Abstract
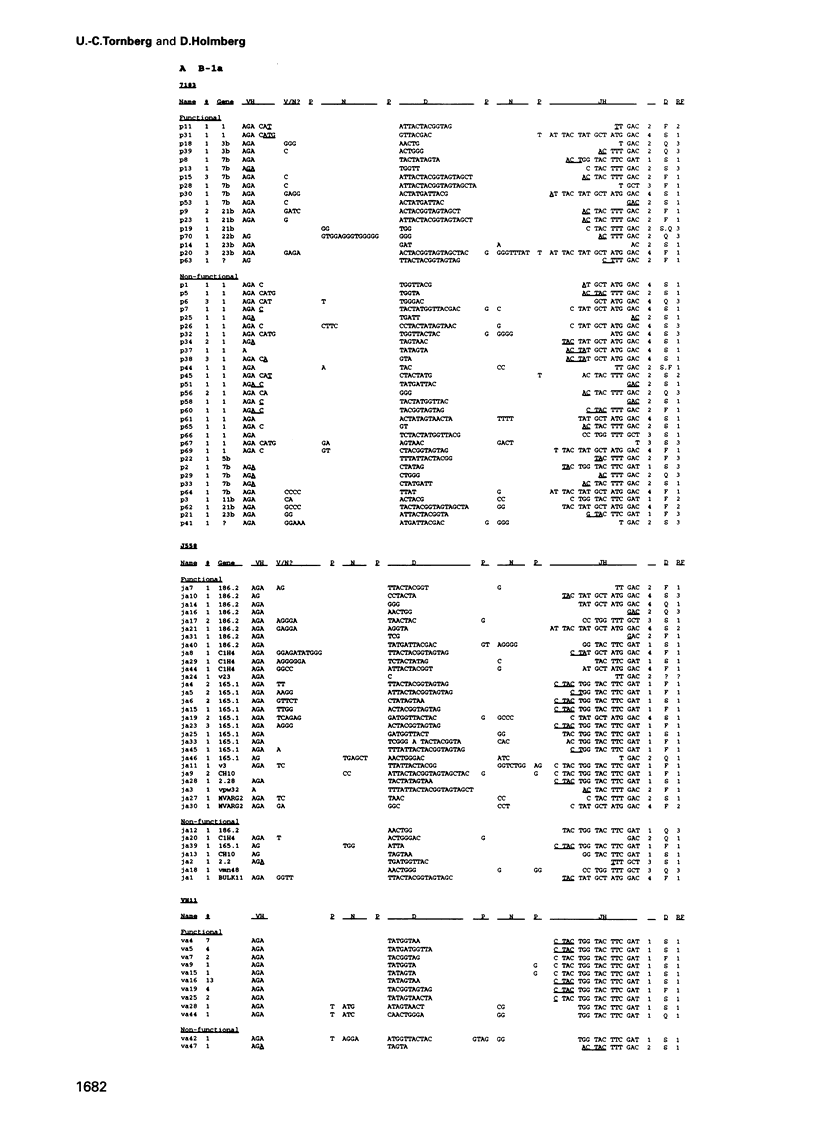
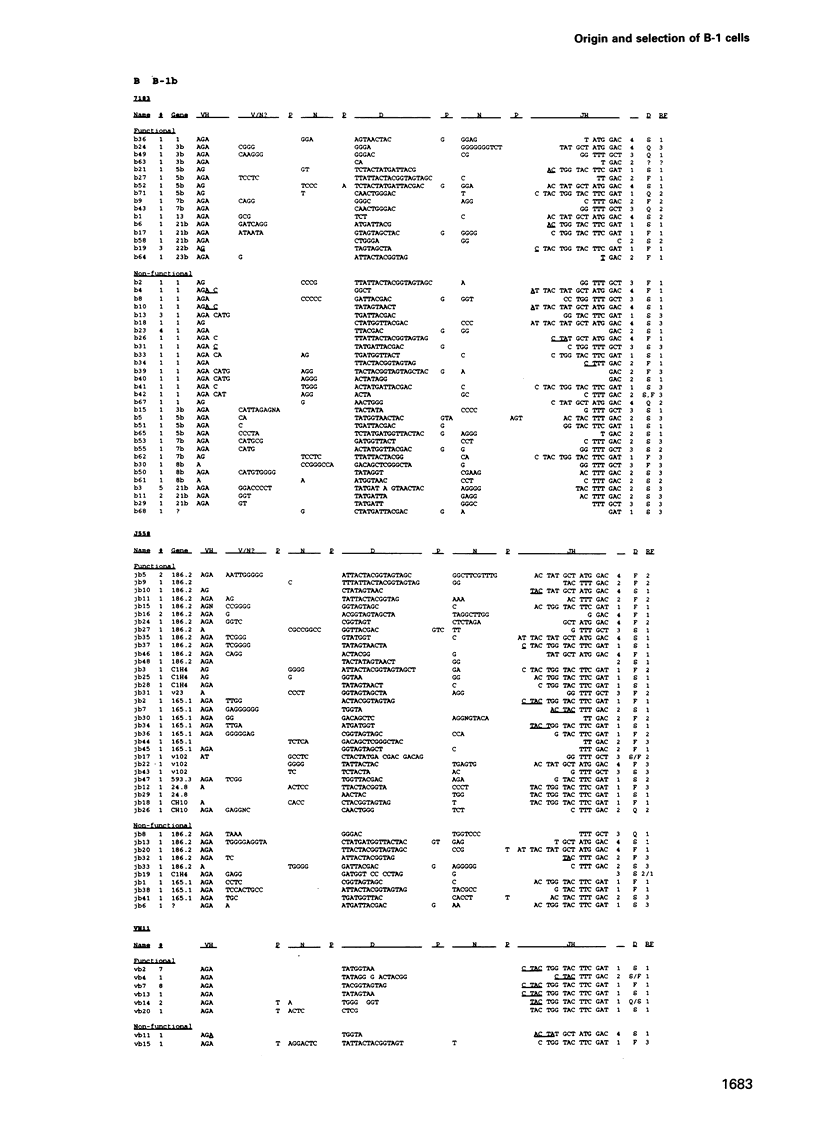
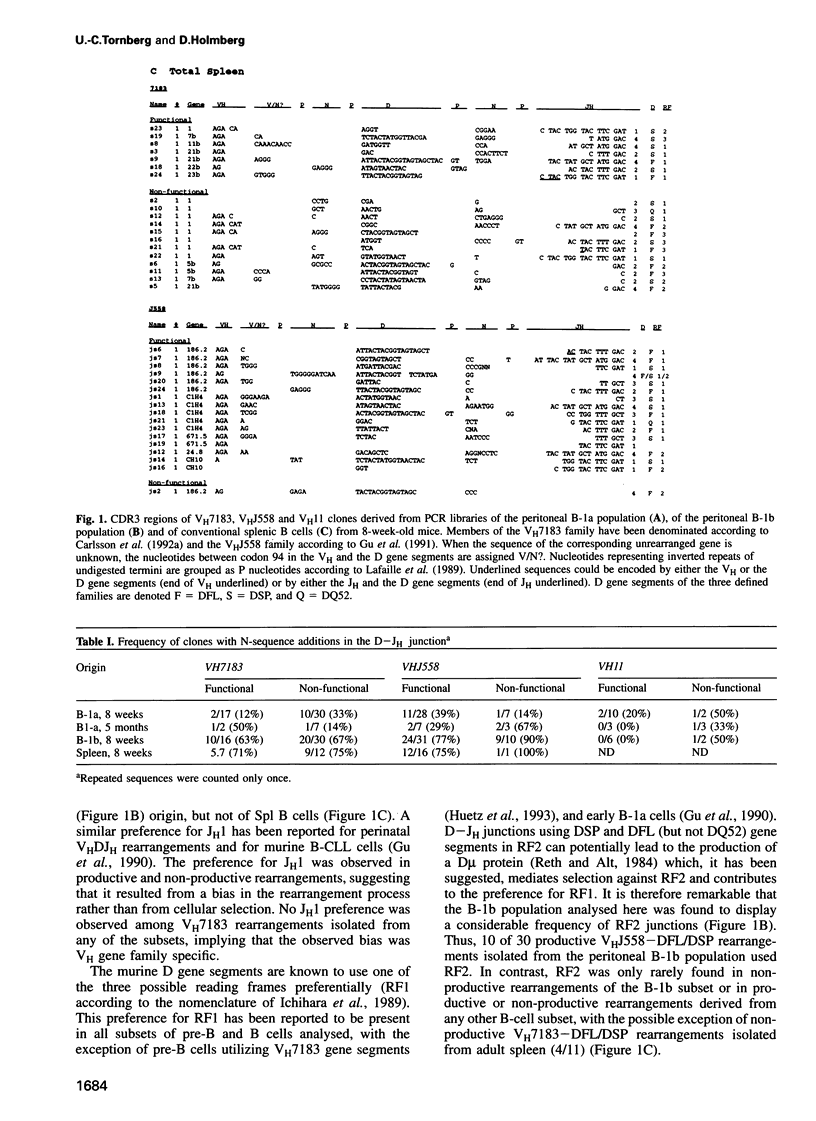
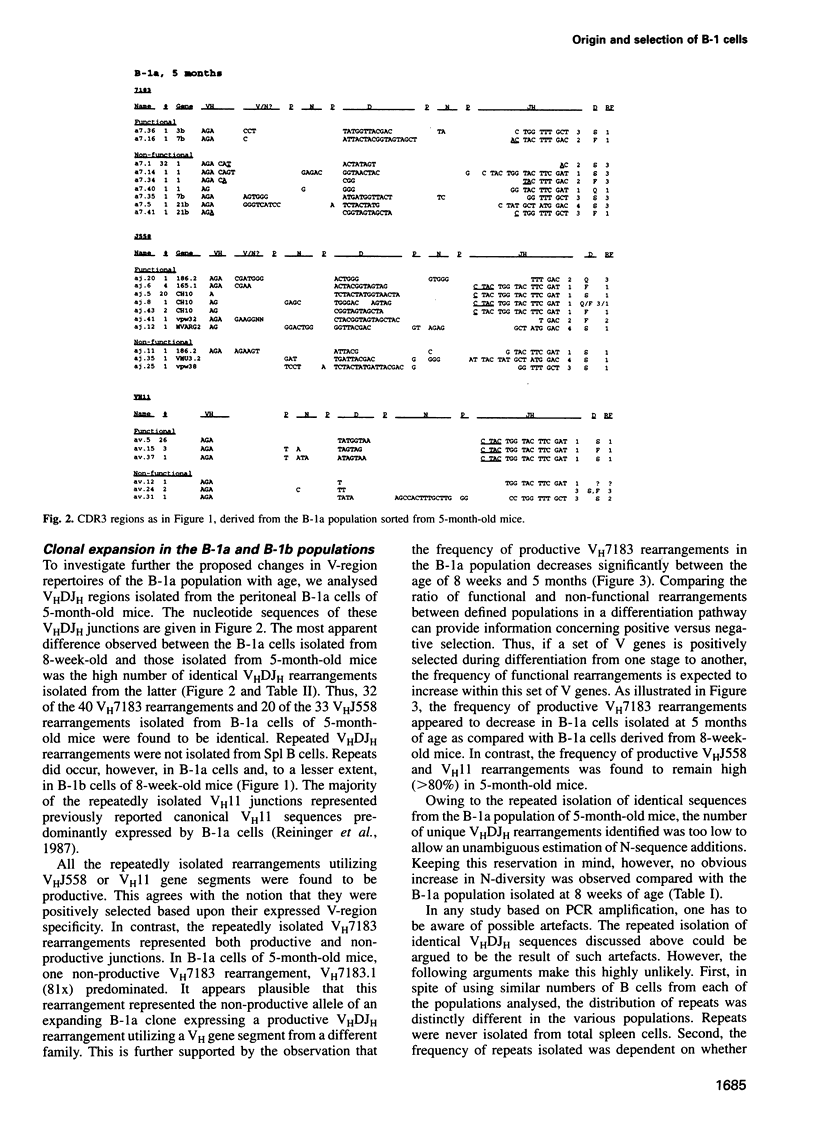
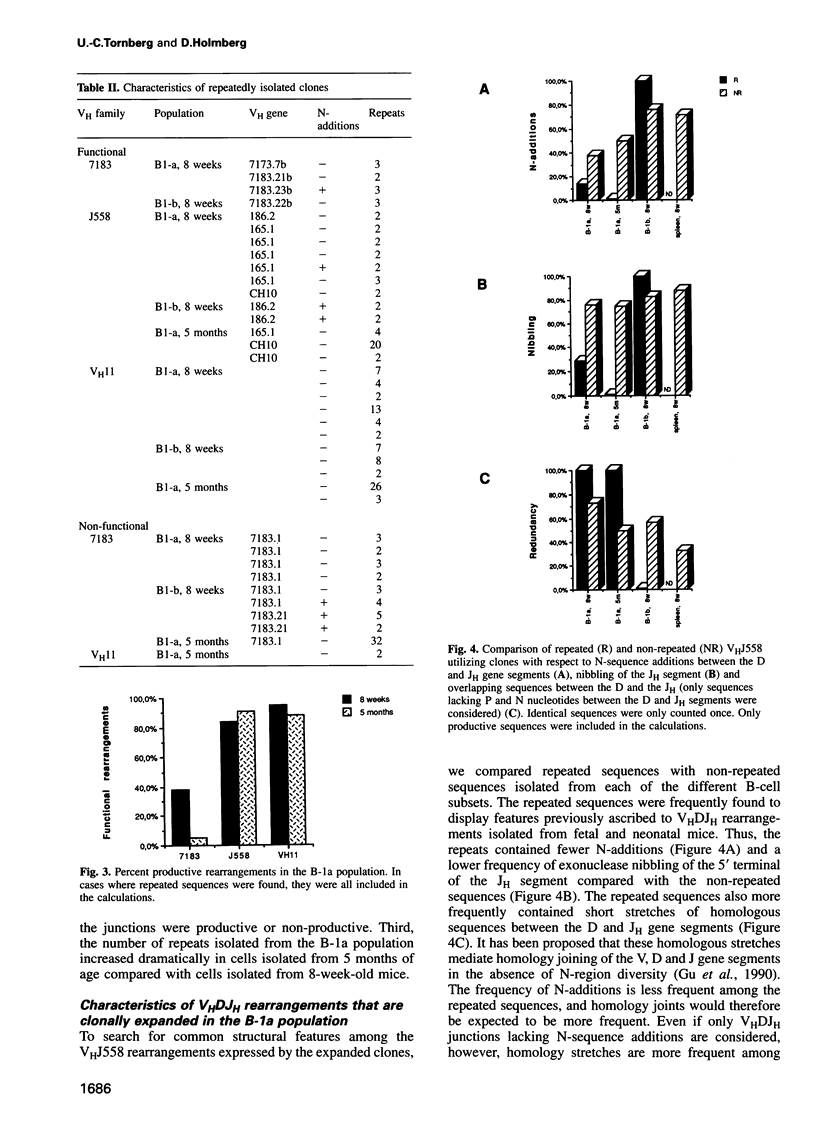
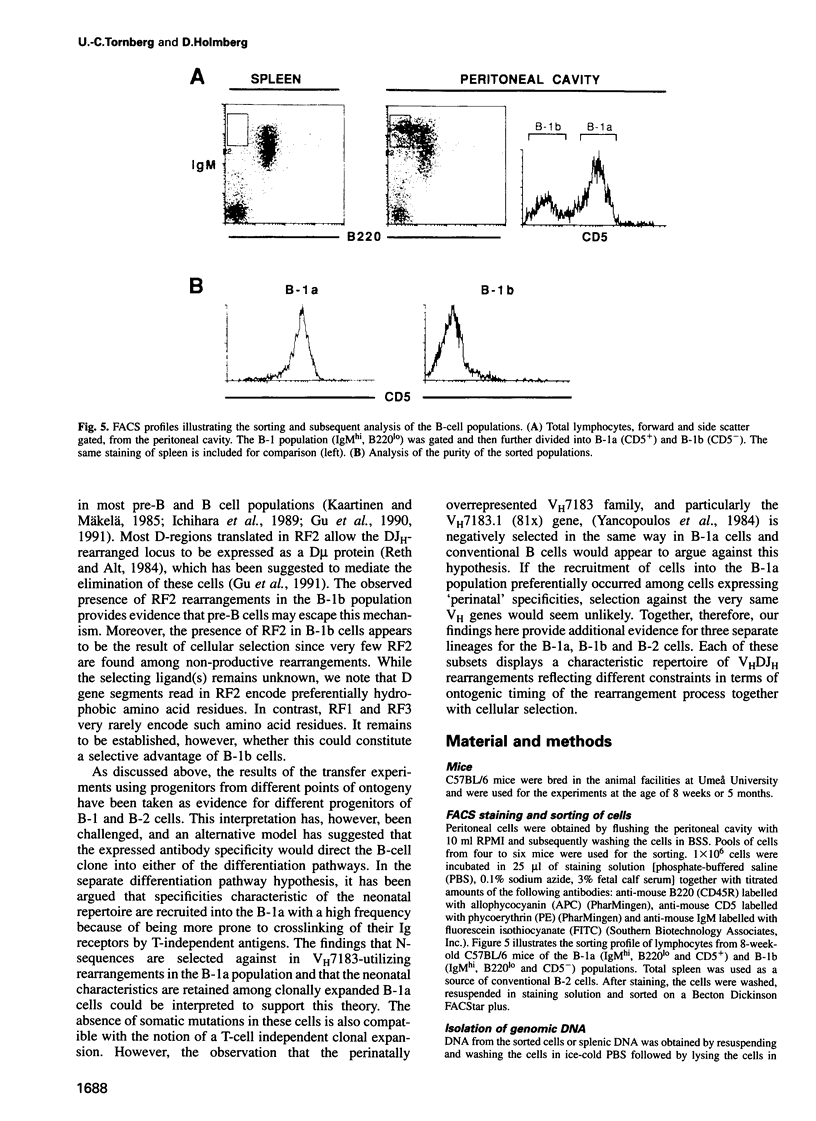
Analyses of VHDJH rearrangements isolated from murine peritoneal B-1a cells (CD5+, IgMhi, B220lo), peritoneal B-1b cells (CD5-, IgMhi, B220lo), and conventional splenic B cells provide evidence that a unique repertoire of VH regions is displayed by each of these B-cell subsets. The B-1a subset is characterized by a low N-region diversity, by a high frequency of sequence homologies in the VH-D and D-JH junctions, and by a limited exonuclease nibbling of the terminals of the joining gene segments. Through expansion in ageing mice, B-1a clones with these properties are favoured. B-1b cells are similar to conventional B-2 cells with respect to N-region diversity, but are unique in terms of D gene expression. Thus, while most murine pre-B and B cells preferentially use DSP and DFL gene segments in a given reading frame (RF1), B-1b cells frequently express D genes in another reading frame (RF2). Together, these findings provide structural evidence for a model where B-1a, B-1b and B-2 cells are produced by separate progenitors that are active at different stages of ontogeny.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Holmberg D. Genetic basis of the neonatal antibody repertoire: germline V-gene expression and limited N-region diversity. Int Immunol. 1990;2(7):639–643. doi: 10.1093/intimm/2.7.639. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Overmo C., Holmberg D. Developmentally controlled selection of antibody genes: characterization of individual VH7183 genes and evidence for stage-specific somatic diversification. Eur J Immunol. 1992 Jan;22(1):71–78. doi: 10.1002/eji.1830220112. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Overmo C., Holmberg D. Selection against N-region diversity in immunoglobulin heavy chain variable regions during the development of pre-immune B cell repertoires. Int Immunol. 1992 May;4(5):549–553. doi: 10.1093/intimm/4.5.549. [DOI] [PubMed] [Google Scholar]
- Carmack C. E., Shinton S. A., Hayakawa K., Hardy R. R. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990 Jul 1;172(1):371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y. Z., Rabin E., Wortis H. H. Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int Immunol. 1991 May;3(5):467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989 Aug;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster I., Gu H., Rajewsky K. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 1988 Dec 1;7(12):3693–3703. doi: 10.1002/j.1460-2075.1988.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Godin I. E., Garcia-Porrero J. A., Coutinho A., Dieterlen-Lièvre F., Marcos M. A. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993 Jul 1;364(6432):67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Whitmore A. C., Clarke S. H. B-1 cells are made, not born. Immunol Today. 1993 Feb;14(2):84–91. doi: 10.1016/0167-5699(93)90064-R. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Carmack C. E., Hyman R., Hardy R. R. Natural autoantibodies to thymocytes: origin, VH genes, fine specificities, and the role of Thy-1 glycoprotein. J Exp Med. 1990 Sep 1;172(3):869–878. doi: 10.1084/jem.172.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986 Apr;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Kantor A. B. B-cell lineages exist in the mouse. Immunol Today. 1993 Feb;14(2):79–90. doi: 10.1016/0167-5699(93)90063-Q. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Andersson A., Carlsson L., Forsgren S. Establishment and functional implications of B-cell connectivity. Immunol Rev. 1989 Aug;110:89–103. doi: 10.1111/j.1600-065x.1989.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Wennerström G., Andrade L., Coutinho A. The high idiotypic connectivity of "natural" newborn antibodies is not found in adult mitogen-reactive B cell repertoires. Eur J Immunol. 1986 Jan;16(1):82–87. doi: 10.1002/eji.1830160116. [DOI] [PubMed] [Google Scholar]
- Huetz F., Carlsson L., Tornberg U. C., Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993 May;12(5):1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y., Hayashida H., Miyazawa S., Kurosawa Y. Only DFL16, DSP2, and DQ52 gene families exist in mouse immunoglobulin heavy chain diversity gene loci, of which DFL16 and DSP2 originate from the same primordial DH gene. Eur J Immunol. 1989 Oct;19(10):1849–1854. doi: 10.1002/eji.1830191014. [DOI] [PubMed] [Google Scholar]
- Kantor A. B., Stall A. M., Adams S., Herzenberg L. A., Herzenberg L. A. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A. A new nomenclature for B cells. Immunol Today. 1991 Nov;12(11):388–388. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986 Dec;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Kehry M. R., Hudak S. A. Characterization of B-cell populations bearing Fc epsilon receptor II. Cell Immunol. 1989 Feb;118(2):504–515. doi: 10.1016/0008-8749(89)90397-3. [DOI] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Herzenberg L. A., Adams S., Stall A. M. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989 Mar;19(3):507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- Manohar V., Brown E., Leiserson W. M., Chused T. M. Expression of Lyt-1 by a subset of B lymphocytes. J Immunol. 1982 Aug;129(2):532–538. [PubMed] [Google Scholar]
- Marcos M. A., Huetz F., Pereira P., Andreu J. L., Martinez-A C., Coutinho A. Further evidence for coelomic-associated B lymphocytes. Eur J Immunol. 1989 Nov;19(11):2031–2035. doi: 10.1002/eji.1830191110. [DOI] [PubMed] [Google Scholar]
- Mercolino T. J., Arnold L. W., Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J Exp Med. 1986 Jan 1;163(1):155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercolino T. J., Arnold L. W., Hawkins L. A., Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988 Aug 1;168(2):687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell C. A., Arnold L. W., Haughton G., Clarke S. H. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J Immunol. 1988 Oct 15;141(8):2788–2796. [PubMed] [Google Scholar]
- Pennell C. A., Maynard E., Arnold L. W., Haughton G., Clarke S. H. High frequency expression of S107 VH genes by peritoneal B cells of B10.H-2aH-4bP/WTS mice. J Immunol. 1990 Sep 1;145(5):1592–1597. [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Reininger L., Kaushik A., Izui S., Jaton J. C. A member of a new VH gene family encodes antibromelinized mouse red blood cell autoantibodies. Eur J Immunol. 1988 Oct;18(10):1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- Reininger L., Ollier P., Poncet P., Kaushik A., Jaton J. C. Novel V genes encode virtually identical variable regions of six murine monoclonal anti-bromelain-treated red blood cell autoantibodies. J Immunol. 1987 Jan 1;138(1):316–323. [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason N., Lehuen A., Kearney J. F. An embryonic source of Ly1 but not conventional B cells. Int Immunol. 1991 Jun;3(6):543–550. doi: 10.1093/intimm/3.6.543. [DOI] [PubMed] [Google Scholar]
- Tarlinton D., Stall A. M., Herzenberg L. A. Repetitive usage of immunoglobulin VH and D gene segments in CD5+ Ly-1 B clones of (NZB x NZW)F1 mice. EMBO J. 1988 Dec 1;7(12):3705–3710. doi: 10.1002/j.1460-2075.1988.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil M., Kearney J. F. Functional characterization of monoclonal auto-anti-idiotype antibodies isolated from the early B cell repertoire of BALB/c mice. Eur J Immunol. 1986 Sep;16(9):1151–1158. doi: 10.1002/eji.1830160920. [DOI] [PubMed] [Google Scholar]
- Waldschmidt T. J., Kroese F. G., Tygrett L. T., Conrad D. H., Lynch R. G. The expression of B cell surface receptors. III. The murine low-affinity IgE Fc receptor is not expressed on Ly 1 or 'Ly 1-like' B cells. Int Immunol. 1991 Apr;3(4):305–315. doi: 10.1093/intimm/3.4.305. [DOI] [PubMed] [Google Scholar]
- Wetzel G. D. Interleukin 5 regulation of peritoneal Ly-1 B lymphocyte proliferation, differentiation and autoantibody secretion. Eur J Immunol. 1989 Sep;19(9):1701–1707. doi: 10.1002/eji.1830190926. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]