Abstract
Objectives
The Health Information Technology for Economic and Clinical Health (HITECH) Act encourages the meaningful use of certified electronic health record technology. A HITECH-compliant core component is nationwide electronic laboratory reporting (ELR) implementation for communicable disease surveillance. In Oklahoma, laboratories with ≥400 positive tests/year for reportable diseases must use ELR. Of 18 such laboratories, two have adopted ELR. We compared completeness and timeliness of ELR reports from these two laboratories with conventional reports from all other Oklahoma laboratories.
Methods
We retrospectively reviewed confirmed reportable disease cases for January 1–December 31, 2011, excluding tuberculosis, hepatitis, sexually transmitted infections, diseases without laboratory diagnoses, and immediately reportable diseases. Probable reportable tickborne disease cases were included. We compared ELR with conventional reporting (i.e., mail, fax, telephone, and Internet). We assessed data completeness based on eight demographic and two laboratory fields in each disease report and timeliness by percentage of cases reported in ≤1 business day.
Results
Overall, 1,867 reports met the inclusion criteria; 24% of these reports had been submitted by ELR. Data completeness was 90% for ELR and 95% for conventional reporting. Patient addresses accounted for 97% of the missing data fields for ELR reports. Timeliness was 91% for ELR and 87% for conventional reports.
Conclusions
Although early in the transition to ELR compliance in Oklahoma, ELR has already yielded improved timeliness for communicable disease surveillance. However, ELR did not yield more complete reports than conventional reporting. Requiring specific demographic data fields for ELR reports can improve the completeness of ELR.
Monitoring disease occurrence is essential for identifying disease outbreaks or bioterrorism threats that demand public health action. Communicable disease surveillance historically involved a paper-based system of laboratory reports delivered to the health department by mail, telephone, or fax, which can be both incomplete and slow.1 However, electronic laboratory reporting (ELR) facilitates more comprehensive and rapid information communication.2 ELR's goal is to establish a central database repository of positive laboratory results for reportable diseases, which will improve the public health surveillance for communicable diseases.
Federal legislation has emphasized the introduction and institution of updated health information technology in the health-care field.3 Creation of the Health Information Technology for Economic and Clinical Health (HITECH) Act, a component of the American Recovery and Reinvestment Act of 2009, encourages the meaningful use of certified electronic health record (EHR) technology.4 Since 2011, the HITECH Act has provided incentive payments, which will total approximately $27 billion during 10 years, for adopting and supporting EHRs.5 Funding eligibility requires health-care providers and hospitals to achieve specific objectives related to EHR use. Implementation of ELR for communicable disease surveillance meets one of these objectives.6 ELR has been demonstrated to improve the completeness and timeliness of communicable disease surveillance and reduce manual data entry errors, which is the justification for its meaningful use.7,8
BACKGROUND
A total of 61 reportable diseases must be reported to the Oklahoma State Department of Health (OSDH) upon suspicion, diagnosis, or positive laboratory test.9 These diseases are typically reported by laboratory personnel, infection preventionists, or clinicians when a positive laboratory test result is obtained. Data from the laboratory test results can be transmitted to OSDH by fax, mail, telephone, or entry into the Public Health Investigation and Disease Detection of Oklahoma (PHIDDO) system, Oklahoma's National Electronic Disease Surveillance System-compatible Internet-based reporting application. These processes are defined as conventional laboratory reporting.
By contrast, ELR is the direct, automated, electronic transmission of positive laboratory test results for reportable diseases to public health agencies.8 OSDH has developed procedures to accept positive laboratory results from a laboratory information system, then encrypt, transmit, decrypt, parse, translate, and insert the data by using Public Health Information Network Messaging System and Eclipsys eLink® software. The results are then linked to PHIDDO.
According to Oklahoma law, all clinical laboratories with ≥400 positive laboratory tests performed on-site per year for reportable diseases are required to use ELR.10 During 2011, a total of 18 laboratories met this criterion in Oklahoma. Two laboratories had ELR in place, five laboratories were transitioning to ELR, and 11 laboratories did not have ELR. The statewide expansion of all Oklahoma laboratories to have full ELR capabilities is planned, primarily because of the impetus provided by the HITECH Act. Thus, we evaluated Oklahoma's ELR-based communicable disease reporting system during the early stages of ELR implementation.
Purpose of evaluation
State health officials from OSDH inquired about the status of ELR in Oklahoma before broad implementation of ELR statewide after HITECH legislation. Multiple previous studies of ELR at the state level (i.e., Hawaii, Pennsylvania, and Indiana) focused on the improved completeness and timeliness of reporting when implementing ELR; these two metrics were chosen for evaluating our system.2,7,11 We compared ELR with conventional laboratory reporting for selected communicable diseases to determine if ELR yielded more complete and timely disease reports than conventional reporting.
METHODS
We performed a retrospective analysis of confirmed reportable disease cases reported during 2011 for which the surveillance case definition required laboratory evidence of infection. Probable reportable tickborne disease cases were also included in the analysis because those reports are generated by positive laboratory tests; probable cases of other reportable diseases were excluded because laboratory evidence of infection is not required. Tuberculosis, hepatitis, and sexually transmitted infections were excluded because these diseases can involve multiple specimens and tests. Immediately reportable diseases, whose rarity and urgency generate special handling, were also excluded from the analysis because these diseases are often reported to the health department on suspicion alone before laboratory confirmation. We included 18 of the 61 reportable diseases in Oklahoma in our analysis: anaplasmosis; brucellosis; campylobacteriosis; cryptosporidiosis; ehrlichiosis; Escherichia coli (E. coli) O157, O157:H7, or a non-O157 Shiga toxin-producing E. coli infection; legionellosis; listeriosis; Lyme disease; malaria; mumps; pertussis; Q fever; spotted fever rickettsioses; salmonellosis; shigellosis; Streptococcus pneumoniae invasive disease among children aged <5 years; and vibriosis, including cholera. All of these diseases are required by Oklahoma law to be reported to OSDH in ≤1 business day.10
We filtered the data by how the case was reported using a drop-down question in the PHIDDO case report form that asks, “How is this case being reported?” Variable options include ELR or conventional reporting methods (e.g., fax, mail, telephone, and Internet). Reports for which the method of reporting was unknown (n=52) were excluded from analysis. We compared ELR reports from the two laboratories that had adopted ELR with conventional reports from the 91 other laboratories that submitted reports to OSDH during 2011.
We assessed completeness by percentage of completed data fields for certain standard variables from the initial disease report. Eight key demographic fields (last name, first name, date of birth, sex, ethnicity, street address, city of residence, and home ZIP Code) and two laboratory fields (laboratory name and laboratory result date) were used as surrogates for completeness.
We assessed timeliness by comparing the time from a positive laboratory test until it was reported to OSDH. Only cases reported in ≤1 business day were considered timely. Because state regulations require cases to be reported in ≤1 business day, we adjusted the analysis for weekends and holidays.
RESULTS
A total of 1,867 reports met the inclusion criteria; 1,322 (71%) involved enteric diseases (Table 1). ELR was used for 454 (24%) reports; conventional reporting was responsible for 1,413 (76%) reports, of which 850 (46%) were reported through online case reporting (PHIDDO) (Figure).
Table 1.
Types of communicable disease reports meeting inclusion criteria for comparing completeness and timeliness of electronic vs. conventional laboratory reporting—Oklahoma, 2011

Figure.
Reporting method for communicable disease reports received by the Oklahoma State Department of Health for comparing completeness and timeliness of electronic vs. conventional laboratory reporting—Oklahoma, 2011 (n=1,867)
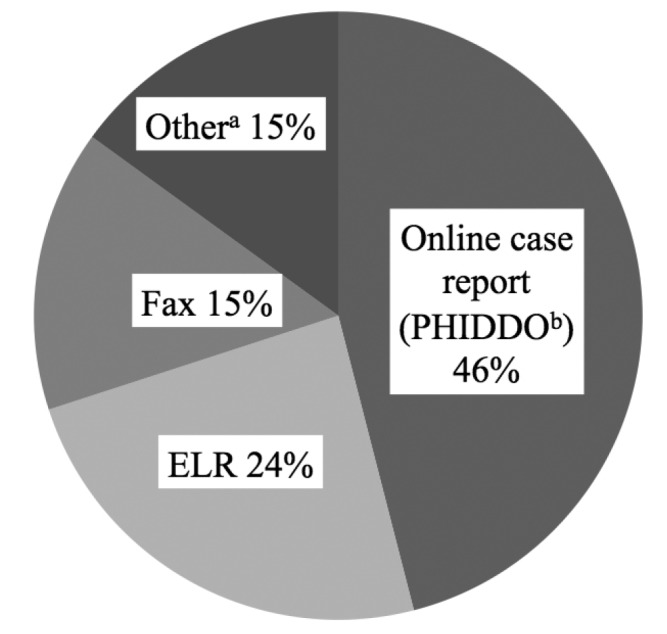
aOther methods include mail, telephone, audit, and from the Oklahoma State Public Health Laboratory.
bOklahoma's National Electronic Disease Surveillance System compatible Web-based reporting application
PHIDDO = Public Health Investigation and Disease Detection of Oklahoma
ELR = electronic laboratory reporting
For ELR reports, 4,103 (90%) of 4,540 data fields were complete. The majority of missing data from ELR reports were patient addresses (i.e., street, city, and ZIP Code); only 313 (69%) of 454 reports were complete for street, city, and ZIP Code. Among the remaining seven data fields, all had ≥98% completion rates. For conventional reports, 13,405 (95%) of 14,130 total data fields were complete. First and last name completion was 100%. For other variables, the completion rate ranged from 82% for laboratory result dates to 99% for date of birth, ethnicity, and laboratory name (Table 2).
Table 2.
Data field completion for reports received by ELR and conventional reporting methods for comparing completeness and timeliness of electronic vs. conventional laboratory reporting—Oklahoma, 2011
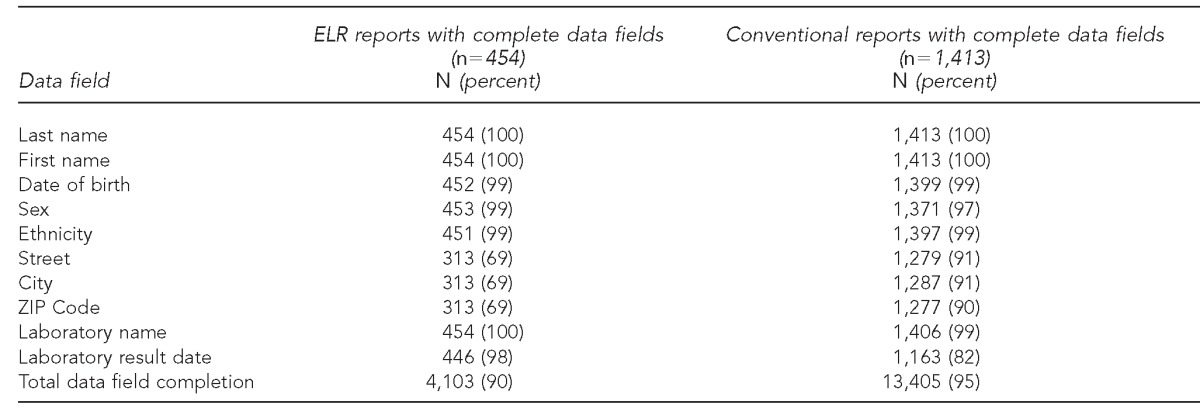
ELR = electronic laboratory reporting
A total of 440 ELR reports and 1,138 conventional reports had a valid date of final result to calculate the time until reported to OSDH. Of these reports, 399 (91%) ELR reports and 995 (87%) conventional reports were considered timely (i.e., reported in ≤1 business day). Telephone (100%) and fax (93%) reports had the highest percentage of timely reports completed in ≤1 business day (Table 3).
Table 3.
ELR (n=440) and conventional (n=1,138) reports that were reported in ≤1 business day for comparing completeness and timeliness of electronic vs. conventional laboratory reporting—Oklahoma, 2011a
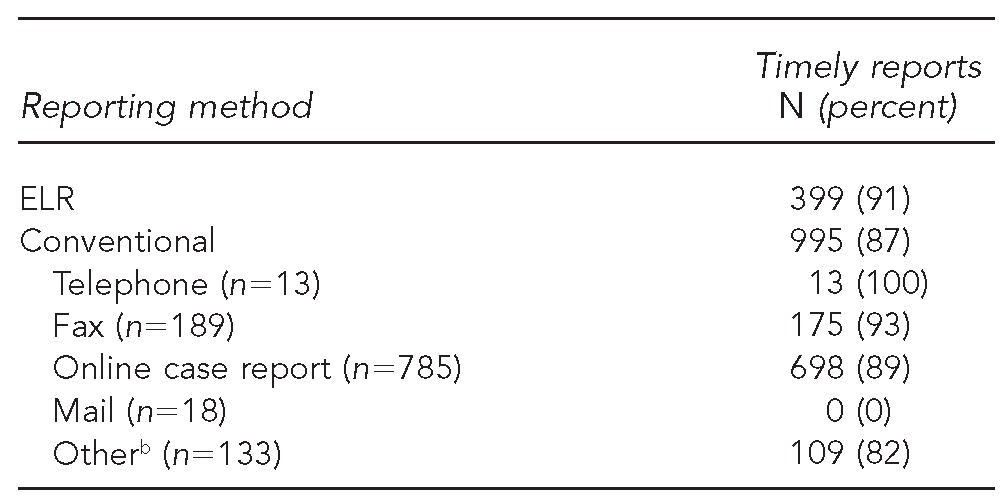
Includes only reports with date of final result listed
bIncludes reports from audits and from the Oklahoma State Public Health Laboratory
ELR = electronic laboratory reporting
DISCUSSION
The HITECH Act creates a unique opportunity to take advantage of the incentives of harnessing ELR technology to improve communicable disease surveillance nationwide. In fact, providers and institutions have considerable motivation to comply with HITECH because those that do not comply with HITECH objectives will eventually be penalized in the form of having a portion of their Medicare and Medicaid payments withheld.6 The adoption and meaningful use of ELR meets one of the HITECH objectives, defined as “submit electronic data on reportable laboratory results to public health agencies.”6 ELR is being adopted -nationwide for communicable disease surveillance because multiple studies have demonstrated ELR to be more complete and timely than conventional reporting methods.1,2,7,11 Despite concerns regarding confidentiality and ownership of health records, in addition to the increased costs and health information technology compatibility problems associated with ELR, it is a versatile and efficient technology that is expected to improve public health surveillance and our nation's health-care system as a whole.3,5,6
At this phase of ELR implementation in Oklahoma, our findings demonstrated that ELR yielded improved timeliness compared with conventional reporting. However, ELR was not more complete than conventional reporting. These results should not be interpreted as discouraging or surprising. ELR implementation is challenging. Certain smaller laboratories do not yet have the standardized message format and coding required for ELR, and state-specific reporting regulations sometimes are not fully structured for ELR. Most importantly, a lack of knowledge and awareness exists about the technology regarding ELR and the merits of implementing it.1
Of 49 states that responded to the 2011 National Electronic Laboratory Reporting Snapshot Survey regarding ELR implementation, two states were in the planning stage of ELR, three states were in the testing stage, 10 states had an operational ELR system receiving 1%–24% of reports electronically, 11 states were receiving 25%–49% of reports electronically, 11 states were receiving 50%–74% of reports electronically, and 12 states were receiving 75%–100% of reports electronically. Oklahoma was listed as having an operational ELR receiving 1%–24% of reports electronically, which is consistent with the findings of our analysis.12 Oklahoma remains in the early phases of ELR implementation, and additional time is necessary for the system to be fully established statewide.
Although ELR reports were not as complete as conventional reports, the missing data were concentrated in the patient address field. This finding indicates that improved completeness of the address field might enhance the overall completeness of ELR. In contrast, conventional reports were more complete but had a wider array of missing data. Seven of the 10 data fields had ≥10 incomplete reports. Thus, despite the greater completeness of conventional reporting in this analysis, seeking a solution to linking patient addresses in the medical record to the ELR report can improve the completeness of ELR, whereas multiple data fields would need to be addressed to improve the completeness of conventional reporting.
Telephone and fax were the timeliest reporting methods. Both are well-established practices that are easy to use and provide a direct and straightforward means of transmitting information. However, all telephone and fax reports need to be reviewed by health department officials and manually entered into the PHIDDO disease reporting system. These steps take time and allow for possible data entry errors. These redundant tasks are eliminated with ELR.
Targeted education by OSDH for laboratories with ELR capabilities about adhering to disease reporting regulations might improve the performance of ELR statewide. In addition, monitoring surveillance data quality by state health departments can ensure that laboratories are reporting in a complete and timely manner. Requiring entry of patient address data fields for all ELR reports can facilitate the capture of essential data and improve the completeness of reporting. For instance, in 2011, Washington State required the inclusion of specific demographic data fields when ordering a laboratory test for any notifiable condition.13
Limitations
This study had several limitations. The ELR assessment was based on only two laboratories that have full ELR capabilities, whereas the assessment of conventional reporting was based on a greater number of laboratories. Also, our analysis only represented 18 of the 61 reportable diseases in Oklahoma and might not be representative of all reportable diseases. Certain prevalent diseases, including chlamydia infection, gonorrhea, and human immunodeficiency virus and acquired immunodeficiency syndrome, were excluded from analysis. Additionally, certain diseases that were included in the analysis (e.g., brucellosis, Q fever, and vibriosis) occur infrequently; as such, their rarity might arouse special attention by laboratorians or public health officials, thereby affecting the timeliness and completeness of reporting.
CONCLUSION
Acquiring and maintaining certified EHR technology is expensive and challenging, but will strengthen public health surveillance. In Oklahoma, ELR is already outperforming conventional reporting in terms of timeliness; however, changes should be made to improve the completeness of ELR. Time is necessary for laboratories to fully implement ELR; therefore, a transition period is expected before ELR is established statewide. In the meantime, requiring specific demographic data fields through revisions to reportable disease regulations can help to improve the completeness of ELR in Oklahoma.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Jernigan DB. Electronic laboratory-based reporting: opportunities and challenges for surveillance. Emerg Infect Dis. 2001;7(3 Suppl):538. doi: 10.3201/eid0707.017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effler P, Ching-Lee M, Bogard A, Ieong MC, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories. JAMA. 1999;282:1845–50. doi: 10.1001/jama.282.19.1845. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal D. Launching HITECH. N Engl J Med. 2010;362:382–5. doi: 10.1056/NEJMp0912825. [DOI] [PubMed] [Google Scholar]
- 4. Pub. L. No. 111-5, 123 Stat. 226 (February 17, 2009), codified at 42 U.S.C. §§300jj et seq.; §§17901 et seq.
- 5.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363:501–4. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services (US) EHR incentive programs. [cited 2013 Jan 3]. Available from: URL: http://www.cms.gov/ehrincentiveprograms/30_Meaningful_Use.asp.
- 7.Overhage JM, Grannis S, McDonald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98:344–50. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Electronic laboratory reporting (ELR) [cited 2013 Jan 3]. Available from: URL: http://www.cdc.gov/ehrmeaningfuluse/elr.html.
- 9. Oklahoma Administrative Code, Title 63, Ch. 1, Art. 5, §§1-503.
- 10. Oklahoma Administrative Code, Title 310, Ch. 515, §§1-8.
- 11.Panackal AA, M'ikanatha NM, Tsui FC, McMahon J, Wagner MM, Dixon BW, et al. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerg Infect Dis. 2002;8:685–91. doi: 10.3201/eid0807.010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnuson JA. Portland (OR): Oregon Health Authority, Acute and Communicable Disease Prevention; 2012. [cited 2013 Jan 3]. 2011 national electronic laboratory reporting (ELR) snapshot survey: summary of results. Also available from: URL: http://coast2coastinformatics.com/2011NationalELRSurvey-DataSummary.pdf. [Google Scholar]
- 13.Washington State Legislature. WAC 246-101-105. Duties of the healthcare provider. 2011. [cited 2013 Jan 3]. Available from: URL: http://apps.leg.wa.gov/wac/default.aspx?cite=246-101-105.


