Abstract
The fluorescence excitation spectra of jet-cooled benzocyclobutane have been recorded and together with its ultraviolet absorption spectra have been used to assign the vibrational frequencies for this molecule in its S1(π,π*) electronic excited state. Theoretical calculations at the CASSCF(6,6)/aug-cc-pVTZ level of theory were carried out to compute the structure of the molecule in its excited state. The calculated structure was compared to that of the molecule in its electronic ground state as well as to the structures of related molecules in their S0 and S1(π,π*) electronic states. In each case the decreased π bonding in the electronic excited states results in longer carbon-carbon bonds in the benzene ring. The skeletal vibrational frequencies in the electronic excited state were readily assigned and these were compared to the ground state and to the frequencies of five similar molecules. The vibrational levels in both S0 and S1(π,π*) states were remarkably harmonic in contrast to the other bicyclic molecules. The decreases in the frequencies of the out-of-plane skeletal modes reflect the increased floppiness of these bicyclic molecules in their S1(π,π*) excited state.
INTRODUCTION
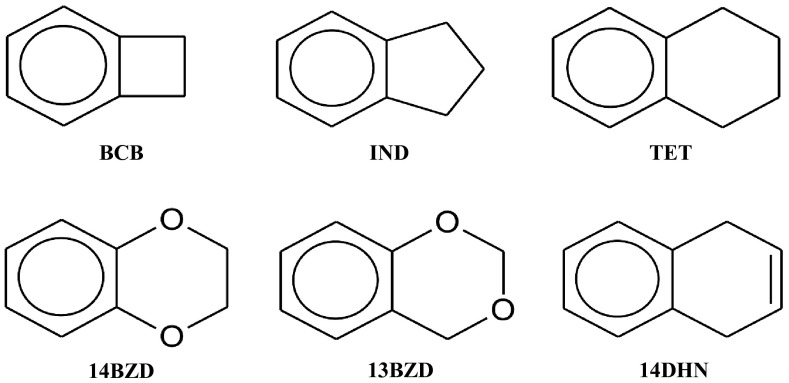
In recent years we have investigated the structures, conformations, and molecular vibrations of bicyclic molecules in their ground and excited states. Among these are indan (IND),1 tetralin (TET),2 1,4-benzodioxan (14BZD),3, 4, 5 1,3-benzodioxan (13BZD),6 and 1,4-dihydronaphthalene (14DHN).7 In the present study we report our results on the S1(π,π*) electronic excited state of benzocyclobutane (BCB) utilizing fluorescence excitation spectroscopy (FES) and ultraviolet absorption spectroscopy (Scheme ch1). The vibrational study of the BCB S0 ground state is reported in Ref. 8.
Figure .
Bicyclic molecules of interest.
The focus of these studies has been not only to determine the structures and vibrational data for these molecules but also to understand what effect the π→π* transition has on the rigidity and conformations of the molecules. Each of these molecules has a ring-flapping vibration whose frequency decreases dramatically in the S1(π,π*) excited states. In addition, many of the vibrational frequencies and bond distances reflect the weakening of the π-bonding in the electronic excited states. The five-membered ring of indan (IND) is puckered with a barrier to planarity of 488 cm−1 (1.40 kcal/mole) in its S0 ground state. This drops to 441 cm−1 (1.26 kcal/mole) in its excited state. The six-membered ring in tetralin (TET) is twisted with a barrier to planarity of about 6000 cm−1 (17 kcal/mole) in its S0 electronic ground state and this drops to about 3500 cm−1 (10 kcal/mole) in its S1(π,π*) state. For 1,4-benzodioxan (14BZD) the calculated twisting barrier values are 11.7 kcal/mole for the ground state and 9.2 kcal/mole for the S1(π,π*) excited state. 1,4-Dihydronaphthalene (14DHN) is planar and relatively floppy in its S0 state but becomes even more so in its S1(π,π*) state. Benzocyclobutane (BCB), the molecule of interest here, is expected to remain planar in both its S0 and S1(π,π*) states, but the difference in the rigidity of the molecule between the two electronic states as reflected by its out-of-plane vibrations will be the focus of the work. BCB has a highly strained four-membered ring attached to the benzene ring and what its effect is on the low-frequency vibrations will be investigated.
EXPERIMENTAL
The BCB sample (98% purity) was purchased from Aldrich Chemical Company and further purified by vacuum distillation. Ultraviolet absorption spectra of the vapor at ambient temperatures were recorded on a Bomem DA8.02 Fourier transform spectrometer using a deuterium lamp source, a quartz beamsplitter, and a silicon detector in the 20 000 to 50 000 cm−1 region. Approximately 3 Torr of sample was contained in a 20 cm glass cell with quartz windows. A resolution of 1 cm−1 was used and 4000 scans were averaged. The fluorescence excitation spectra (FES) were recorded using a Continuum Powerlite 9020 Nd:YAG laser which pumped a Continuum Sunlite optical parametric oscillator (OPO) and an FX-1 ultraviolet extension unit. The laser was scanned under computer control and the fluorescence was detected with a photomultiplier tube whenever resonance with a vibronic level was achieved. The FES were obtained at 0.5 cm−1 resolution and recorded under supersonic jet-cooled conditions. More details on the laser system are provided in Refs. 9, 10, 11, 12, 13, 14, 15, 16.
COMPUTATIONS
In the present study, geometry optimizations and harmonic vibrational frequency computations for BCB in its lowest singlet excited state, S1(π,π*), as well as in its ground state, were performed using the complete-active-space self-consistent-field (CASSCF) method with the aug-cc-pVTZ basis sets. The active space for the CASSCF computations consisted of six π electrons distributed in six π orbitals on the six-membered ring of BCB, as depicted in Figure 1. All CASSCF(6,6)/aug-cc-pVTZ computations were performed using the GAMESS program.17, 18 A scaling factor of 0.905 was used for excited state calculated frequencies.19 The MacMolPlt20 program was used to visualize the excited state vibrational motions of BCB.
Figure 1.
Molecular orbital diagram for the π and π* orbitals of BCB.
We have previously reported our electronic ground state results for BCB.8 MP2/cc-pVTZ calculations were used to compute the structures of BCB and related molecules using the Gaussian 09 package.21 The geometrical parameters calculated in the present work for the BCB electronic ground state using the CASSCF method were very similar to the previous MP2/cc-pVTZ results.8 In the previous work B3LYP/cc-pVTZ calculations were utilized to compute the vibrational spectra and these were compared to the experimental vapor and liquid phase infrared and Raman spectra. Frequencies for the ground state below 1450 cm−1 were scaled with a factor of 0.985, frequencies in the 1450–2000 cm−1 region were scaled by a factor of 0.973, and frequencies above 2000 cm−1 by 0.961 based on previous work.22, 23, 24, 25, 26 The Semichem AMPAC/AGUI 9.227 along with the potential energy distribution (PED) was used to assign the vibrations for the electronic ground state.
In addition to the computational results reported here, we have also carried out CIS/6-311++G(d,p) calculations for the excited state of BCB. Since the calculated bond distances and angles did not differ significantly from our results from the CASSCF computations, the results are not presented here.
STRUCTURES
Figure 2 shows the calculated structure of BCB for both its ground (S0) and S1(π,π*) excited states using the CASSCF(6,6)/aug-cc-pVTZ level. The determinants with the largest contributions to the CASSCF wave function for the S1(π,π*) state of BCB correspond to excitation from the π3 to π4* orbitals and from the π2 to π5* orbitals. As a result, the electronic excitation from the S0 to S1(π,π*) states increases all the C–C bonds of the benzene ring. Note that the C–C bonds between the benzene ring and the CH2 group are slightly shorter in the S1(π,π*) state than in the ground state. This may be due to an increased hyperconjugative interaction between the C–H σ bonding orbitals on the methylene group and the half-filled π bonding orbitals.
Figure 2.

Calculated structures of BCB in its S0 ground and S1(π,π*) excited states (CASSCF(6,6)/aug-cc-pVTZ).
Figure 3 shows the structures for both IND and TET with selected bond distances and angles from the MP2/cc-pVTZ calculations performed on the ground state.8 Selected bond distances and angles of IND1 and TET2 in their S1(π,π*), electronic excited state are also shown. The calculations performed for IND in the excited state1 utilized the CIS/6-31G(d) basis set, whereas the basis set used for the excited state calculations for TET2 was CIS/6-311++G(d,p). Figure 1 presents a representation of the molecular orbitals of BCB so that the π→π* electronic transition can be visualized. As discussed in Ref. 8, the structure for the ground state is quite typical for what is expected for a bicyclic molecule containing the benzene ring. Namely, the carbon-carbon bond distances are all 1.39 ± 0.01 Å for BCB as well as for IND and TET. The ∠C–C–C angles in the benzene ring are all 120° ± 1° for IND and TET, but the small ring angles of the four-membered ring in BCB cause the ∠C–C–C angle at the carbon joined to the external ring to open up to 122°. In the S1(π,π*) excited states, as expected, all of the C–C bonds in the benzene rings increase. For BCB they are 1.43 ± 0.01 Å reflecting an average increase of 0.03 Å. For IND they are 1.42 ± 0.02 Å with an increase of 0.02 Å and for TET they are 1.42 ± 0.01 Å, also with an increase of 0.02 Å. This shows that greater angle strain in the external ring of BCB results in somewhat larger increases in the carbon-carbon bond lengths of the benzene ring. It is also interesting to note that the C–C bond from the benzene ring to an external carbon decreases slightly in the S1(π,π*) excited state by 0.006 Å for BCB and by 0.009 Å for IND and TET.
Figure 3.
Calculated structures of IND and TET in their S0 ground and S1(π,π*) excited states. The S0 structures were calculated using MP2/cc-pVTZ.8 The S1 structure for IND1 utilized CIS/6-31G(d). The S1 structure for TET2 utilized CIS/6-311++G(d,p).
RESULTS AND DISCUSSION
Figure 4 presents the fluorescence excitation spectrum (FES) of jet-cooled BCB and its ultraviolet (UV) absorption spectrum of BCB at room temperature in the −320 to 1700 cm−1 region, relative to the electronic band origin at 37 093.6 cm−1.
Figure 4.
Fluorescence excitation spectrum and ultraviolet absorption spectrum of BCB. The band origin is at 37 093.6 cm−1.
Table 1 compares the BCB electronic band origin to those of related molecules. The BCB transition frequency is the highest among these except for 13BZD which shows the effect of attaching an oxygen atom to the benzene ring. Figure 5 shows the UV spectrum expanded in the −340 to 25 cm−1 region. A number of assignments are shown in the Figures 45. Table 2 presents a summary of the vibrational assignments for the ground and excited states of BCB. The ground state data were presented in Ref. 8 whereas the excited state data have been derived from Table 3, which presents the experimental FES and UV absorption data for the most significant transitions. A more complete listing of the spectral data is available in Table S1 in the supplementary material.28 Figure 6 presents an energy map for several of the low-frequency vibrations of BCB in its ground and excited electronic states. This energy map is consistent with the data and assignments of Table 3. Table 2 also shows the calculated frequencies for both the S0 and S1(π,π*) states. As can be seen, these provide excellent guidance for assigning the experimental spectra. In order to allow comparison with the related molecules, Table 4 shows a listing of selected vibrational frequencies of BCB, IND, TET, 14BZD, 13BZD, and 14DHN in their ground (S0) and excited (S1) states.
Table 1.
Observed electronic transition frequencies (cm−1) and average calculated benzene ring bond distances (Å) of BCB and related molecules.
| Average benzene ring bond distance |
||||
|---|---|---|---|---|
| Transition frequency | S0 | S1(π,π*) | Reference1 | |
| BCB | 37 093.6 | 1.3952 | 1.4293 | This work |
| IND | 36 903.7 | 1.3952 | 1.4164 | 1 |
| TET | 36 789.7 | 1.3962 | 1.4155 | 2 |
| 14BZD | 35 563.1 | 1.3912 | 1.4195 | 3, 4, 5 |
| 13BZD | 38 885.6 | 1.3942 | 1.4056 | 6 |
| 14DHN | 36 788.6 | 1.3932 | … | 7 |
References for transition frequencies and S1 state bond distances. All So state bond distances are from Ref. 8.
MP2/cc-pVTZ.
CASSCF(6,6)/aug-cc-pVTZ.
CIS/6-31G(d).
CIS/6-311++G(d,p).
CIS/6-311+G(d,p).
Figure 5.
UV absorption spectra of BCB in the −340 to 21 cm−1 region. The band origin is at 37 093.6 cm−1.
Table 2.
Vibrational frequencies (cm−1) of BCB in its ground and excited electronic states.
| S0 |
S1(π,π*) |
|||||
|---|---|---|---|---|---|---|
| Experimental1 | Calculated1 | Experimental2 | Calculated3 | |||
| A1 | ν11 | CH sym. stretch | 3077 | 3061 | 3068 | 3045 |
| ν2 | CH sym. stretch | 3052 | 3043 | 3015 | 3024 | |
| ν3 | CH2 sym. stretch | 2949 | 2928 | 2845 | 2876 | |
| ν4 | Benzene C–C stretch | 1605 | 1599 | 1643 | 1679 | |
| ν5 | Benzene C–C stretch | 1466 | 1461 | 1491 | 1484 | |
| ν6 | CH2 deformation | 1444 | 1440 | 1402 | 1454 | |
| ν7 | Benzene C–C stretch4 | 1344 | 1345 | 1370 | 1360 | |
| ν8 | CH wag4 | 1195 | 1203 | 1167 | 1170 | |
| ν9 | CH2 wag4 | 1182 | 1189 | 1208 | 1209 | |
| ν10 | CH wag | 1152 | 1160 | 1113 | 1112 | |
| ν11 | Benzene C–C stretch | 1001 | 1009 | 892 | 843 | |
| ν12 | 4 mem. ring C–C stretch | 895 | 893 | 900 | 868 | |
| ν13 | 4 mem. ring C–C stretch4 | 765 | 782 | 740 | 713 | |
| ν14 | Benzene angle bend | 539 | 542 | 464 | 465 | |
| A2 | ν15 | CH2 antisym. stretch | 2966 | 2957 | … | 2893 |
| ν16 | CH2 twist | 1170 | 1169 | 1150 | 1169 | |
| ν17 | CH2 rock | 1054 | 1034 | 966 | 962 | |
| ν18 | CH out-of-plane wag | 993 | 981 | 656 | 635 | |
| ν19 | CH out-of-plane wag | 865 | 873 | 549 | 502 | |
| ν20 | Benzene ring twist | 670 | 710 | 440 | 450 | |
| ν21 | Benzene ring twist | 486 | 485 | 314 | 312 | |
| ν22 | Skeletal twist | 208 | 189 | 153 | 157 | |
| B1 | ν23 | CH stretch | 3066 | 3052 | … | 3039 |
| ν24 | CH stretch | 3031 | 3035 | … | 3012 | |
| ν25 | CH2 sym. stretch | 2942 | 2923 | … | 2866 | |
| ν26 | Benzene C–C stretch | 1596 | 1593 | 1500 | 1502 | |
| ν27 | Benzene C–C stretch | 1458 | 1455 | 1364 | 1366 | |
| ν28 | CH2 deformation | 1429 | 1427 | 1426 | 1438 | |
| ν29 | CH wag in-plane | 1282 | 1292 | 1266 | 1261 | |
| ν30 | CH2 wag | 1208 | 1218 | 1188 | 1174 | |
| ν31 | CH wag in-plane | 1132 | 1138 | 972 | 972 | |
| ν32 | 4 mem. ring C–C stretch4 | 1091 | 1089 | 1085 | 1083 | |
| ν33 | Benzene ring bend4 | 855 | 854 | 819 | 825 | |
| ν34 | Benzene ring bend | 634 | 637 | 564 | 567 | |
| ν35 | Skeletal deformation | 404 | 409 | 366 | 375 | |
| B2 | ν36 | CH2 antisym. stretch | 2975 | 2972 | … | 2911 |
| ν37 | CH2 twist | 1068 | 1079 | 1035 | 1038 | |
| ν38 | CH out-of-plane wag | 928 | 936 | 567 | 568 | |
| ν39 | CH2 rock4 | 781 | 785 | 493 | 483 | |
| ν40 | CH out-of-plane wag4 | 715 | 720 | 737 | 747 | |
| ν41 | Benzene ring bend | 386 | 397 | 245 | 250 | |
| ν42 | Ring flap | 209 | 213 | 105 | 109 | |
Reference 8.
The experimental frequencies given for vibrations of A2, B1, and B2 symmetry in the S1(π,π*) electronic excited state were determined from overtone or combination bands.
CASSCF(6,6)/aug-cc-pVTZ calculations. Scaling factor: 0.905.
Represent pairs of coupled vibrations.
Table 3.
Fluorescence excitation and UV spectra (cm−1) of BCB selected bands.
| FES1 | UV1 | Assignment | Inferred2 | Calculated3 | ||
|---|---|---|---|---|---|---|
| −276 | ms | −172 – 105 = −277 | ||||
| −262 | ms | −54 – 204 = −258 | ||||
| −209 | ms | −417 + 209 = −208 | ||||
| −183 | ms | 440 – 624 = −184 | ||||
| −172 | ms | 314 – 486 = −172 | ||||
| −154 | s | −54 – 105 = −159 | ||||
| −144 | s | 245 – 386 = −141 | ||||
| −114 | ms | 301 – 417 = −116 | ||||
| −105 | s | 105 – 209 = −104 | ||||
| −86 | m | 20 – 105 = −85 | ||||
| −76 | s | 464 – 539 = −75 | ||||
| −71 | mw | 564 – 634 = −70 | ||||
| −54 | mw | 153 – 208 = −55 | ||||
| −48 | mw | 440 – 486 = −46 | ||||
| −38 | s | 366 – 404 = −38 | ||||
| −28 | mw | −38 + 8 = −30 | ||||
| −18 | mw | 656 – 670 = −14 | ||||
| 0 | vs | 0 | vs | |||
| 8 | mw | 420 – 411 = 9 | ||||
| 20 | w | 737 – 715 = 22 | ||||
| 63 | mw | 549 – 486 = 63 | ||||
| 105 | ms | 209 – 105 = 104 | ||||
| 314 – 208 = 106 | ||||||
| 209 | ms | 209 | ms | 2 × 109 = 218 | ||
| 301 | w | 301 | w | 2 × 157 = 314 | ||
| 420 | vw | 420 | mw | 2 × 209 = 418 | ||
| 463 | mw | 464 | vw | 465 | ||
| 466 | mw | 467 | m | 314 + 153 = 467 | ||
| 485 | m | 484 | mw | 2 × 250 = 500 | ||
| 626 | vw | 626 | mw | 2 × 312 = 624 | ||
| 733 | ms | 731 | mw | 2 × 375 = 750 | ||
| 1364 – 634 = 730 | ||||||
| 740 | s | 740 | s | 713 | ||
| 875 | m | 875 | mw | 2 × 450 = 900 | ||
| 893 | ms | 892 | w | 843 | ||
| 900 | s | 900 | s | 868 | ||
| 940 | mw | 940 | vw | 731 + 209 = 940 | ||
| 949 | w | 949 | w | 464 + 485 = 949 | ||
| 740 + 209 = 949 | ||||||
| 977 | vw | 978 | w | 737 + 245 = 982 | ||
| 986 | vvw | 987 | w | 2 × 483 | ||
| 1094 | m | 1094 | ms | 2 × 502 = 1004 | ||
| 1113 | mw | 1113 | m | 1112 | ||
| 1130 | m | 1129 | w | 2 × 567 = 1134 | ||
| 1133 | ms | 1133 | m | 2 × 568 = 1136 | ||
| 1169 | w | 1167 | m | 1170 | ||
| 1208 | s | 1208 | s | 1209 | ||
| 1246 | vw | 2 × 626 = 1252 | ||||
| 1313 | mw | 1313 | w | 2 × 635 = 1270 | ||
| 1343 | mw | 1342 | vw | 1133 + 209 = 1342 | ||
| |
|
1363 |
mw |
|
900 + 464 = 1364 |
|
| 1370 | m | 1370 | mw | 1360 | ||
| 1404 | mw | 1402 | w | 1454 | ||
| 1457 | w | 1458 | vw | 2 × 731 = 1462 | ||
| 1470 | w | 1471 | w | 2 × 747 = 1494 | ||
| 740 + 731 = 1471 | ||||||
| 1477 | s | 1477 | ms | 2 × 740 = 1480 | ||
| 1493 | mw | 1491 | w | 1484 | ||
| 1517 | m | 1517 | w | 892 + 626 = 1518 | ||
| 1593 | vw | 1594 | vvw | 464 + 1129 = 1593 | ||
| 1597 | w | 1599 | vvw | 464 + 1133 = 1597 | ||
| 1623 | mw | 1623 | mw | 892 + 731 = 1623 | ||
| 1629 | vw | 892 + 740 = 1632 | ||||
| 1167 + 464 = 1631 | ||||||
| 1633 | w | 1633 | w | 2 × 825 = 1650 | ||
| 1640 | w | 1639 | vw | 900 + 740 = 1640 | ||
| 1644 | s | 1643 | s | 1679 | ||
| 1646 | w | 1646 | mw | 1085 + 564 = 1649 | ||
| 1765 | w | 892 + 875 = 1767 | ||||
| 1785 | w | 2 × 892 = 1784 | ||||
| 1793 | m | 892 + 900 = 1792 | ||||
| 1810 | mw | 2222 – 411 = 1811 | ||||
| 1833 | mw | 1370 + 464 = 1834 | ||||
| 740 +1094 = 1834 | ||||||
| 1871 | m | 740 + 1133 = 1873 | ||||
| 1904 | w | 1085 + 819 = 1904 | ||||
| 1910 | vw | 1167 + 740 = 1907 | ||||
| 1931 | w | 2 × 962 = 1924 | ||||
| 1943 | m | 2 × 972 = 1944 | ||||
| 1946 | m | 1208 + 740 = 1948 | ||||
| 1957 | vvw | 1491 + 464 = 1955 | ||||
| 2003 | w | 1113 + 892 = 2005 | ||||
| 2015 | vw | 1113 + 900 = 2013 | ||||
| 2031 | mw | −38 + 2069 = 2031 | ||||
| 2041 | m | 1113 + 927 = 2040 | ||||
| 2053 | vw | 972 + 1085 = 2057 | ||||
| 740 + 1313 = 2053 | ||||||
| 2069 | vw | 2 × 1038 = 2076 | ||||
| 2092 | vvw | 1633 + 464 = 2097 | ||||
| 2105 | mw | 1370 + 740 = 2110 | ||||
| 2116 | m | 2222 – 105 = 2117 | ||||
| 2153 | vvw | 1167 + 987 = 2154 | ||||
| 2222 | vw | 2 × 1113 = 2226 | ||||
| 1491 + 731 = 2222 | ||||||
| 2239 | w | 927 + 1313 = 2240 | ||||
| 1113 + 1129 = 2242 | ||||||
| 2256 | vw | 2 × 1129 = 2258 | ||||
| 2300 | vvw | 2 × 1169 = 2338 | ||||
| 2376 | vw | 2 × 1174 = 2378 | ||||
| 2384 | w | 1643 + 740 = 2383 | ||||
| 2411 | vvw | 464 + 1943 = 2407 | ||||
| |
|
2521 |
vvw |
|
|
2 × 1261 = 2522 |
| 2531 | w | 1402 + 1129 = 2531 | ||||
| 2605 | vvw | 1477 + 1129 = 2606 | ||||
| 2606 | vvw | 1477 + 1133 = 2610 | ||||
| 2679 | vw | 740 + 1943 = 2683 | ||||
| 2725 | vw | 2 × 1366 = 2732 | ||||
| 2845 | w | 2876 | ||||
| 2851 | w | 2 × 1438 = 2876 | ||||
| 2999 | w | 2 × 1502 = 3004 | ||||
| 3015 | vvw | 3024 | ||||
| 3068 | vvw | 3045 |
Relative to band at 37 093.6 cm−1; the relative intensities are indicated s-stong, m-medium, w-weak, v-very.
Inferred from other spectroscopic transitions (FES, IR, Raman, and ultraviolet).
CASSCF(6,6)/aug-cc-pVTZ calculations. Scaling factor: 0.905.
Figure 6.
Energy map for the low frequency vibrations of BCB in its S0 ground and S1(π,π*) excited states. The levels at 417 and 411 cm−1 are in Fermi resonance.
Table 4.
Skeletal vibrational frequencies (cm−1) of BCB and related molecules in the ground (S0) and excited (S1) state.
| S0 |
S1(π,π*) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCB | IND | TET | 14BZD | 13BZD | 14DHN | BCB | IND | TET | 14BZD | 13BZD | 14DHN | ||
| A1 | Benzene C–C stretch | 1605 | 1589 | 1581 | 1599 | 1587 | 1571 | 1646 | (1627) | (1640) | (1502) | (1608) | (1625) |
| Benzene C–C stretch | 1466 | 1474 | 1496 | 1500 | 1503 | 1497 | 1491 | (1493) | (1531) | (1498) | (1559) | (1518) | |
| Benzene C–C stretch | 1344 | 1318 | 1295 | 1307 | 1309 | 1288 | 1370 | (1409) | (1435) | (1391) | (1381) | 1337 | |
| Benzene C–C stretch | 1001 | 1025 | 1035 | 1028 | 1035 | 1041 | 892 | (934) | 971 | 994 | (1051) | 951 | |
| Benzene angle bend | 539 | 610 | 578 | 566 | 588 | 586 | 464 | 460 | 480 | 531 | 525 | 459 | |
| A2 | Benzene ring twist | 670 | (714)1 | 700 | 717 | 712 | 703 | 440 | 485 | 521 | (502) | 682 | (447) |
| Benzene ring twist | 486 | 499 | 501 | 553 | 536 | 510 | 314 | 345 | 330 | 363 | 374 | (360) | |
| Skeletal twist | 208 | 178 | 142 | 166 | 158 | 148 | 153 | 137 | 94 | 140 | 102 | 92 | |
| B1 | Benzene C–C stretch | 1596 | 1610 | 1598 | 1602 | 1614 | 1603 | 1500 | (1446) | (1449) | (1566) | (1665) | (1415) |
| Benzene C–C stretch | 1458 | 1460 | 1437 | 1462 | 1460 | 1460 | 1364 | (1394) | (1406) | (1437) | (1481) | (1406) | |
| Benzene ring bend | 855 | 832 | 804 | 834 | 853 | 885 | 819 | (821) | (764) | 888 | (750) | 867 | |
| Benzene ring bend | 634 | 579 | 586 | 643 | 698 | 765 | 564 | 535 | 545 | (530) | (574) | (707) | |
| B2 | Benzene ring bend | 386 | 412 | 436 | (466) | 430 | 427 | 245 | 273 | 270 | (370) | 255 | 298 |
| Ring flap | 209 | 248 | 257 | 297 | 274 | 231 | 105 | 176 | (140) | (119) | 194 | 115 | |
| Reference | 8 | 1 | 2 | 3, 4, 5 | 6 | 7 | This work | 1 | 2 | 3, 4, 5 | 6 | 7 | |
Frequencies in parentheses are calculated values.
There are a number of noteworthy features regarding the BCB vibrations. The molecule is rigidly planar in its S0 ground state but still has two very low-frequency out-of-plane ring modes, ν22 (A2) skeletal twisting between the two rings at 208 cm−1 and ν42 (B2) ring flapping at 209 cm−1. The near coincidence of these frequencies was demonstrated by observation of their infrared and Raman band types.8 From the UV spectrum in our present study we find that the overtone levels of these vibrations are in Fermi resonance and occur at 411 and 417 cm−1 with mixed 2ν22 and 2ν42 character. Aside from the Fermi interaction, as can be seen in Figure 6, both ν22 and ν42, as well as two other relatively low frequency vibrations ν41 (B2), the benzene ring out-of-plane bending, and ν21 (A2), the benzene ring twisting, are all nearly harmonic. This is quite different from the other bicyclic molecules under discussion where the external rings display highly anharmonic character. As can be seen in Table 4, the skeletal twisting frequency for BCB is the highest at 208 cm−1 whereas for the other five molecules it is in the 142–178 cm−1 range. On the other hand, the ring-flapping vibration, which is also a reflection of the rigidity of the molecule, has the lowest value of 209 cm−1 while the frequencies for the related molecules are between 231 and 297 cm−1. This is in part be due to coupling with the out-of-plane benzene ring bending of the same symmetry which in fact is lower for BCB (386 cm−1) than for the related molecules (412–466 cm−1). Of particular interest for us is what happens to the vibrational frequencies in the S1(π,π*) excited states of all these molecules. And, specifically, we are most interested in the low-frequency out-of-plane modes. Table 4 shows, as expected, that for the most part the benzene ring modes show modest frequency decreases in the S1(π,π*) states as the extent of π bonding is decreased. The out-of-plane modes, however, show the greatest changes. For BCB the skeletal twisting drops from 208 to 153 cm−1 while the related molecules show drops of 26–56 cm−1 for the same mode. The ring-flapping frequency of BCB drops even more dramatically from 209 to 105 cm−1. Similarly, large frequency drops of 72–178 cm−1 occur for the other bicyclic molecules. The out-of-plane benzene ring modes which are ν20 (A2), ν21 (A2), and ν41 (B2) for BCB also show sizable frequency differences between the S0 and S1(π,π*) states due to the decrease in ring rigidity resulting from the π → π* transition. For BCB the frequency decreases are 670–440 cm−1, 486–314 cm−1, and 386–245 cm−1, respectively. The other bicyclic systems show similar decreases.
CONCLUSIONS
Ab initio computations have been carried out to calculate the structure of benzocyclobutane in its S1(π,π*) excited state and to compare it with the S0 ground state. The results were compared to similar computational results for the ground and excited states of five related molecules. The ab initio calculations were also helpful in assigning the fluorescence excitation and ultraviolet absorption spectra. The majority of the vibrational frequencies, including all of the low-frequency modes, were assigned for the excited state and compared to the ground state data. Of special interest were the large frequency decreases of the out-of-plane skeletal and benzene ring modes in the excited state reflecting the decreased molecular rigidity resulting from a decrease in the π bonding.
ACKNOWLEDGMENTS
J.L. wishes to thank the Robert A. Welch Foundation (Grant No. A-0396) for financial support. S.K. received funding from the Intramural Research Program of the National Institutes of Health (NIH), National Library of Medicine (NLM). Computations by J.L. and E.J.O. were carried out on the Texas A&M University Department of Chemistry Medusa computer system funded by the National Science Foundation, Grant No. CHE-0541587 and S.K. utilized the Biowulf Linux cluster at the NIH, Bethesda, MD (http://biowulf.nih.gov). The Laboratory for Molecular Simulation provided the Semichem AMPAC/AGUI software used by J.L. and E.J.O.
References
- Arp A., Meinander N., Choo J., and Laane J., J. Chem. Phys. 116, 6648 (2002). 10.1063/1.1458926 [DOI] [Google Scholar]
- Yang J., Wagner M., and Laane J., J. Phys. Chem. A 111, 8429 (2007). 10.1021/jp073752p [DOI] [PubMed] [Google Scholar]
- Yang J., Choo J., Kwon O., and Laane J., Spectrochim. Acta A 68, 1170 (2007). 10.1016/j.saa.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Yang J., Wagner M., and Laane J., J. Phys. Chem. A 110, 9805 (2006). 10.1021/jp062474b [DOI] [PubMed] [Google Scholar]
- Autrey D., Yang J., and Laane J., J. Mol. Struct. 661–662, 23 (2003). 10.1016/j.molstruc.2003.07.015 [DOI] [Google Scholar]
- McCann K., Wagner M., Guerra A., Coronado P., Villareal J. R., Choo J., Kim S., and Laane J., J. Chem. Phys. 131, 044302-1–044302-9 (2009). 10.1063/1.3169504 [DOI] [PubMed] [Google Scholar]
- Rishard M., Wagner M., Choo J., and Laane J., J. Phys. Chem. A 113, 7753 (2009). 10.1021/jp902672x [DOI] [PubMed] [Google Scholar]
- Ocola E. J., Shin H. W., and Laane J., “Infrared and Raman spectra and theoretical calculations for benzocyclobutane in its electronic ground state,” Spectrochim. Acta (to be published). [DOI] [PubMed]
- Morris K., Ph.D. thesis, Texas A&M University, 1998. [Google Scholar]
- Arp Z., Ph.D. thesis, Texas A&M University, 2001. [Google Scholar]
- Laane J., J. Phys. Chem. A 104, 7715 (2000). 10.1021/jp0009002 [DOI] [Google Scholar]
- Sakurai S., Meinander N., Morris K., and Laane J., J. Am. Chem. Soc. 121, 5056 (1999). 10.1021/ja9844433 [DOI] [Google Scholar]
- Chiang W.-Y., and Laane J., J. Chem. Phys. 100, 8755 (1994) and references therein. 10.1063/1.466730 [DOI] [Google Scholar]
- Cheatham C. M., Huang M.-H., and Laane J., J. Mol. Struct. 377, 93 (1996). 10.1016/0022-2860(95)09114-9 [DOI] [Google Scholar]
- Cheatham C. M., Huang M.-H., Meinander N., Kelly M. B., Haller K., Chiang W.-Y., and Laane J., J. Mol. Struct. 377, 81 (1996). 10.1016/0022-2860(95)09113-0 [DOI] [Google Scholar]
- Laane J., Zhang J., Chiang W.-Y., Sagear P., and Cheatham C. M., “Jet-cooled fluorescence excitation spectra and carbonyl wagging potential energy functions of cyclic ketones in their electronic excited states,” in Topics in Molecular Organization and Engineering, Vol. 12: Structure and Dynamics of Non-Rigid Molecular Systems, edited by Smeyers Y. G. (Kluwer Academic Publishers, Dordrecht, Netherlands, 1995), pp. 181–201. [Google Scholar]
- Schmidt M. W., Baldridge K. K., Boatz J. A., Elbert S. T., Gordon M. S., Jensen J. H., Koseki S., Matsunaga N., Nguyen K. A., Su S., Windus T. L., Dupuis M., and Montgomery J. A., J. Comput. Chem. 14, 1347 (1993). 10.1002/jcc.540141112 [DOI] [Google Scholar]
- Gordon M. S. and Schmidt M. W., “Advances in electronic structure theory: GAMESS a decade later,” in Theory and Applications of Computational Chemistry: The First Forty Years, edited by Dykstra C. E., Frenking G., Kim K. S., and Scuseria G. E. (Elsevier Publishing, Amsterdam, Netherlands, 2005), Chap. 41, pp. 1167–1189. [Google Scholar]
- Boopalachandran P., Kim S., Choo J., and Laane J., Chem. Phys. Lett. 514, 214 (2011). 10.1016/j.cplett.2011.08.054 [DOI] [Google Scholar]
- Bode B. M. and Gordon M. S., J. Mol. Graphics Mod. 16, 133 (1998). 10.1016/S1093-3263(99)00002-9 [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B.et al. , Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009.
- Autrey D., Choo J., and Laane J., J. Phys. Chem. A. 105, 10230 (2001). 10.1021/jp012170j [DOI] [Google Scholar]
- Ocola E. J., Bauman L. E., and Laane J., J. Phys. Chem. A 115, 6531 (2011). 10.1021/jp2032934 [DOI] [PubMed] [Google Scholar]
- Ocola E. J., Al-Saadi A. A., Mlynek C., Henning H., and Laane J., J. Phys. Chem. A 114, 7457 (2010). 10.1021/jp103406c [DOI] [PubMed] [Google Scholar]
- Ocola E. J., Brito T., McCann K., and Laane J., J. Mol. Struct. 978, 74 (2010). 10.1016/j.molstruc.2009.11.026 [DOI] [Google Scholar]
- Autrey D., Arp Z., Choo J., and Laane J., J. Chem. Phys. 119, 2557 (2003). 10.1063/1.1586697 [DOI] [Google Scholar]
- See http://www.semichem.com for the information about AGUI program from Semichem, Inc., Shawnee, KS 66216.
- See supplementary material at http://dx.doi.org/10.1063/1.4858412 for fluorescence excitation and UV transition frequencies (cm−1) of BCB.