Abstract
Several bacterial species express surface proteins with affinity for the constant region (Fc) of immunoglobulin (Ig) G. The biological consequences of the interaction with IgG are poorly understood but it has been demonstrated that genes encoding different IgG Fc-binding proteins have undergone convergent evolution, suggesting that these surface molecules are connected with essential microbial functions. One of the molecules, protein H, is present in some strains of Streptococcus pyogenes, the most significant streptococcal species in clinical medicine. In contrast to other Ig-binding bacterial proteins tested, protein H was found to interact also with the neural cell adhesion molecule (N-CAM), a eukaryotic cell surface glycoprotein mediating homo- and heterophilic cell-cell interactions. The affinity for the interaction between protein H and N-CAM was 1.6 x 10(8)/M and the binding site on protein H was mapped to the NH2-terminal 80 amino acid residues. N-CAM and IgG are both members of the Ig superfamily and analogous to N-CAM, IgG binds to the NH2-terminal part of protein H. However, the binding sites for the two proteins were found to be separate, an unexpected result which was explained by the observation that the fibronectin type III (FNIII) domains and not the Ig-like domains of N-CAM are responsible for the interaction with protein H. Thus, the binding of N-CAM to protein H was blocked with fibronectin but not with IgG. Moreover, apart from fibronectin itself and N-CAM, fragments of fibronectin and the matrix protein cytotactin/tenascin containing FNIII domains also showed affinity for protein H.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

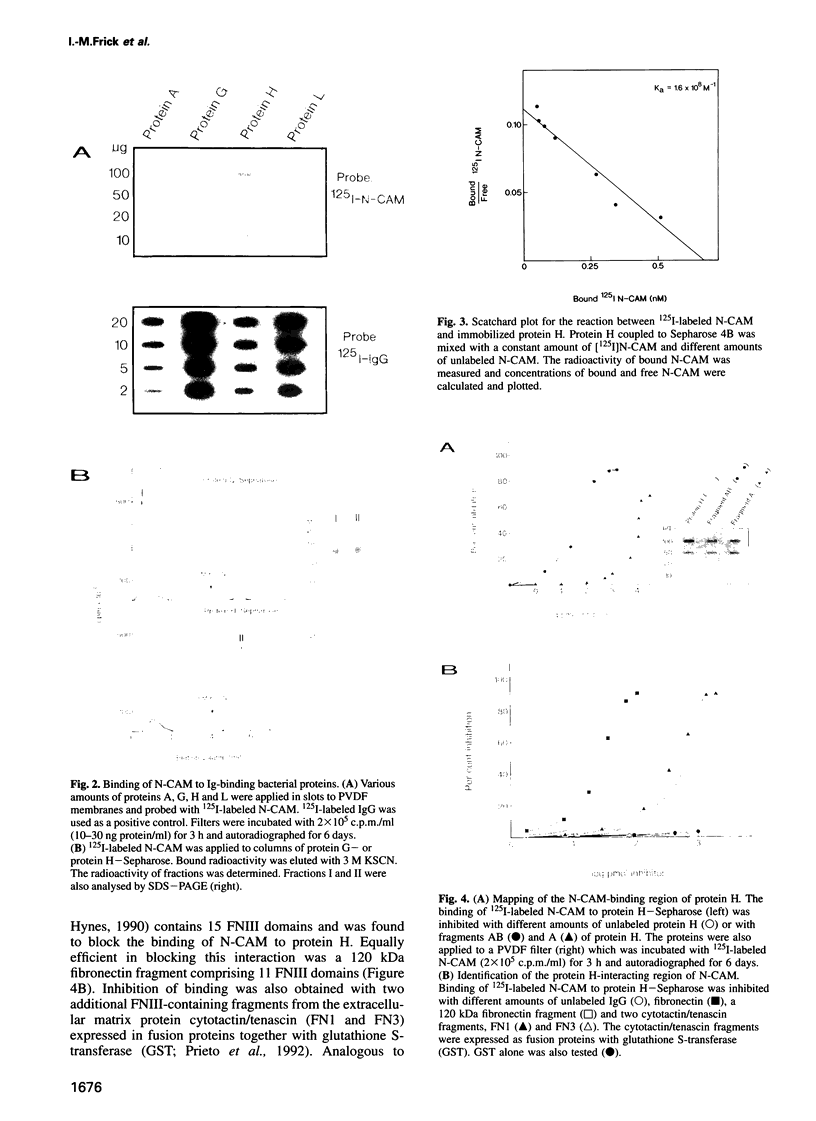
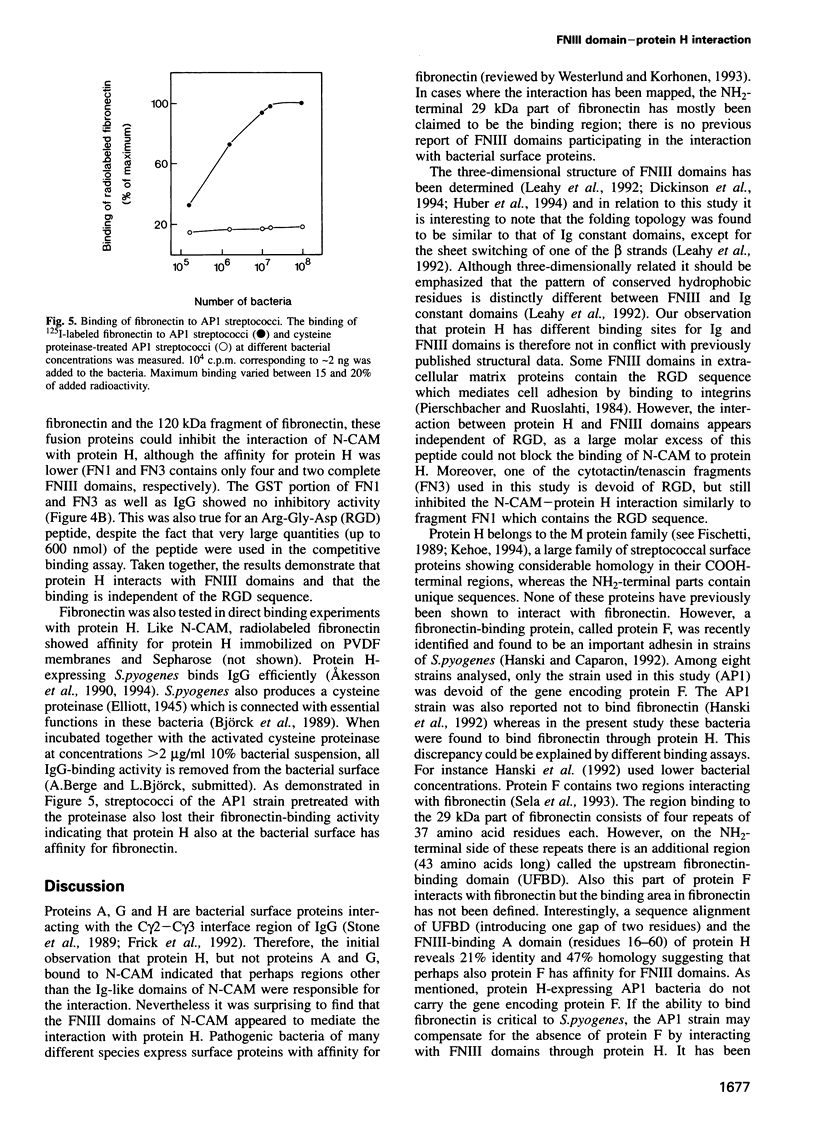


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986 Aug 5;261(22):10240–10247. [PubMed] [Google Scholar]
- Akerström B., Björck L. Protein L: an immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J Biol Chem. 1989 Nov 25;264(33):19740–19746. [PubMed] [Google Scholar]
- Akerström B., Lindahl G., Björck L., Lindqvist A. Protein Arp and protein H from group A streptococci. Ig binding and dimerization are regulated by temperature. J Immunol. 1992 May 15;148(10):3238–3243. [PubMed] [Google Scholar]
- Akesson P., Cooney J., Kishimoto F., Björck L. Protein H--a novel IgG binding bacterial protein. Mol Immunol. 1990 Jun;27(6):523–531. doi: 10.1016/0161-5890(90)90071-7. [DOI] [PubMed] [Google Scholar]
- Akesson P., Schmidt K. H., Cooney J., Björck L. M1 protein and protein H: IgGFc- and albumin-binding streptococcal surface proteins encoded by adjacent genes. Biochem J. 1994 Jun 15;300(Pt 3):877–886. doi: 10.1042/bj3000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck L., Akesson P., Bohus M., Trojnar J., Abrahamson M., Olafsson I., Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989 Jan 26;337(6205):385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- Björck L., Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984 Aug;133(2):969–974. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Doolittle R. F. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8990–8994. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994 Feb 3;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Dickinson C. D., Veerapandian B., Dai X. P., Hamlin R. C., Xuong N. H., Ruoslahti E., Ely K. R. Crystal structure of the tenth type III cell adhesion module of human fibronectin. J Mol Biol. 1994 Mar 4;236(4):1079–1092. doi: 10.1016/0022-2836(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Frick I. M., Akesson P., Cooney J., Sjöbring U., Schmidt K. H., Gomi H., Hattori S., Tagawa C., Kishimoto F., Björck L. Protein H--a surface protein of Streptococcus pyogenes with separate binding sites for IgG and albumin. Mol Microbiol. 1994 Apr;12(1):143–151. doi: 10.1111/j.1365-2958.1994.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Frick I. M., Wikström M., Forsén S., Drakenberg T., Gomi H., Sjöbring U., Björck L. Convergent evolution among immunoglobulin G-binding bacterial proteins. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8532–8536. doi: 10.1073/pnas.89.18.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H., Hozumi T., Hattori S., Tagawa C., Kishimoto F., Björck L. The gene sequence and some properties of protein H. A novel IgG-binding protein. J Immunol. 1990 May 15;144(10):4046–4052. [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Horwitz P. A., Caparon M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992 Dec;60(12):5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Edelman G. M., Cunningham B. A. cDNA clones of the neural cell adhesion molecule (N-CAM) lacking a membrane-spanning region consistent with evidence for membrane attachment via a phosphatidylinositol intermediate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9822–9826. doi: 10.1073/pnas.83.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Murray B. A., Edelman G. M., Cunningham B. A. Sequence of a cDNA clone encoding the polysialic acid-rich and cytoplasmic domains of the neural cell adhesion molecule N-CAM. Proc Natl Acad Sci U S A. 1986 May;83(9):3037–3041. doi: 10.1073/pnas.83.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Huber A. H., Wang Y. M., Bieber A. J., Bjorkman P. J. Crystal structure of tandem type III fibronectin domains from Drosophila neuroglian at 2.0 A. Neuron. 1994 Apr;12(4):717–731. doi: 10.1016/0896-6273(94)90326-3. [DOI] [PubMed] [Google Scholar]
- Kastern W., Sjöbring U., Björck L. Structure of peptostreptococcal protein L and identification of a repeated immunoglobulin light chain-binding domain. J Biol Chem. 1992 Jun 25;267(18):12820–12825. [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Prieto A. L., Andersson-Fisone C., Crossin K. L. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol. 1992 Nov;119(3):663–678. doi: 10.1083/jcb.119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeder R., Otten R. A., Chamberlin L., Boyle M. D. Functional and serological analysis of type II immunoglobulin G-binding proteins expressed by pathogenic group A streptococci. J Clin Microbiol. 1992 Dec;30(12):3074–3081. doi: 10.1128/jcm.30.12.3074-3081.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis K. J., Ayoub E. M., Boyle M. D. Streptococcal Fc receptors. I. Isolation and partial characterization of the receptor from a group C streptococcus. J Immunol. 1984 Jun;132(6):3091–3097. [PubMed] [Google Scholar]
- Retnoningrum D. S., Podbielski A., Cleary P. P. Type M12 protein from Streptococcus pyogenes is a receptor for IgG3. J Immunol. 1993 Mar 15;150(6):2332–2340. [PubMed] [Google Scholar]
- Rothbard J. B., Brackenbury R., Cunningham B. A., Edelman G. M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982 Sep 25;257(18):11064–11069. [PubMed] [Google Scholar]
- Sela S., Aviv A., Tovi A., Burstein I., Caparon M. G., Hanski E. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993 Dec;10(5):1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Stenberg L., O'Toole P., Lindahl G. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol. 1992 May;6(9):1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Stone G. C., Sjöbring U., Björck L., Sjöquist J., Barber C. V., Nardella F. A. The Fc binding site for streptococcal protein G is in the C gamma 2-C gamma 3 interface region of IgG and is related to the sites that bind staphylococcal protein A and human rheumatoid factors. J Immunol. 1989 Jul 15;143(2):565–570. [PubMed] [Google Scholar]
- Westerlund B., Korhonen T. K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993 Aug;9(4):687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]