Abstract
Purpose
Although patients with neurofibromatosis are predisposed to multiple nerve sheath tumors that can develop anywhere in the body and cause significant morbidity (e.g., hearing loss; pain), little research has examined emotional correlates of neurofibromatosis. The purpose of this study was to examine emotional functioning among adult patients with neurofibromatosis.
Methods
A total of 248 patients with neurofibromatosis (neurofibromatosis 1, neurofibromatosis 2, or schwannomatosis) who received care at a specialized clinic completed validated measures to assess symptoms of depression and anxiety, level of perceived stress, and self-esteem.
Results
Patients with neurofibromatosis reported significantly more symptoms of depression and anxiety, higher levels of perceived stress, and lower levels of self-esteem as compared with general population norms. No significant differences were found among patients with neurofibromatosis 1, neurofibromatosis 2, and schwannomatosis, and emotional functioning was not significantly associated with disease severity. However, increased symptoms of depression and anxiety, higher levels of perceived stress, and lower levels of self-esteem were associated with a higher frequency of self-reported medical visits in the past year (P values ≤0.05).
Conclusion
Neurofibromatosis appears to be associated with reduced emotional functioning. Although further research is needed, these findings suggest a role for a multidisciplinary treatment approach to address emotional distress among adult patients with neurofibromatosis.
Keywords: emotional function, neurofibromatosis 1, neurofibromatosis 2, schwannomatosis, tumor suppressor syndromes
Introduction
The neurofibromatoses (neurofibromatosis (NF)1, NF2, and schwannomatosis; OMIM #162200, #101000 and 162091, respectively) are the most common neurologic tumor suppressor syndromes and are a group of related neurogenetic conditions characterized by a predisposition to develop multiple nerve sheath tumors. NF1 is the most common of these conditions with a birth incidence of ∼1:2,700.1 NF2 and schwannomatosis are less common than NF1. The estimated birth incidence of NF2 is 1:33,0001; schwannomatosis may have an incidence rate similar to that of NF2.2 These genetic conditions are transmitted in autosomal dominant fashion with 50% risk to offspring of affected parents. Symptom severity varies widely among patients; however, the hallmark manifestation of NF1, NF2, and schwannomatosis is nerve sheath tumors, including neurofibromas and schwannomas. These histologically benign tumors can occur anywhere in the body and often cause significant morbidity including disfiguring cutaneous tumors (NF1), complete hearing loss, facial weakness, and poor gait (NF2), and chronic disabling pain (schwannomatosis). In addition to the increased risk of benign tumors, patients with these genetic syndromes may have or develop malignant tumors and nontumor manifestations that affect the nervous system, eyes, and skin.3,4 Currently, there are only a handful of clinics in the United States that provide specialized care for adult patients with NF; the majority of such patients will be cared for by primary-care physicians. Given the limited number of specialized clinics, as well as the substantial morbidity associated with the neurofibromatoses, it is imperative for clinicians to be familiar with these conditions.
Anecdotal evidence is that patients with NF1, NF2, and schwannomatosis express concern about their present and future health status, and about the possibility of transmitting NF to their offspring. Research with non-NF patient populations also highlights the emotional distress that may accompany chronic medical conditions.5 To date, however, research regarding the potential psychological impact of NF in adulthood has been generally restricted to a few studies that have examined quality of life among patients with NF16–8 or NF29 and to a few that have assessed psychiatric morbidity among patients with NF1 only.10–12 For example, 12-year follow-up of 48 Swedish patients with NF1 (37 were evaluated for psychiatric disorders at follow-up) found that one-third of the patients met criteria for at least one psychiatric disorder.11 Psychiatric disorders were significantly more common among patients as compared with controls. At least one study also examined responses to receiving a diagnosis among 20 patients with NF2 and found that eight patients reported negative emotional responses such as anxiety.13 To our knowledge, however, in-depth studies of psychological and emotional functioning are rare in the NF literature and no such studies have included patients with schwannomatosis.
To address this gap in the literature, we examined emotional functioning in adult patients with NF1, NF2, and schwannomatosis (both in comparison with population norms and between patient groups). The term emotional functioning was used in this study to refer to patients' self-reported symptoms of depression, symptoms of anxiety, and levels of stress and self-esteem. We also examined whether clinical factors—specifically, skin manifestation (NF1), hearing impairment, walking status, and facial function (NF2), and pain (schwannomatosis)—were associated with patients' levels of emotional functioning. Finally, given that decreased emotional functioning is associated with increased use of health-care services among patients with chronic medical conditions,14 we assessed whether level of emotional functioning was associated with the number of self-reported medical visits (a proxy of health-care utilization) within the past year. Although we expected patients to report poorer levels of emotional functioning as compared with population norms, this study was mainly exploratory and descriptive in nature.
Materials and Methods
This project was a cross-sectional study examining emotional functioning among adult patients with NF. Patients who were 18 years or older with a clinical diagnosis of NF1, NF2, or schwannomatosis and who were seen in The Family Center for Neurofibromatosis at Massachusetts General Hospital between January and November 2010 were recruited. This clinic specializes in the care of adult patients with NF and sees a large volume of patients annually (435 adult patients in 2010). Patients who were unable to comprehend the consent form because of cognitive limitations (determined by the medical provider), who were non-English speaking, or who had a medical condition that interfered with the ability to complete the study procedures (determined by the medical provider) were excluded from participating.
Eligible patients were asked to complete a paper survey during a scheduled clinic visit. Specifically, study personnel approached patients waiting in an examination room to inform them about the study. Interested and eligible patients subsequently completed the self-administered survey, which took ∼20 min to complete. Participants who did not complete the survey during their scheduled clinic visit (n = 10) were provided with a pre-addressed, stamped envelope in which to return the survey and were asked to complete the survey at home. The study was approved by the Massachusetts General Hospital/Partners Review Board and all participants provided written informed consent.
Demographic and emotional functioning measures
Demographics and medical information
This questionnaire assessed age, gender, race, ethnicity, level of education, and marital status. Health-care utilization was assessed by the number of medical visits in the past 12 months.
Center for Epidemiologic Studies Depression Scale
The Center for Epidemiologic Studies Depression Scale (CES-D) contains 20 items that measure the frequency with which symptoms of depression were experienced during the past week.15 Items are rated using a scale from 0 (rarely or none of the time; <1 day) to 3 (most or all of the time; 5–7 days); possible scores range from 0 to 60 and higher scores indicate more symptoms. The CES-D has been shown to discriminate between general and psychiatric patient samples, and although the CES-D is not a diagnostic tool, a score of 16 or greater is indicative of clinically significant symptoms of depression. The general population scores reported by Radloff15 were utilized for comparison purposes in our study. One item (i.e., I feel fearful) was imputed for the first 200 patients using means replacement because of a typographical error.
Perceived Stress Scale-4
The Perceived Stress Scale-4 contains four items that assess the degree to which situations in one's life during the past month are viewed as stressful.16 Items are rated using a scale from 0 (never) to 4 (very often). Possible scores range from 0 to 16, with higher scores indicating higher levels of perceived stress. In this study, general population norms based on a probability sample of US adults were used for comparison purposes.17
Rosenberg Self-Esteem Scale
The Rosenberg Self-Esteem Scale consists of 10 items that assess global self-esteem (overall evaluation of one's value or worth).18 Items are rated using a scale from 0 (strongly disagree) to 3 (strongly agree), and possible scores range from 0 to 30. Higher scores on the Rosenberg Self-Esteem Scale indicate higher levels of self-esteem. General population norms based on a recent probability sample of US adults were used for comparison purposes in our study.19
State-Trait Anxiety Inventory for Adults form Y2
The State-Trait Anxiety Inventory for Adults (STAI) form Y2, containing 20 items rated on a four-point scale from 1 (almost never) to 4 (almost always), was administered to assess trait anxiety.20 Higher scores on the STAI indicate higher levels of anxiety. General population norms reported by Spielberger et al.20 were used for comparison purposes.
To contextualize the results, we identified several studies that utilized the CES-D and STAI (trait version) to study other medical populations, and have selected some of those studies as comparison for our results in NF patients.21–32
Measures of NF-related clinical factors
Skin severity
For all participants with NF1, skin severity was rated by their medical provider. Specifically, the medical provider was asked to rate, “How severe is this patient's main skin problem today (compared with other patients with the same skin problem)?” on the following 5-point scale: no skin problem, mild case, moderate case, severe case, or extremely severe case. This item has been used in previous research to assess condition severity among patients with NF1.33 For analysis, skin score ratings were dichotomized: patients with none and mild ratings received a score of 0, and patients with moderate, severe, and extremely severe ratings received a score of 1.
Pain
The average intensity of pain was assessed for all participants with schwannomatosis. Patients rated their pain using a numeric rating scale from 0 (“no pain”) to 10 (“worst pain”).
Hearing, walking, and facial function
Clinical data on hearing, walking, and facial function were collected from the medical records of patients with NF2 only. Hearing function was assessed using the most recent audiology report in the patient's medical record. Patient hearing level was categorized according to the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology–Head and Neck Surgery; this is a four-level scale in which hearing is rated based on accuracy of word recognition and pure-tone threshold.34 Walking status for patients with NF2 was determined from the most recent physical examination noted in the medical record and coded as ambulatory, needing an assistive device, or nonambulatory. Facial function was assessed according to the House–Brackmann grading system, which ranges from normal function (grade 1) to complete paralysis (grade 6).35
Statistical analyses
One-sample t-tests were conducted to examine potential group differences between patients with NF and published norms for each measure of emotional functioning (CES-D, STAI, Rosenberg Self-Esteem Scale, and Perceived Stress Scale). All emotional functioning measures except for the CES-D reported population norms by gender; as such, the overall population norm for the CES-D was used for all analyses comparing patients with NF to general population norms on symptoms of depression. Subsequently, we examined potential differences with regard to emotional functioning between patients with NF1, NF2, and schwannomatosis using Kruskal–Wallis tests.
Two-sided Wilcoxon tests were conducted to examine the relationship between skin severity and each measure of emotional functioning among patients with NF1. Among patients with NF2, Kruskal–Wallis tests were conducted to examine the relationships between hearing, walking status, and facial function and each measure of emotional functioning. Spearman rank order correlation coefficients were calculated to examine the relationship between pain and each measure of emotional functioning in patients with schwannomatosis. Finally, Spearman rank order correlation coefficients were conducted to examine the relationship between health-care utilization among all patients with NF in the past 12 months and each measure of emotional functioning.
Results
Patient characteristics
Among 292 potential participants, 16 patients were not eligible: 6 were non-English speaking, 6 had a medical condition that interfered with the ability to complete the questionnaire, and 4 lacked a diagnosis of NF1, NF2, or schwannomatosis. Of the 276 eligible patients approached for this study, 248 patients agreed to participate and completed the baseline survey (90% response rate; see Table 1 for patient demographic and medical characteristics). The final sample included 133 patients with NF1 (n = 54 men; n = 79 women), 94 patients with NF2 (n = 44 men; n = 50 women), and 21 patients with schwannomatosis (n = 15 men; n = 6 women). In terms of medical characteristics, a small number of participants in each patient group had been diagnosed with a malignant tumor in their lifetime, two (both patients with NF1) of whom had a malignancy that was related specifically to NF (i.e., malignant peripheral nerve sheath tumors). No significant differences were found with regard to the demographic characteristics of the patient groups.
Table 1. Demographics of the study population (n = 248).
| Characteristica | Neurofibromatosis (NF) diagnosis | ||
|---|---|---|---|
| NF1 | NF2 | Schwannomatosis | |
| Number, n (%) | 133 (54) | 94 (38) | 21 (8) |
| Mean age (SD) | 40.0 (12.2) | 39.9 (14.9) | 40.7 (12.2) |
| Male | 41.1 (12.1) | 40.5 (14.2) | 41.9 (12.5) |
| Female | 39.3(12.3) | 39.5 (15.6) | 37.7 (12.2) |
| Marital status, n (%) | |||
| Married | 52 (39) | 38 (40) | 11 (52) |
| Not married, but living with a partner | 6 (5) | 7 (7) | 2 (10) |
| Single, never married | 63 (47) | 33 (35) | 6 (29) |
| Widowed | 2 (2) | 1 (1) | 0 (0) |
| Divorced, separated, other | 10 (8) | 15 (16) | 2 (10) |
| Educational level, n (%) | |||
| Less than high school | 1 (1) | 1 (1) | 0 (0) |
| Some high school | 6 (5) | 0 (0) | 1 (5) |
| High school graduate/GED | 33 (25) | 16 (17) | 1 (5) |
| Some college | 32 (24) | 23 (24) | 8 (38) |
| Completed college | 40 (30) | 34 (36) | 6 (29) |
| Completed post-college graduate work | 21 (16) | 20 (21) | 5 (24) |
| Ethnicity, n (%) | |||
| American Indian or Alaska native | 1 (1) | 0 (0) | 0 (0) |
| Asian | 3 (2) | 3 (3) | 0 (0) |
| Black or African American | 5 (4) | 0 (0) | 0 (0) |
| White | 116 (87) | 88 (94) | 18 (86) |
| Hispanic | 4 (3) | 1 (1) | 0 (0) |
| Other | 4 (3) | 2 (2) | 3 (14) |
| Current or previous malignant tumor, n (%) | 6 (5) | 2 (2) | 3 (14) |
Note that percentages may not add up to 100 due to rounding.
GED, general educational development.
Demographic characteristics did not differ significantly between NF1, NF2, and schwannomatosis groups.
Emotional functioning: comparison of NF patients with population norms and comparisons between NF patient groups
Means and SDs of the emotional functioning outcomes are presented in Table 2. In comparison with published norms, both male and female patients with NF (NF1, NF2, or schwannomatosis) reported significantly more symptoms of depression than the general population (P values <0.001). Over one-third of male patients (n = 41) and approximately 46% (n = 62) of female patients scored at or above the clinical cut-off score on the CES-D as compared with 19% in the general population. Both male and female patients with NF also reported significantly more symptoms of anxiety (P values <0.001), higher levels of perceived stress (P values <0.001), and lower levels of self-esteem (males: P < 0.05; females: P < 0.001) as compared with population norms. These results do not appear to have been driven by one patient group in particular: there were no significant differences between patients with NF1, NF2, and schwannomatosis for either males or females on any of the emotional functioning measures (P values >0.05).
Table 2. Comparisons of measures of emotional functioning in NF patients and the general population.
| NF sample | General population | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) | t value | P value | |
| CES-Da | ||||||
| Male | 112 | 15.3 (11.9) | 2,514 | 9.3 (8.6) | 5.4 | <0.001 |
| Female | 134 | 16.4 (10.9) | 2,514 | 9.3 (8.6) | 7.6 | <0.001 |
|
| ||||||
| STAI | ||||||
| Male | 113 | 40.8 (12.0) | 1,387 | 34.9 (9.2) | 5.2 | <0.001 |
| Female | 133 | 40.7 (10.9) | 451 | 34.8 (9.2) | 6.3 | <0.001 |
|
| ||||||
| RSES | ||||||
| Male | 113 | 21.1 (6.0) | 242 | 22.4 (6.2) | −2.3 | 0.02 |
| Female | 134 | 20.9 (6.3) | 261 | 22.8 (5.4) | −3.6 | <0.001 |
|
| ||||||
| PSS | ||||||
| Male | 113 | 6.3 (3.4) | 949 | 4.2 (2.8) | 6.4 | <0.001 |
| Female | 134 | 6.4 (2.7) | 1,406 | 4.7 (3.1) | 7.6 | <0.001 |
CES-D, Center for Epidemiological Studies Depression scale; NF, neurofibromatosis; PSS, Perceived Stress Scale; RSES, Rosenberg Self-Esteem Scale; STAI, State-Trait Anxiety Inventory for Adults.
As CES-D population norms were not available by gender, the same general population mean was used for both male and female t-tests.
Associations between NF-specific clinical factors and emotional functioning
No significant relationships were found between skin score severity and any of the measures of emotional functioning for patients with NF1 (P values >0.05) Among patients with NF2, there was a significant relationship between facial function and anxiety only (K = 12.81, P = 0.03); walking status and hearing function were not significantly related to any of the measures of emotional functioning (P values >0.05). Among the patients with schwannomatosis, there was a trend for pain and symptoms of depression to be significantly correlated (rs = 0.41; P = 0.06); pain did not correlate significantly with symptoms of anxiety, levels of perceived stress, or levels of self-esteem for patients with schwannomatosis (P values >0.05).
Associations between emotional functioning and health-care utilization
The median number of medical visits reported by NF patients in the last 12 months was 4. Significant correlations were found between patients' self-reported level of emotional functioning and number of medical visits in the past year. Specifically, a higher number of medical visits in the past year was significantly related to increased symptoms of depression (rs = 0.19; P = 0.005) and anxiety (rs = 0.18; P = 0.006), higher levels of perceived stress (rs = 0.16; P = 0.015), and lower levels of self-esteem (rs = −0.13; P = 0.05).
Discussion
We surveyed adults with NF to understand how emotional functioning may be affected among such patients, as well as to examine how such functioning is associated with common clinical manifestations of NF. We also compared emotional functioning among the different types of NF to understand whether any single patient group experienced poorer functioning. We found a consistent pattern of reduced emotional well-being in NF patients as compared with the general population. Of note, despite the different clinical manifestations associated with the various types of NF, this pattern did not appear to be restricted to a single type of NF. Our findings, therefore, suggest that living with NF in any form may be associated with negative emotional consequences. Indeed, there are experiences that are common to all forms of NF, including the risk of current and future medical problems and the possibility of having affected children, and it may be that such experiences contribute to decreased emotional functioning among some patients. The levels of anxiety and depressive symptoms in our NF sample were were often higher than those reported by patients with other chronic diseases, including coronary artery disease and cancer (Figure 1).
Figure 1. Symptoms of anxiety and depression in the current sample of patients with neurofibromatosis as compared with other medical populations.
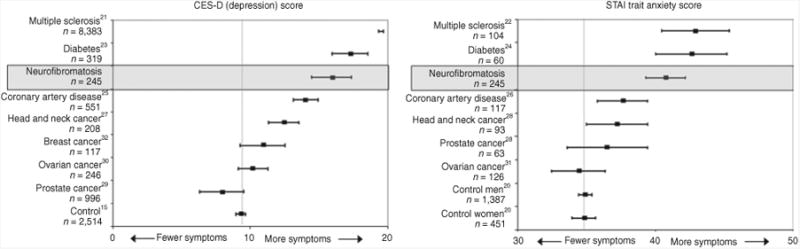
Scores shown are mean scores (with 95% confidence interval) on the Center for Epidemiologic Studies Depression Scale and State-Trait Anxiety Inventory for Adults for patients with neurofibromatosis as compared with published population norms and published scores of patients with multiple sclerosis,21,22 diabetes,23,24 coronary artery disease,25,26 head and neck cancer,27,28 prostate cancer,28,29 ovarian cancer,30,31 and breast cancer.32
Our findings also suggest that increased severity of disease is not necessarily indicative of reduced emotional functioning among patients with NF. For example, in contrast to previous studies that found more visible symptoms of NF1 to be associated with greater effects on patients' emotional symptoms,6–8 our study did not find a relationship between NF1 skin severity and emotional functioning. This difference may reflect how skin severity was rated in our study (with one item vs. multi-item validated measure). However, our findings also align with previous research that suggests that severity of disfigurement (in patients with various forms of visible disfigurement) is not necessarily predictive of the extent of psychosocial distress.36 Similarly, neither hearing loss, the cardinal feature of NF2, nor walking status was associated with self-reported emotional functioning in our sample of patients with NF2. Among patients with schwannomatosis, however, there was a trend for pain and symptoms of depression to be significantly associated. There is high comorbidity between chronic pain and depression;37 health-care providers should be aware of the need to assess for and monitor symptoms of depression among patients with schwannomatosis and pain and to refer to a qualified mental health professional when appropriate.
Our study also found that poorer emotional functioning was significantly associated with an increased number of patient medical visits in the previous year. Although little is known about NF and health-care utilization patterns, previous research has documented more frequent health-care use among patients with emotional and psychiatric problems.38 Further research is needed to understand the link between emotional functioning and health-care utilization among patients with NF, and to explore whether treating emotional problems could reduce such use, and associated medical costs, in this population. To date, psychological treatment protocols with demonstrated effectiveness for reducing emotional distress and targeting specific problems such as depression (e.g., cognitive behavioral therapy; mindfulness) have not been tested with patients with NF, and future research is needed to assess the application of psychological treatments for helping patients with NF.
There are limitations of our study. Although we were able to enroll a large number of patients with NF1, we were not able to recruit an equal number of patients with NF2 and schwannomatosis because of the lower prevalence of these diseases. Therefore, it is possible that our study may have missed true associations between emotional functioning and clinical factors such as hearing, balance, and pain. Second, this study relied upon normative data for comparison purposes, most of which were published years ago (e.g., CES-D, STAI, and Perceived Stress Scale norms) and may not reflect accurate levels of distress in the current US population. Our patients were also drawn from a single NF clinic in the United States; although the sample was representative of the patient population at this clinic, the results of this study may not be generalizable to other regions or populations with different sociodemographic characteristics. In addition, medical provider ratings were used to determine skin severity ratings for our NF1 sample and may not reflect patients' perceptions regarding severity of their disease; Benjamin et al.,39 for example, did not find significant correlations between patients' perceptions and medical classification of NF1 disease severity. Finally, although valid and reliable self-reported measures were used to assess emotional functioning, future work should assess psychological and emotional functioning among patients using structured diagnostic evaluations.
In sum, NF appears to be associated with reduced emotional functioning. Going forward, it is critical for physicians, caregivers, and patients to be aware of the emotional factors associated with NF in adults. As well, it will be important for future research to examine whether established psychological interventions are effective in helping patients with NF address their emotional concerns. We also see a need for comprehensive programs that incorporate psychoeducation, stress management, and support workshops to improve patients' quality of life. The development of such programs, modeled on cancer survivorship clinics, would provide opportunities for NF patients to (i) meet in groups to discuss the potential negative emotional consequences of living with NF; (ii) learn about advancements in the field; and (iii) receive services that address both their medical and psychological needs. Although our study is the first to examine emotional functioning among adult patients with schwannomatosis, it builds upon previous NF1 and NF2 research and underscores the importance of assessing for and addressing emotional distress among patients with NF.
Acknowledgments
Kelly B. Smith is currently supported by a Clinical Research Trainee Award from the Michael Smith Foundation for Health Research. The authors thank Vanessa Merker for her helpful comments regarding the manuscript.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Evans DG, Ramsden RT, Shenton A, et al. What are the implications in individuals with unilateral vestibular schwannoma and other neurogenic tumors? J Neurosurg. 2008;108:92–96. doi: 10.3171/JNS/2008/108/01/0092. [DOI] [PubMed] [Google Scholar]
- 2.Antinheimo J, Sankila R, Carpén O, Pukkala E, Sainio M, Jääskeläinen J. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000;54:71–76. doi: 10.1212/wnl.54.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Lu-Emerson C, Plotkin SR. The neurofibromatoses. Part 1: NF1 Rev Neurol Dis. 2009;6:E47–E53. [PubMed] [Google Scholar]
- 4.Lu-Emerson C, Plotkin SR. The neurofibromatoses. Part 2: NF2 and schwannomatosis. Rev Neurol Dis. 2009;6:E81–E86. [PubMed] [Google Scholar]
- 5.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–981. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- 6.Page PZ, Page GP, Ecosse E, Korf BR, Leplege A, Wolkenstein P. Impact of neurofibromatosis 1 on Quality of Life: a cross-sectional study of 176 American cases. Am J Med Genet A. 2006;140:1893–1898. doi: 10.1002/ajmg.a.31422. [DOI] [PubMed] [Google Scholar]
- 7.Kodra Y, Giustini S, Divona L, et al. Health-related quality of life in patients with neurofibromatosis type 1. A survey of 129 Italian patients. Dermatology. 2009;218:215–220. doi: 10.1159/000187594. [DOI] [PubMed] [Google Scholar]
- 8.Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplège A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch Dermatol. 2001;137:1421–1425. doi: 10.1001/archderm.137.11.1421. [DOI] [PubMed] [Google Scholar]
- 9.Neary WJ, Hillier VF, Flute T, Stephens SD, Ramsden RT, Evans DG. The relationship between patients' perception of the effects of neurofibromatosis type 2 and the domains of the Short Form-36. Clin Otolaryngol. 2010;35:291–299. doi: 10.1111/j.1749-4486.2010.02176.x. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson B, Samuelsson S. Neurofibromatosis in Gothenburg, Sweden. I. Background, study design and epidemiology. Neurofibromatosis. 1989;2:6–22. [PubMed] [Google Scholar]
- 11.Zöller ME, Rembeck B. A psychiatric 12-year follow-up of adult patients with neurofibromatosis type 1. J Psychiatr Res. 1999;33:63–68. doi: 10.1016/s0022-3956(98)00052-1. [DOI] [PubMed] [Google Scholar]
- 12.Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplège A. Visibility of neurofibromatosis 1 and psychiatric morbidity. Arch Dermatol. 2003;139:103–104. doi: 10.1001/archderm.139.1.103. [DOI] [PubMed] [Google Scholar]
- 13.Neary W, Stephens D, Ramsden RT, Evans DG. Psychosocial effects of neurofibromatosis type 2 (Part 1): general effects. Audiol Med. 2006;4:202–210. [Google Scholar]
- 14.Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385–401. [Google Scholar]
- 16.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 17.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Sage Publications; Newbury Park, CA: 1988. pp. 31–67. [Google Scholar]
- 18.Rosenberg M. Society and the Adolescent Self-Image. Wesleyan University Press; Middletown, CT: p. 1989. [Google Scholar]
- 19.Sinclair SJ, Blais MA, Gansler DA, Sandberg E, Bistis K, LoCicero A. Psychometric properties of the Rosenberg Self-Esteem Scale: overall and across demographic groups living within the United States. Eval Health Prof. 2010;33:56–80. doi: 10.1177/0163278709356187. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: p. 1983. [Google Scholar]
- 21.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Mult Scler. 2009;15:385–392. doi: 10.1177/1352458508099477. [DOI] [PubMed] [Google Scholar]
- 22.Goretti B, Portaccio E, Zipoli V, et al. Coping strategies, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2009;30:15–20. doi: 10.1007/s10072-008-0009-3. [DOI] [PubMed] [Google Scholar]
- 23.Sundaram M, Kavookjian J, Patrick JH, Miller LA, Madhavan SS, Scott VG. Quality of life, health status and clinical outcomes in Type 2 diabetes patients. Qual Life Res. 2007;16:165–177. doi: 10.1007/s11136-006-9105-0. [DOI] [PubMed] [Google Scholar]
- 24.Surwit RS, van Tilburg MA, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34. doi: 10.2337/diacare.25.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Ried LD, Tueth MJ, Taylor MD, Sauer BC, Lopez LM, Pepine CJ. Depressive symptoms in coronary artery disease patients after hypertension treatment. Ann Pharmacother. 2006;40:597–604. doi: 10.1345/aph.1G438. [DOI] [PubMed] [Google Scholar]
- 26.Astin F, Jones K, Thompson DR. Prevalence and patterns of anxiety and depression in patients undergoing elective percutaneous transluminal coronary angioplasty. Heart Lung. 2005;34:393–401. doi: 10.1016/j.hrtlng.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 28.Fischer DJ, Villines D, Kim YO, Epstein JB, Wilkie DJ. Anxiety, depression, and pain: differences by primary cancer. Support Care Cancer. 2010;18:801–810. doi: 10.1007/s00520-009-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giesler RB, Given B, Given CW, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse-driven intervention. Cancer. 2005;104:752–762. doi: 10.1002/cncr.21231. [DOI] [PubMed] [Google Scholar]
- 30.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78(3 Pt 1):302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 31.Parker PA, Kudelka A, Basen-Engquist K, Kavanagh J, de Moor J, Cohen L. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol Oncol. 2006;100:495–500. doi: 10.1016/j.ygyno.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 33.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Otolaryngology-Head and Neck Surgery Foundation I. Committee on hearing and equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma) Otolaryngol Head Neck Surg. 1995;113:179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 35.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 36.Rumsey N, Clarke A, White P, Wyn-Williams M, Garlick W. Altered body image: appearance-related concerns of people with visible disfigurement. J Adv Nurs. 2004;48:443–453. doi: 10.1111/j.1365-2648.2004.03227.x. [DOI] [PubMed] [Google Scholar]
- 37.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 38.Katon W, Von Korff M, Lin E, et al. Distressed high utilizers of medical care. DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry. 1990;12:355–362. doi: 10.1016/0163-8343(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin CM, Colley A, Donnai D, Kingston H, Harris R, Kerzin-Storrar L. Neurofibromatosis type 1 (NF1): knowledge, experience, and reproductive decisions of affected patients and families. J Med Genet. 1993;30:567–574. doi: 10.1136/jmg.30.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]


