Abstract
In this paper we unveil a new sensing strategy for sensitive and selective detection of Hg2+ through surface-enhanced Raman scattering (SERS) using Ag2Te nanoparticles (NPs) as a substrate and recognition element and rhodamine 6G (R6G) as a reporter. Ag2Te NPs prepared from tellurium dioxide and silver nitrate and hydrazine in aqueous solution containing sodium dodecyl sulfate at 90°C with an average size of 26.8 ± 4.1 nm (100 counts) have strong SERS activity. The Ag2Te substrate provides strong SERS signals of R6G with an enhancement factor of 3.6 × 105 at 1360 cm−1, which is comparable to Ag NPs. After interaction of Ag2Te NPs with Hg2+, some HgTe NPs are formed, leading to decreases in the SERS signal of R6G, mainly because HgTe NPs relative to Ag2Te NPs have weaker SERS activity. Under optimum conditions, this SERS approach using Ag2Te as substrates is selective for the detection of Hg2+, with a limit of detection of 3 nM and linearity over 10–150 nM. The practicality of this approach has been validated for the determination of the concentrations of spiked Hg2+ in a pond water sample.
Keywords: surface-enhanced Raman scattering, nanoparticles, silver telluride, mercury telluride, mercury ions
Introduction
Monitoring the level of mercury ions in ecological systems is an extremely important issue, mainly because they are highly toxic, non-biodegradable, and bioaccumulated (Zahir et al., 2005; Clarkson et al., 2008). Several techniques such as atomic absorption/emission spectrometry, atomic fluorescence spectrometry, inductively coupled plasma spectrometry (ICP-MS) have been applied to detect Hg2+ in environmental and biological samples (Leermakers et al., 2005; Butler et al., 2006; Li et al., 2006). Among them, ICP-MS provides the highest sensitivity and a widest linear range, but the system is expensive because it requires expensive noble gas. In addition, it is not easy for the in-field analysis.
With advantages of simplicity, low cost, sensitivity, and selectivity, a number of optical and electrochemical sensors have been demonstrated for the detection of Hg2+ (Huang and Chang, 2007; Darbha et al., 2008; Jena and Raj, 2008; Zhu et al., 2009). Having extremely high stability, specificity, and ease in preparation, DNA-based optical sensors have become popular for the detection of Hg2+ (Chiang et al., 2008; Stewart et al., 2008; Dave et al., 2010). Polythymines (Tn) that are specific toward Hg2+ ions through T–Hg2+–T coordination have been used for the selective and sensitive detection of Hg2+ ions through fluorescence detection based on the analyte induced changes in the DNA conformation, leading to enhanced efficiency in the fluorescence resonance energy transfer between the donor and the acceptor or increased quantum yield of the fluorophore. In addition, Tn conjugated with gold nanoparticles (NPs) have been used for the detection of Hg2+ ions through absorption or fluorescence modes (Huang et al., 2007; Lee et al., 2007; Wang et al., 2008; Xue et al., 2008). The detections are mainly based on the analyte induced changes in the DNA conformation and charge density on the surfaces of the Au NPs, leading to changes in absorbance (red shift upon aggregation) or fluorescence intensity (either decreases or increases). Surface-enhanced Raman scattering (SERS) using Ag NPs conjugated with Tn and organic dyes (reporter) are alternative for the sensitive detection of Hg2+ (Wang et al., 2009, 2011). Upon interaction with Hg2+, the DNA conformation changes, leading to changes in the distance of the reporter from the surfaces of Ag NPs and thus changes in the SERS signal (Grubisha et al., 2003; Doering et al., 2007). Although these sensing systems are sensitive and suitable for in-field analysis, the DNA is expensive and their sensitivity and selectivity are highly dependent on the ionic strength of the samples, limiting their wide practicality.
Relative to normal Raman scattering, SERS can provide enhancement factors (EFs) up to 1015 theoretically through a long-range electromagnetic (EM) effect such as “hot spots,” and/or chemical effect due to the charge-transfer excitation of chemisorbed molecules (Aravind et al., 1981; Kneipp et al., 1997; Nie and Emery, 1997). The EF values are dependent on the compositions, sizes, and shapes of the SERS substrates. Relative to spherical shaped silver NPs, silver plates and silver nanowires provides higher EF values; EF values up to 108 have been demonstrated for the SERS signals of common reporters such as Rhodamine 6G (R6G), 4-mercaptobenzoic acid, and 5,5′-dithiobis(2-nitrobenzoic acid) (Tao et al., 2003; Yang et al., 2007). Gold-tellurium nanodumbbells, gold-tellurium nanopeapods, and gold pearl-necklace nanomaterials (Au PNNs) providing EF values of R6G up to 5.6 × 109 have been used for the selective detection of human serum albumin down to 70 pM using AB 580 as a reporter (Lin and Chang, 2008; Lin et al., 2011).
In this study, we developed a simple SERS approach using silver telluride (Ag2Te) NPs as substrates for sensitive and selective detection of Hg2+ in aqueous solutions. Ag2Te NPs were prepared from tellurium dioxide and silver nitrate in the presence of hydrazine and sodium dodecyl sulfate (Samal and Pradeep, 2009). The Ag2Te NPs provided SERS enhancement effect of R6G. Upon increasing the concentration of Hg2+ ions, the SERS signal of R6G decreased. The novel SERS approach was further validated by the determination of the concentrations of Hg2+ in pond water samples, showing advantages of sensitivity, selectivity, and simplicity.
Materials and methods
Chemicals
Hydrazine monohydrate (80%) and tellurium dioxide powder (99.9%) were purchased from SHOWA (Tokyo, Japan). Sodium phosphate monobasic, dibasic, and tribasic, and sodium dodecyl sulfate were purchased from Acros (Geel, Belgium). Mercury chloride, R6G, silver nitrate, and other metal salts [Ca2+, Co2+, Cu2+, Cd2+, K+, Mg2+, Mn2+, Ni2+, Cr3+, Fe3+, Fe2+, Pd2+, Zn2+, Pb2+, and Na+ (chlorides)] used in this study were purchased from Sigma Aldrich (Missouri, USA). Ultrapure water was obtained using a Milli-Q ultrapure (18.2 MΩ-cm) system.
Synthesis of Ag2Te NPs
Hydrazine (1 mL) was added slowly to a sample vial containing aqueous solution (9 mL) of tellurium dioxide (5 mM), silver nitrate (10 mM), and sodium dodecyl sulfate (30 mM). The mixture was then subjected to constant magnetic stirring at 90°C. The solution changed color from colorless to dark brown after 8 h, indicating the formation of Ag2Te NPs. To terminate the reaction and to remove most of the matrix (e.g., hydrazine), the Ag2Te NPs were subjected to three cycles of centrifugation [relative centrifugation force (RCF): 12000 g for 10 min] and wash (3 × 10 mL of water). For simplicity, the concentration of the as-prepared Ag2Te NPs in 10 mL H2O is represented as 1 X.
Characterization
JEOL JSM-1230 and FEI Tecnai-G2-F20 transmission electron microscopes (TEM) were used to measure the sizes and shapes of the as-prepared Ag2Te NPs. The re-dispersed Ag2Te NPs were separately placed on formvar/carbon film Cu grids (200 mesh; Agar Scientific) and dried at ambient temperature (25°C). An energy dispersive X-ray (EDAX) system (Inca Energy 200, Oxford) was used to determine the composition of the as-prepared NMs. Raman spectra were recorded using a Raman spectrometer (DongWoo 500i, Korea) equipped with a 50× objective and a charge-coupled detector. The excitation wavelength was 532 nm and the spectral aperture was 50 μm. The signal collection time for each sample was 30 s.
Detection of Hg2+ ions using Ag2Te NPs
Ag2Te NPs (0.01 ×, 100 μL), phosphate buffer (PB) (1 mM, pH 4.0, 100 μ L), and SDS (0.1 mM, 100 μ L) were added to aqueous solutions (0.7 mL) containing various concentrations of HgCl2 (final concentrations 10–150 nM). The mixtures were equilibrated under constant stirring at 37°C for 10 min. After centrifugation at 12000 g for 10 min, the supernatants were discarded and the pellets were dispersed in R6G solution (10 μ M, 20 μ L). Finally, drops (1 μ L) of the R6G mixtures were added onto separate silica wafers and dried at ambient temperature (25°C) prior to SERS measurement.
Analysis of real sample
Pond water sample was collected from the National Taiwan University campus, and subsequently filtered through a 0.45 μm membrane. For comparison, aliquots (0.1 mL) of the pond water sample was mixed with HNO3 (0.9 mL, final concentration 2%) prior to ICP-MS analysis. Aliquots of the pond water (100 μ L) were spiked with standard solutions (100 μ L) containing Hg2+ at various concentrations (0.3–1.5 μM). Next, PB (1 mM, pH 4.0, 100 μ L), SDS (0.1 mM, 100 μ L), Ag2Te NPs (0.01 ×, 100 μ L), and water (500 μ L) were added to the mixture to give final volumes of 1 mL. The mixtures were equilibrated under constant stirring at 37°C for 10 min. After centrifugation at 12000 g for 10 min, the supernatants were discarded and the pellets were dispersed in R6G solution (10 μ M, 20 μ L). Finally, drops of solutions (1 μ L) were added onto separate silica wafers and dried at ambient temperature prior to SERS measurement.
Results and discussion
Sensing strategy
Scheme 1 shows the detection of Hg2+ based on differential SERS EFs of Ag2Te and HgTe NPs. Ag2Te relative to HgTe provides a higher EF value. The displacement reaction between Hg2+ and Ag2Te NPs leads to the formation of HgTe nanostructures and decomposition of Ag2Te NPs. As a result, the SERS signal of R6G decreases upon increasing the concentration of Hg2+, mainly because Ag2Te relative to HgTe NPs provides a greater SERS EF value. Although the Ksp values of Ag2Te and HgTe are unavailable, the latter has a small one based on that of the metal selenides (Moon et al., 2010). For example, Ag2Se relative to HgSe has a higher Ksp value (1 × 10−54 vs. 4 × 10−59) (Wang et al., 2007). It has been reported that the reaction of Ag2Te nanostructures with Hg2+ions is spontaneous and fast (Samal and Pradeep, 2011).
Scheme 1.
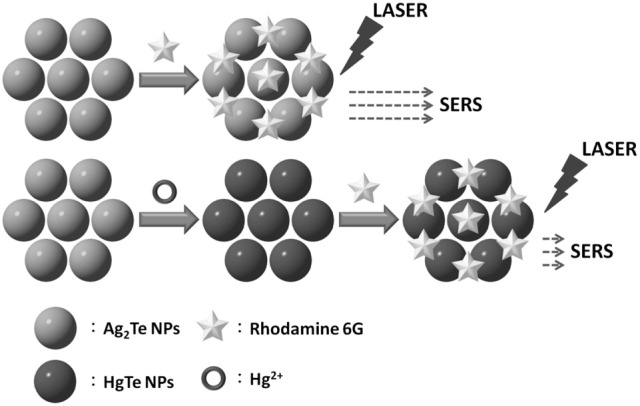
Schematic representation of the sensing of Hg2+ through SERS using Ag2Te NPs as a substrate and recognition element.
To confirm the formation of HgTe NPs from the reaction of Ag2Te NPs with Hg2+, we conducted TEM, EDX, and XRD measurements. Figure 1A shows the TEM image of as-prepared Ag2Te NPs with an average size of 26.8 ± 4.1 nm (100 counts). On the other hand, the TEM image displayed in Figure 1B shows HgTe NPs with an average size of 37.7 ± 6.8 nm. Different morphologies in the two TEM images reveal the changes in the Ag2Te NPs. The EDX patterns (Figures 1C,D) confirm the displacement reaction between Hg2+ and Ag2Te NPs. The XRD patterns of Ag2Te NPs (Figure 1E) and its reaction product (Figure 1F) with Hg2+ agree with the literature data of Ag2Te (JCPDS: 34-0142) and HgTe (JCPDS: 75-2084), respectively.
Figure 1.
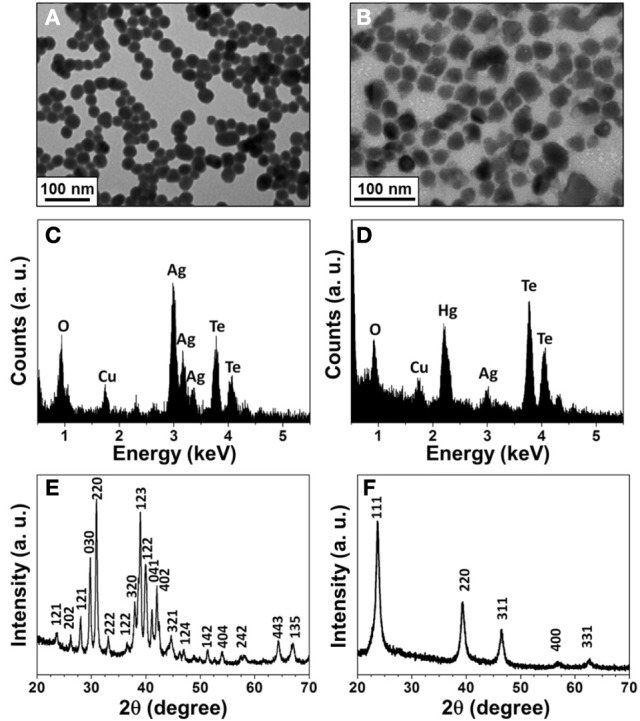
Characterization of the synthesized Ag2Te and HgTe NPs. (A) and (B) TEM images, (C) and (D) EDAX spectra, (E) and (F) XRD spectra of Ag2Te and HgTe NPs, respectively. The concentrations of Ag2Te and Hg2+ ions are 1 × and 5 mM, respectively.
The EF value of Ag2Te NPs (0.1 X) was investigated using R6G as a reporter. The limits of detection (LODs) at a signal-to-noise (S/N) 3 for R6G using silica wafers and Ag2Te NPs as SERS substrates were 1.8 mM and 5 nM, respectively, which reveals that the Ag2Te substrate provided an EF value of 3.6 × 105. The EF value of Ag2Te NPs is comparable with that of Ag NPs (Wang et al., 1980; Kerker, 1987). The SERS signal of R6G on the Ag2Te substrate was about 5-fold greater than that on an HgTe substrate that had been formed from the reaction of Ag2Te NPs with 100 nM Hg2+ ions. The decreased SERS signal of R6G at a constant concentration (10 μ M) is related to the concentration of Hg2+ ions (to be discussed later), revealing the potential use of this approach for the determination of the concentration of Hg2+ ions. The decrease in SERS signal was due to the decreased amount of Ag2Te NPs.
Optimization of detection conditions
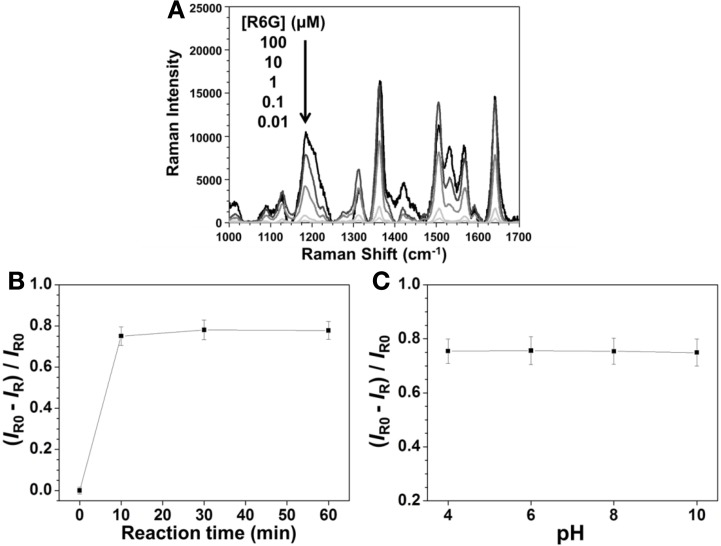
In order to optimize the sensing condition, we investigated several important parameters, including concentration of R6G, reaction time, and pH. The strongest SERS signal of R6G at 1361 cm−1 (aromatic C-C stretching) was used to evaluate the effects of these parameters. Figure 2A shows that the SERS signal of R6G at 1361 cm−1 increased upon increasing its concentration, with a saturated concentration of 10 μ M. Figure 2B displays the reaction was completed within 10 min when using Hg2+ at the concentration of 100 nM. Figure 2C displays that pH is not an important factor over the investigated pH range (4.0–10.0), mainly because the replacement reaction between Ag2Te and Hg2+ ions and the SERS signal of R6G are both not pH sensitive. Although larger Ag2Te NPs provided greater SERS EF values, poor reproducibility of SERS signals due to their instability in aqueous solution is problematic.
Figure 2.
Effects of (A) R6G concentration, (B) reaction times, and (C) pH on the SERS signal of R6G at 1361 cm−1 when using Ag2Te NPs as a substrate. The concentrations of Ag2Te NPs were both 0.001 × in (A) and (B), of Hg2+ were 0 in (A) and 100 nM in (B) and (C), and of R6G were both 10 μM in (B) and (C). The pH values were both 4.0 in (A) and (B) and reaction times were both 10 min in (A) and (C). IR0 and IR are the SERS intensities of R6G at 1361 cm−1 in the absence and presence of Hg2+ (100 nM), respectively, in (B) and (C).
Sensitivity and selectivity of Hg2+ detection
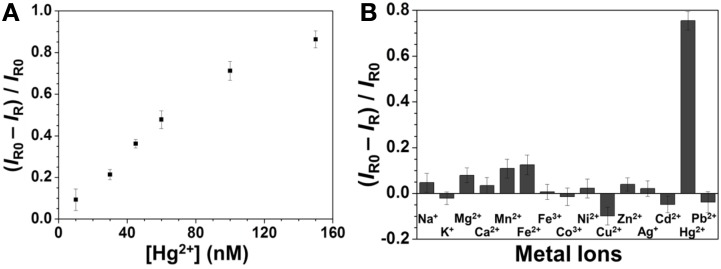
Figure 3A shows that the SERS signal of R6G decreased upon increasing the Hg2+ concentration, with a linear relationship between the SERS ratios ((IR0—IR)/IR0) at 1361 cm−1 and the Hg2+ concentration ranging from 10 to 150 nM (R2 = 0.98). This approach provided an LOD at an S/N = 3 of 3 nM for Hg2+ ions. The sensitivity is better than that provided by SERS approaches using different reporters (Zamarion et al., 2008; Han et al., 2010; Senapati et al., 2011; Luo et al., 2012). Control experiments were carried out to test the specificity of the developed approach for Hg2+ ions (100 nM) under optimal conditions in the presence of various metal ions (each at a concentration of 1 μM). The results displayed in Figure 3B reveal that the sensing approach is selective to Hg2+ ions. The potential interferences could not replace Ag+ ions from the Ag2Te NPs, resulting in negligible changes in the SERS signal of R6G at 1361 cm−1.
Figure 3.
(A) Sensitivity and (B) selectivity of the SERS approach for Hg2+. Concentrations: 100 nM for Hg2+ and 1 μM for the other metal ions. Reaction time was 10 min and pH value was 4.0. The other conditions are the same as in Figure 2.
Real sample analysis
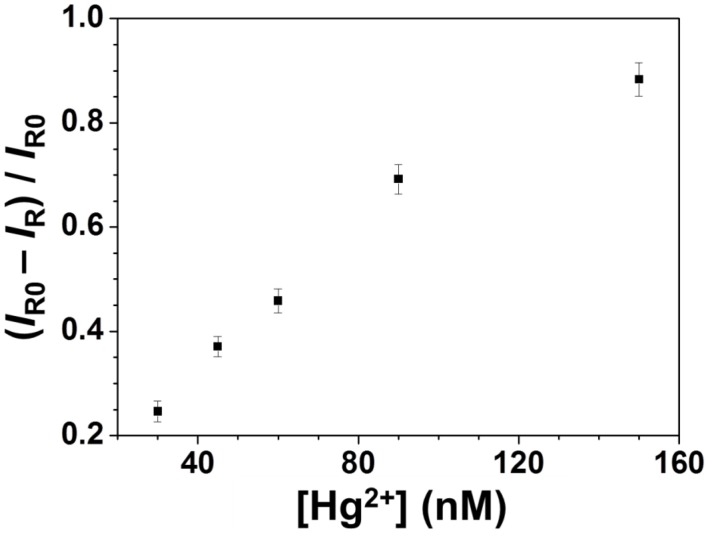
To examine the practicality of our approach, the concentration of Hg2+ in a pond water sample was determined. Our ICP-MS result showed that no Hg2+ was detected. By applying a standard addition method, the Raman ratios against the concentration of Hg2+ was found to be linear (R2 = 0.98) over 30–150 nM (Figure 4). The recovery percentage values of Hg2+ over the concentration range (30–150 nM) were 96–103%, showing low matrix interference. Our results reveal that this approach holds great potential for the determination of the concentrations of Hg2+ in environmental samples.
Figure 4.
Detection of Hg2+ in a spiked pond water sample through SERS using Ag2Te NPs as a substrate. Concentrations of Hg2+ ions ranged from 30 to 150 nM.
Conclusions
We have demonstrated a SERS-based approach for the detection of Hg2+ ions using Ag2Te NPs as a substrate and recognition element and R6G as a reporter. To the best of our knowledge, this is the first example using a single material (Ag2Te) as the substrate and recognition element in SERS technology. Relative to Ag2Te, HgTe is a less SERS active substrate, thus the SERS signals of R6G decreased upon increasing Hg2+ concentration when using Ag2Te NPs as a substrate. This novel approach is sensitive (LOD 3 nM) and selective for the detection of Hg2+ ions over a wide pH range. With its high sensitivity, selectivity, and simplicity, the SERS-based approach holds great potential for the determination of the concentrations of Hg2+ in environmental samples.
Author contributions
Chia-Wei Wang: He did the characterization of Ag2Te nanoparticles and the detection of Hg2+. Zong-Hong Lin: He developed the method to synthesize Ag2Te nanoparticles and designed the sensing strategy. Prathik Roy: He gave some advice about synthesis of Ag2Te nanoparticles and sensing strategy of Hg2+. Huan-Tsung Chang: He is the advisor of this group.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Science Council of Taiwan under contracts NSC 101-2113-M-002-002-MY3.
References
- Aravind P. K., Nitzan A., Metiu H. (1981). The interaction between electromagnetic resonances and its role in spectroscopic studies of molecules adsorbed on colloidal particles or metal spheres. Surf. Sci. 110, 189–204 10.1016/0039-6028(81)90595-1 [DOI] [Google Scholar]
- Butler O. T., Cook J. M., Harrington C. F., Hills S. J., Rieuwerts J., Miles D. L. (2006). Atomic spectrometry update. Environmental analysis. J. Anal. At. Spectrom. 21, 217–243 10.1039/B516025C9839683 [DOI] [Google Scholar]
- Chiang C.-K., Huang C.-C., Liu C.-W., Chang H. T. (2008). Oligonucleotide-based fluorescence probe for sensitive and selective detection of mercury(II) in aqueous solution. Anal. Chem. 80, 3716–3721 10.1021/ac800142k [DOI] [PubMed] [Google Scholar]
- Clarkson T. W., Magos L., Myers G. J. (2008). The toxicology of mercury-current exposures and clinical manifestations. N. Engl. J. Med. 349, 1731–1737 10.1056/NEJMra022471 [DOI] [PubMed] [Google Scholar]
- Darbha G. K., Singh A. K., Rai U. S., Yu E., Yu H., Ray P. C. (2008). Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J. Am. Chem. Soc. 130, 8038–8043 10.1021/ja801412b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave N., Chan M. Y., Huang P.-J. J., Smith B. D., Liu J. (2010). Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J. Am. Chem. Soc. 132, 12668–12673 10.1021/ja106098j [DOI] [PubMed] [Google Scholar]
- Doering W. E., Piotti M. E., Natan M. J., Freeman R. G. (2007). SERS as a foundation for nanoscale, optically detected biological labels. Adv. Mater. 19, 3100–3108 10.1002/adma.200701984 [DOI] [Google Scholar]
- Grubisha D. S., Lipert R. J., Park H.-Y., Driskell J., Porter M. D. (2003). Femtomolar detection of prostate-specific antigen: an immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal. Chem. 75, 5936–5943 10.1021/ac034356f [DOI] [PubMed] [Google Scholar]
- Han D., Lim S. Y., Kim B. J., Piao L., Chung T. D. (2010). Mercury(II) detection by SERS based on a single gold microshell. Chem. Commun. 46, 5587–5589 10.1039/C0CC00895H [DOI] [PubMed] [Google Scholar]
- Huang C.-C., Chang H. T. (2007). Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem. Commun. 1215–1217 10.1039/B615383F [DOI] [PubMed] [Google Scholar]
- Huang C.-C., Yang Z., Lee K.-H., Chang H.-T. (2007). Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew. Chem. Int. Ed. 46, 6824–6828 10.1002/anie.200700803 [DOI] [PubMed] [Google Scholar]
- Jena B. K., Raj C. R. (2008). Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 80, 4836–4844 10.1021/ac071064w [DOI] [PubMed] [Google Scholar]
- Kerker M. (1987). Estimation of surface-enhanced Raman scattering from surface-averaged electromagnetic intensities. J. Colloid Interface Sci. 118, 417–421 10.1016/0021-9797(87)90477-2 [DOI] [Google Scholar]
- Kneipp K., Wang Y., Kneipp H., Perelman L. T., Itzkan I., Dasari R. R., et al. (1997). Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 78, 1667–1670 10.1103/PhysRevLett.78.1667 [DOI] [Google Scholar]
- Lee J.-S., Han M. S., Mirkin C. A. (2007). Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem. Int. Ed. 46, 4093–4096 10.1002/anie.200700269 [DOI] [PubMed] [Google Scholar]
- Leermakers M., Baeyens W., Quevauviller P., Horvat M. (2005). Mercury in environmental samples: speciation, artifacts and validation. Trends Anal. Chem. 24, 383–393 10.1016/j.trac.2004.01.001 [DOI] [Google Scholar]
- Li Y., Chen C., Li B., Sun J., Wang J., Gao Y., et al. (2006). Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. J. Anal. At. Spectrom. 21, 94–96 10.1039/B511367A [DOI] [Google Scholar]
- Lin Z.-H., Chang H.-T. (2008). Preparation of gold-tellurium hybrid nanomaterials for surface-enhanced Raman spectroscopy. Langmuir 24, 365–367 10.1021/la702543k [DOI] [PubMed] [Google Scholar]
- Lin Z.-H., Chen I.-C., Chang H.-T. (2011). Detection of human serum albumin through surface-enhanced Raman scattering using gold “pearl necklace” nanomaterials as substrates. Chem. Commun. 47, 7116–7118 10.1039/C1CC11818H [DOI] [PubMed] [Google Scholar]
- Luo Y., Li K., Wen G., Liu Q., Liang A., Jiang Z. (2012). A rapid surface-enhanced Raman scattering method for the determination of trace Hg2+ using rhodamine 6G-aggregated nanosilver as Probe. Plasmonics 7, 461–468 10.1007/s11468-012-9329-522967639 [DOI] [Google Scholar]
- Moon G. D., Ko S., Xia Y., Jeong U. (2010). Chemical transformations in ultrathin chalcogenide nanowires. ACS Nano 4, 2307–2319 10.1021/nn9018575 [DOI] [PubMed] [Google Scholar]
- Nie S., Emery S. R. (1997). Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 10.1126/science.275.5303.1102 [DOI] [PubMed] [Google Scholar]
- Samal A. K., Pradeep T. (2009). Room-temperature chemical synthesis of silver telluride nanowires. J. Phys. Chem. C 113, 13539–13544 10.1021/jp901953f [DOI] [Google Scholar]
- Samal A. K., Pradeep T. (2011). Hybrid A–B–A type nanowires through cation exchange. Nanoscale 3, 4840–4847 10.1039/C1NR11124H [DOI] [PubMed] [Google Scholar]
- Senapati T., Senapati D., Singh A. K., Fan Z., Kanchanapally R., Ray P. C. (2011). Highly selective SERS probe for Hg(II) detection using tryptophan-protected popcorn shaped gold nanoparticles. Chem. Commun. 47, 10326–10328 10.1039/C1CC13157E [DOI] [PubMed] [Google Scholar]
- Stewart M. E., Anderton C. R., Thompson L. B., Maria J., Gray S. K., Rogers J. A., et al. (2008). Nanostructured plasmonic sensors. Chem. Rev. 108, 494–521 10.1021/cr068126n [DOI] [PubMed] [Google Scholar]
- Tao A., Kim F., Hess C., Goldberger J., He R., Sun Y., et al. (2003). Langmuir-Blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Nano Lett. 3, 1229–1233 10.1021/nl0344209 [DOI] [Google Scholar]
- Wang D.-S., Chew H., Kerker M. (1980). Enhanced Raman scattering at the surface (SERS) of a spherical particle. Appl. Opt. 19, 2256–2257 10.1364/AO.19.002256 [DOI] [PubMed] [Google Scholar]
- Wang G., Lim C., Chen L., Chon H., Choo J., Hong J., et al. (2009). Surface-enhanced Raman scattering in nanoliter droplets: towards high-sensitivity detection of mercury (II) ions. Anal. Bioanal. Chem. 39, 1827–1832 10.1007/s00216-009-2832-7 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang Y., Jin J., Yang R. (2008). Gold nanoparticle-based colorimetric and “turn-on” fluorescent probe for mercury(II) ions in aqueous solution. Anal. Chem. 80, 9021–9028 10.1021/ac801382k [DOI] [PubMed] [Google Scholar]
- Wang X., Peng Q., Li Y. (2007). Interface-mediated growth of monodispersed nanostructures. Acc. Chem. Res. 40, 635–643 10.1021/ar600007y [DOI] [PubMed] [Google Scholar]
- Wang X., Shen Y., Xie A., Li S., Cai Y., Wang Y., et al. (2011). Assembly of dandelion-like Au/PANI nanocomposites and their application as SERS nanosensors. Biosen. Bioelectron. 26, 3063–3067 10.1016/j.bios.2010.11.044 [DOI] [PubMed] [Google Scholar]
- Xue X., Wang F., Liu X. (2008). One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 130, 3244–3245 10.1021/ja076716c [DOI] [PubMed] [Google Scholar]
- Yang Y., Shi J., Tanaka T., Nogami M. (2007). Self-assembled silver nanochains for surface-enhanced Raman scattering. Langmuir 23, 12042–12047 10.1021/la701610s [DOI] [PubMed] [Google Scholar]
- Zahir F., Rizwi S. J., Haq S. K., Khan R. H. (2005). Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 20, 351–360 10.1016/j.etap.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Zamarion V. M., Timm R. A., Araki K., Toma H. E. (2008). Ultrasensitive SERS nanoprobes for hazardous metal ions based on trimercaptotriazine-modified gold nanoparticles. Inorg. Chem. 47, 2934–2936 10.1021/ic800122v [DOI] [PubMed] [Google Scholar]
- Zhu Z., Su Y., Li J., Li D., Zhang J., Song S., et al. (2009). Highly sensitive electrochemical sensor for mercury(II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based amplification. Anal. Chem. 81, 7660–7666 10.1021/ac9010809 [DOI] [PubMed] [Google Scholar]