillary acidic protein gene that directs astrocyte-specific transcription. J Neurochem. 1993 Jul;61(1):160–166. doi: 10.1111/j.1471-4159.1993.tb03551.x. [DOI] [PubMed] [Google Scholar]
- Miura M., Tamura T., Mikoshiba K. Cell-specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis- and trans-acting promoter elements for astrocyte-specific expression. J Neurochem. 1990 Oct;55(4):1180–1188. doi: 10.1111/j.1471-4159.1990.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Ohara O., Gahara Y., Miyake T., Teraoka H., Kitamura T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol. 1993 Apr;121(2):387–395. doi: 10.1083/jcb.121.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Basarsky T. A., Liu F., Jeftinija K., Jeftinija S., Haydon P. G. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994 Jun 30;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Genetic and infectious prion diseases. Arch Neurol. 1993 Nov;50(11):1129–1153. doi: 10.1001/archneur.1993.00540110011002. [DOI] [PubMed] [Google Scholar]
- Rothnagel J. A., Dominey A. M., Dempsey L. D., Longley M. A., Greenhalgh D. A., Gagne T. A., Huber M., Frenk E., Hohl D., Roop D. R. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992 Aug 21;257(5073):1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- Rugg E. L., McLean W. H., Lane E. B., Pitera R., McMillan J. R., Dopping-Hepenstal P. J., Navsaria H. A., Leigh I. M., Eady R. A. A functional "knockout" of human keratin 14. Genes Dev. 1994 Nov 1;8(21):2563–2573. doi: 10.1101/gad.8.21.2563. [DOI] [PubMed] [Google Scholar]
- Sarid J. Identification of a cis-acting positive regulatory element of the glial fibrillary acidic protein gene. J Neurosci Res. 1991 Feb;28(2):217–228. doi: 10.1002/jnr.490280209. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Cowan N. J. Intragenic sequences affect the expression of the gene encoding glial fibrillary acidic protein. J Neurochem. 1991 Aug;57(2):675–684. doi: 10.1111/j.1471-4159.1991.tb03799.x. [DOI] [PubMed] [Google Scholar]
- Toggas S. M., Masliah E., Rockenstein E. M., Rall G. F., Abraham C. R., Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994 Jan 13;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Torpey N., Wylie C. C., Heasman J. Function of maternal cytokeratin in Xenopus development. Nature. 1992 Jun 4;357(6377):413–415. doi: 10.1038/357413a0. [DOI] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Weinstein D. E., Shelanski M. L., Liem R. K. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J Cell Biol. 1991 Mar;112(6):1205–1213. doi: 10.1083/jcb.112.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S., Haug H., Budka H. Astroglial changes in the cerebral cortex of AIDS brains: a morphometric and immunohistochemical investigation. Neuropathol Appl Neurobiol. 1993 Aug;19(4):329–335. doi: 10.1111/j.1365-2990.1993.tb00448.x. [DOI] [PubMed] [Google Scholar]