Summary
Purpose
The orbitofrontal (OF) region is one of the least explored regions of the cerebral cortex. There are few studies on patients with electrophysiologically and surgically confirmed OF epilepsy and a negative MRI. We aimed to examine the neuroimaging characteristics of MRI-negative OF epilepsy with the focus on a voxel-based morphometric MRI post-processing technique.
Methods
We included 6 patients with OF epilepsy, who met the following criteria: surgical resection of the OF lobe with/without adjacent cortex, seizure-free for ≥ 12 months, invasive video-EEG monitoring showing ictal onset from the OF area, and pre-operative MRI regarded negative. Patients were investigated in terms of their image postprocessing and functional neuroimaging characteristics, electroclinical characteristics obtained from noninvasive and invasive evaluations, and surgical pathology. MRI postprocessing on T1-weighted high-resolution scans was implemented with a Morphometric Analysis Program (MAP) in MATLAB SPM5.
Key findings
Single MAP+ abnormalities were found in 4 patients; three were in the OF region and one in the ipsilateral mesial frontal area. These abnormalities were included in the resection. One patient had bilateral MAP+ abnormalities in the OF region, with the ipsilateral one completely removed. The MAP+ foci were concordant with invasive electrophysiological data in the majority of MAP+ patients (4 of 5). The localization value of FDG-PET and ictal SPECT is low in this cohort. Surgical pathology included focal cortical dysplasia, remote infarct, rosenthal fiber formation and gliosis.
Significance
Our study highlights the importance of MRI post-processing in the process of presurgical evaluation of patients with suspected orbitofrontal epilepsy and “normal” MRI. Using MAP, we were able to positively identify subtle focal abnormalities in the majority of the patients. MAP results need to be interpreted in the context of their electroclinical findings and can provide valuable targets in the process of planning invasive evaluation.
Keywords: epilepsy, MRI, presurgical evaluation, MRI post-processing, MRI-negative epilepsy, voxel-based morphometry, orbitofrontal epilepsy, focal cortical dysplasia
Introduction
Among all cerebral regions, the orbitofrontal (OF) cortex is one of the least studied and understood region. It has vast bidirectional connectivity to a widely distributed network involving the frontal lobe, the temporal lobe and the limbic system, making localization of ictal foci to this region particularly difficult (Alexopoulos & Tandon 2008, Cavada & Schultz 2000). Because of the hidden, deep location of the OF region in relation to the scalp electrode sampling, scalp EEG can be at times misleading with the lack of distinct ictal manifestations (Burgess, et al. 2005, Ebner 2001, Pedley, et al. 2003)
High-resolution structural magnetic resonance imaging (MRI), when interpreted by experienced neuro-radiologists, can be very helpful to search for focal abnormalities in the OF region. When a patient presents with a negative pre-operative MRI, imaging with higher magnetic field or three-dimensional volume sequence, post-processing analysis, as well as functional neuroimaging such as positron emission tomography (PET), single-photon emission computed tomography (SPECT) and magnetoencephalography, may be important complements to the test battery of pre-surgical evaluation (Bien, et al. 2009, Knowlton, et al. 2008). In the literature, there are only rare cases documenting clear OF epilepsy with a negative pre-operative MRI (Kriegel, et al. 2012, Rugg-Gunn, et al. 2002, Smith, et al. 2004). Moreover, there was no study specifically reporting post-processing neuroimaging characteristics of OF epilepsies in the setting of a normal pre-operative MRI.
Recently, a voxel-based MRI post-processing technique has been used to detect subtle cortical malformations that can be missed with conventional MRI visual inspection (Huppertz, et al. 2005, Huppertz, et al. 2008, Wagner, et al. 2011). This technique compares each individual patient with a normal control database, and yields 3D feature maps of gray-white junction, cortical gyration, and cortical thickness. Abnormalities in these feature maps can indicate underlying focal cortical dysplasia (FCD) (Krsek, et al. 2008). The yield and specificity of the findings using this post-processing technique in patients with OF epilepsy have not been reported before.
In this study, we propose to examine the image post-processing and functional neuroimaging (FDG-PET and ictal SPECT) characteristics of a strictly defined and highly selected, invasive EEG confirmed group of pharmacoresistant OF epilepsy patients with negative pre-operative MRI. All patients were rendered seizure-free after resection of the OF region with/without adjacent cortex. In addition, we report the electroclinical features obtained from noninvasive and invasive studies and pathological findings in these patients.
Methods
Patients
This retrospective study was approved by the Cleveland Clinic institutional review board. From the consecutive surgical series of Cleveland Clinic Epilepsy Center between January 1999 and December 2011, patients with pharmacoresistant epilepsy were identified with the following criteria: 1) invasive video-EEG monitoring confirmed focal OF ictal onset during recorded habitual seizures; 2) surgical resection of the OF region with/without adjacent cortex rendered the patient seizure-free for more than 12 months (Engel class 1 outcome) (Engel, et al. 1993); 3) pre-operative MRI were interpreted as normal; and 4) preoperative and postoperative MRI data were available. Clinical data were collated from medical charts, archived scalp and invasive video-EEG monitoring databases and neuroimaging records.
Pre-surgical Evaluations
All patients underwent one or more MRI 1.5 Tesla (1.5T) or 3 Tesla (3T) studies with a standard epilepsy protocol with Siemens scanners (Erlangen, Germany). Detailed imaging parameters can be found elsewhere (Wang, et al. 2012). All patients underwent scalp video-EEG monitoring, PET, and subsequently intracranial electrode implantation. Ictal SPECT was performed whenever possible. Implantation and resection decisions were made at a multi-disciplinary patient management conference (PMC) where all modalities were presented and consensus recommendations were made. MRI Postprocessing was not available at the time of the evaluations and therefore was only studied retrospectively. Generally, patients who have no MRI lesion, present with poorly localized scalp EEG and hypermotor seizures, and those with seizures manifesting as temporal lobe seizures in the absence of temporal lobe pathology, are usually investigated with intracranial electrodes sampling from the OF region in our center.
PET scans were obtained after injection of 5 to 10mCi 18FDG (fluorodeoxyglucose) during the interictal state, with patient resting in dimly lit room with eyes open. Scans were performed on Biograph PET CT (Siemens AG, Munich, Germany). Scans were coregistered to the volumetric MRI using vendor software. Visual analysis was performed first independent of other modalities, and then confirmed at PMC by a nuclear medicine physician. Presence and localization of relative focal hypometabolism were then reported.
SPECT images were acquired on a Siemens (Erlangen, Germany) Symbia dual-head camera (SPECT: 15s per stop x60 stops, 128x128 matrix, iterative reconstruction with attenuation correction; CT: 30mAs, 5mm slice). Interictal injection occurred after the patient was free of seizures for a minimum of 24-hours. Ictal injection of radioisotope 99mTc-ECD (ethyl-cysteinate dimer; 1.6 mL) was administered by a specially trained nurse who remained at the bedside, when the patient underwent scalp video-EEG monitoring. Within 2 hours after injection, SPECT images were acquired. The interictal image was subtracted from the ictal image and analyzed following methodology of SISCOM (subtraction ictal SPECT coregistered to MRI) (O'Brien, et al. 1998).
MRI Post-processing
MRI post-processing was performed using a Morphometric Analysis Program (MAP) in SPM (Wellcome Department of Cognitive Neurology, London, UK) within MATLAB 2007a (MathWorks, Natick, Massachusetts) on pre-operative T1-weighed Magnetization Prepared Rapid Acquisition with Gradient Echo (MPRAGE) images (Huppertz, et al. 2005, Huppertz, et al. 2008). Due to the time span of the patients selected, 3 patients had 1.5T MRI (best available at that time), and 3 patients had 3T MRI. To ensure maximal sensitivity (Huppertz, et al. 2010), we used different normal databases for 1.5T and 3T images. For 3T MRI, we used a database comprised of 90 normal controls (41 female, 49 male, mean age 42.0 years, range 22–80 years), whose MRIs were acquired on the same 3T Siemens Trio scanner using the same MPRAGE sequence as the patients. For 1.5T MRI, we used an average database of 1.5T and 3T average normal database of 150 controls, 70 female, 80 male, mean age at MRI 30.9 years, range 15–77 years, with MRIs acquired on five different MRI scanners, which was kindly provided along with the MAP program (Huppertz, et al. 2008). The output of MAP consists of three z-score feature maps in which brain structures deviating from the average normal brain have high z-scores, thus highlighting subtle abnormalities on the MRI. The junction file is sensitive to the blurring of the gray-white matter junction; the extension file is sensitive to abnormal gyration and extension of gray matter into white matter; the thickness file is sensitive to abnormal cortical thickness. More details can be found elsewhere (Huppertz, et al. 2008). On a standard PC, each MAP processing took approximately 40 minutes.
MAP abnormalities were searched for in the entire brain. A blinded reviewer (ZIW) used the z-score of 4 to identify highlighted areas on the junction file. If an abnormality is detected on the junction file, the reviewer examined whether there was an accompanying z>4 region on the extension file and the thickness file. Then with the guidance from these candidate areas of abnormality, a neuroradiologist (SEJ) conducted a focused re-review of the pre-surgical clinical MRI (with T1-weighted MPRAGE, T2-weighted FLAIR and TSE sequences), in order to confirm or dismiss each candidate MAP abnormality. Only the confirmed abnormalities were regarded as MAP+. This methodology is consistent with our previous report (Wang, et al. 2012) and literature (Wagner, et al. 2011). Images for this study were processed and reviewed as part of a large ongoing retrospective study, which examines the sensitivity and specificity of MAP to detect subtle abnormalities in a consecutive cohort of epilepsy patients with a negative MRI. Mixed with patient scans were control scans obtained from normal subjects. Neither the MAP reviewer nor the neuroradiologist was given prior information about the type of epilepsy, or whether it was a patient or control.
Results
Patient population
From January 1999 to December 2011, there were 1841 resective surgeries of which 290 involved lobar or sublobar resections of the frontal lobe. A total of 28 patients were identified who underwent resections that involved the orbitofrontal region with or without additional sublobar areas. Among these 28 patients, only 9 had a clear MRI lesion within the orbitofrontal area. Six MRI-negative patients were identified who fulfilled the selection criteria (5 right-handed patients, 5 females, mean age at surgery = 32.0 ± 16.1 years, age range: 8 – 51 years, mean epilepsy duration = 18.2 ± 12.7 years, epilepsy duration range: 6 – 40 years). Seizures were defined according to the semiologic seizure classification (Luders, et al. 1998). Detailed clinical information is summarized in Table 1.
Table 1.
Summary of data used during presurgical evaluation, including demographics data, semiology, seizure frequency, scalp-EEG evaluation and subsequent ICEEG localization.
| Pat-No. | Age | Sex | Hand | ED (y) | Seizure Semiology (Luders, et al. 1998) | Seizure Frequency | Interictal scalp EEG | Ictal scalp EEG onset | Invasive procedure (n electrodes / n contacts) | Invasive EEG onset |
|---|---|---|---|---|---|---|---|---|---|---|
| P1* | 51 | F | R | 14 | Nonspecific aura → Automotor seizure | 10 / month | Regional L temporal (max T7) | Regional L temporal | SEEG (10/92) | L orbitofrontal |
| P2 | 38 | F | R | 24 | Type 1: Nonspecific aura → Hypermotor seizure Type 2: No aura → Hypermotor seizure |
3-4 / month | Regional L frontal (max SP1) | Nonlocalizable | SDG (6/120) | L orbitofrontal |
| P3 | 20 | F | R | 18 | Abdominal aura → Hypermotor seizure | 10 / month | Regional R temporal (max SP2, 70%) Regional L temporal (max SP1, 30%) |
Regional R temporal | SDG (7/174) DE (3/24) |
R orbitofrontal |
| P4 | 45 | M | R | 40 | Abdominal aura → Axial tonic seizure → Hypermotor seizure | 20 / month | Regional L temporal (max SP1 =T7) | Regional L frontocentral 26 szs | SEEG (14/126) | L orbitofrontal |
| P5 | 8 | F | R | 6 | No aura → Hypermotor seizure | up to 150 / day | Regional R frontal (max Fp2=F4=F8) | Regional R Frontal | SDG (7/210) | R orbitofrontal |
| P6 | 30 | F | L | 7 | Abdominal and gustatory aura → Hypermotor seizure | 12 / month | Regional R frontotemporal (max T8/FT10/F10=T10/FT8/SP2) | Regional R frontotemporal | SEEG (12/112) | R orbitofrontal |
F=female; M=male; L=left; R=right; ED=epilepsy duration; y=years; SEEG=stereotactic EEG; SDG=subdural grids; DE=depth electrodes
Patient 1 had prior (13 years before) L anterior temporal lobectomy with seizures recurring shortly after.
Scalp EEG and invasive EEG Findings
On scalp EEG, interictal epileptiform discharges were recorded in all 6 patients (ipsilateral temporal = 2, ipsilateral frontal = 2, ipsilateral frontotemporal = 1, bilateral temporal =1). In the majority of the patients, ictal onset of scalp EEG was mapped to the ipsilateral frontal and/or temporal region (temporal = 2, frontal = 1, frontocentral = 1, frontotemporal = 1). Ictal onset in one patient was non-localizable.
Three patients had subdural grid implantation with depth electrodes, and 3 patients underwent stereotactic EEG depth electrodes (SEEG) implantations. In all patients, invasive video-EEG confirmed ipsilateral OF focal ictal onset during their habitual seizures (see Table 1, Figures 1, 2 and 3).
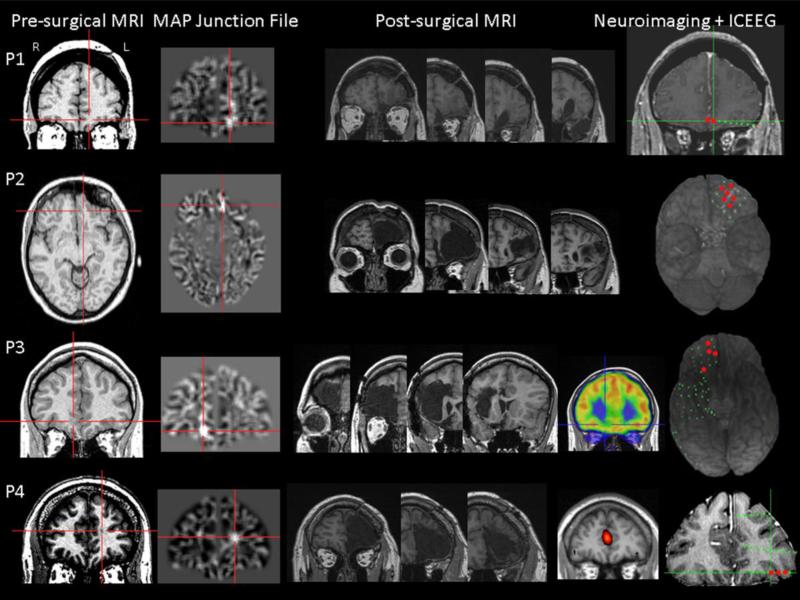
Figure 1.
Four OF epilepsy patients with positive MAP findings shown on gray-white junction file. In this and Figure 2, first column: pre-surgical T1-weighted image; second column: coregistered MAP junction file; third column: post-surgical MRI indicating resection of the OF region. The red crosses show the location of the subtle abnormalities. The rest of the column(s) contain illustrations of neuroimaging/intracranial EEG (ICEEG) findings when applicable (non-localizable or discordant findings were not shown but reported in Tables 1 and 2). Red electrode contacts indicate ictal onset during invasive monitoring.
Figure 2.
An OF epilepsy patient who had three abnormalities indicated by MAP junction file; each abnormality is shown on a separate row. Surgical resection occurred only in the right OF region, concordant with PET and ictal onset on intracranial EEG.
Figure 3.
An OF epilepsy patient with negative MAP findings. The left column shows the post-surgical MRI, the middle column is an illustration of SPECT localization, and the right column indicates intracranial EEG ictal onset zone from the OF region.
MAP Findings
MAP+ abnormalities were found in 5 of 6 patients. In P1, P2 and P3, MAP gray-white junction file pinpointed a subtle abnormality in the OF area, concordant with ictal invasive findings. In P4, MAP+ abnormality was in the mesial frontal area, but separated from the OF region. Resection included the MAP+ abnormality in all 4 patients. Given that all patients were seizure-free, these lesions were considered true positives. P5 was unique: she is the only patient with bilateral OF MAP+ changes. A contralateral epileptogenic focus was not suspected by the electroclinical and other noninvasive data. The patient underwent exploration with subdural electrodes targeting the right hemisphere and remains seizure-free following resection restricted to the right OF region. P2, P3 and P4 were found to have abnormalities on the MAP extension file in the same or adjacent region as the junction file, among which only P2 and 3 had accompanying abnormalities in the MAP thickness file. Details of neuroimaging, surgical and pathology data can be found in Table 2 and the MAP+ abnormalities are shown in Figures 1 and 2, side-by-side with coregistered T1-weighted images. Figure 3 shows neuroimaging and SEEG data for the MAP-negative patient, who did not have any abnormalities identified in OF or other cortical areas.
Table 2.
Summary of neuroimaging data (including MRI post-processing, FDG-PET and ictal SPECT), area of surgical resection and surgical pathology.
| Pat-No. | MAP | FDG-PET (S/L/M) | Ictal SPECT (injection time) (S/L/M) | Surgery | Pathology |
|---|---|---|---|---|---|
| P1* | L orbitofrontal | R anterior mesial temporal (S) | NA | L orbitofrontal resection | FCD IA (orbitofrontal specimen) |
| P2 | L orbitofrontal | Normal | R parietoccipital cortex (27s) (M) | L orbitofrontal resection extending to the L frontal pole | Nonspecific gliosis |
| P3 | R orbitofrontal | R temporal pole R mesial frontal (M) | R middle to anterior temporal lobe (10s) (L) | R orbitofrontal resection extending to the R frontal pole | Nonspecific gliosis |
| P4 | L mesial frontal | Bilateral frontoparietal (M) | L mesial frontal (11s) (S) | L orbitofrontal resection extending to the L frontal pole | Remote infarct with gliosis and Rosenthal fiber formation |
| P5 | R and L orbitofrontal | L superior parietal, bilateral temporal and occipital, R orbitofrontal (M) | NA | R orbitofrontal resection | FCD IIA |
| P6 | Negative | Bilateral mild hypometabolism in anterior and mesial temporal, minimally worse on the R (M) | R anterior mesial temporal and orbitofrontal (9s) (M) | R orbitofrontal resection plus temporal lobe and mesial structures | FCD IA (both orbitofrontal and temporal specimens) No HS |
L=left; R=right; S=sublobar; L=lobar; M=multilobar; FCD=focal cortical dysplasia; HS=hippocampal sclerosis
Patient 1 had prior (13 years before) L anterior temporal lobectomy with seizures recurring shortly after.
Functional Neuroimaging Findings
FDG-PET and ictal SPECT findings are summarized in Table 2 and illustrated in Figures 1, 2 and 3, when concordant with the OF region. Interictal FDG-PET falsely localized to ipsilateral mesial temporal region in one patient and was non-localizing in the remaining patients (normal FDG-PET = 1; multilobar glucose hypometabolism = 4). Upon retrospective analysis, the MAP+ focus was concordant with hypometabolism on PET in P3; in P5, one of the MAP+ foci was concordant with PET, was subsequently resected, and rendered the patient seizure-free.
Ictal SPECT was successfully obtained in 4 of the 6 patients (ictal injection time: 9-27 seconds). In P6, SISCOM analysis showed ictal hyperperfusion in ipsilateral OF and mesial temporal region. Hyperperfusion areas in the other patients included the ipsilateral frontal lobe, contralateral parieto-occipital cortex and ipsilateral temporal lobe, suggesting rapid propagation of seizures from the OF region. MAP+ focus was concordant with ictal SPECT hyperperfusion in only one patient (P4).
Surgery and Pathology
Left sided surgical resection was performed in 3 patients. Temporal structures were removed along with the OF region in P6. Anterior temporal resection of P1 was performed 13 years earlier without seizure improvement. Post-surgical MRI is shown in Figures 1, 2 and 3 for each patient. Surgical pathology revealed FCD in 3 patients, including FCD type IIA (n=1) and type IA (n=2) (Palmini, et al. 2004). In P4, pathology in tissue resected near the MAP+ focus was consistent with remote infarct with gliosis and Rosenthal fiber formation; in the OF region non-specific gliosis was found. Surgical Pathology of P2 and P3 showed non-specific gliosis (Table 2).
Discussion
We present here the largest series to date of MRI-negative orbitofrontal epilepsy. Our cohort of OF epilepsy was confirmed by invasive EEG recordings and postoperative seizure freedom. Our data show that voxel-based morphometric MRI post-processing can identify subtle abnormalities in the majority of patients we studied. Our results suggest that a MAP+ abnormality may guide a more focused invasive EEG strategy and a subsequent tailored surgical resection.
Despite the significant improvements in MRI technology, a significant number of patients with frontal lobe (and in particular orbitofrontal) epilepsy continue to have “normal” MRI studies upon visual analysis (Lorenzo, et al. 1995, Noe, et al. 2013, See, et al. 2013). The difficulty in identifying lesions within the OF region in particular may be due to the following possibilities: (1) the sulcation pattern in the OF region is highly variable and densely packed; (2) some MRI sequences (in particular, T2-weighted sequences) have higher susceptibility artifacts/geometric distortion due to the proximity of the OF region to the air-filled sinuses, therefore not providing adequate anatomic information at times (Kringelbach & Rolls 2004). For these reasons, subtle lesions in the OF region may not be obvious upon conventional MRI visual inspection. When non-invasive evaluation data (e.g. scalp EEG) do not point to a specific area of interest, MRI readers lack a testable anatomic hypothesis and subsequently the study may be read as negative. Under these circumstances, a whole-brain MRI post-processing technique that directs the reader's attention to suspicious abnormalities may prove to be helpful.
Focal cortical dysplasia, a common substrate in patients with refractory epilepsies, is frequently associated with blurring in gray-white matter junction (Krsek, et al. 2008). Therefore, it is not surprising to find abnormalities on MAP junction file to be associated with FCD histopathology in 2 patients (P1 and P5). Interestingly, pathology findings also showed 2 MAP+ patients had nonspecific gliosis, and the MAP-negative patient had FCD in the resected specimen. One explanation could be the limited sampling issue of the pathological tissue undergoing examination, under which circumstances single/isolated/small lesions could be missed. Another possible explanation is the hypothesis that MAP, as a post-processing technique of structural MRI, is not sensitive strictly to FCD per se, but to any pathological substrate (or combinations of them) causing T1 signal alteration leading to a blurred gray-white junction. This hypothesis is also consistent with the non-FCD pathology finding in P4. Consistent with our study, a 2004 study also reported one “nonlesional” OF epilepsy patient whose surgical pathology only showed gliosis; the patient remained seizure-free at 5-year follow-up (Smith, et al. 2004). A 2002 case study presented another patient without abnormality on conventional MRI but with increased diffusivity in the OF region. The patient underwent inferior frontal lobe resection and remained Engel class 2a. Pathology also showed gliosis but not dysplasia (Rugg-Gunn, et al. 2002).
Although most identified MAP+ abnormalities were correlated with in situ epileptogenicity, our data show that the presence of MAP+ abnormalities does not always directly correlate with active epileptogenicity. These abnormalities were not resected and no information regarding the histological characteristics can be obtained. As observed in P5, the resection of the structurally abnormal and EEG-proven epileptic abnormality leads to long lasting seizure control (> 5 years). The finding of structural abnormalities in areas outside the presumed epileptic focus is consistent with the literature (Colliot, et al. 2006, Fauser, et al. 2009, Salmenpera, et al. 2007, Yasuda, et al. 2010). These postprocessing imaging changes could be due to potentially epileptic/proepileptic abnormalities that were not epileptic at the time of invasive recordings, and may be the underlying cause of late seizure recurrence following epilepsy surgery (Najm, et al. 2013). Another explanation of the presence of “non-epileptic” focal abnormalities on MAP is the possibility of false positive changes. Previously published automated voxel-based morphometry studies had reported false positive findings in the control group, particularly when sensitivity of the patient group was maximized (Focke, et al. 2008). The significance of MAP+ regions should therefore be interpreted in the context of all other clinical test results.
Interictal and ictal scalp EEG rarely provide localizing information in OF epilepsy due to the large distance between the epileptogenic zone and the electrodes (Alexopoulos & Tandon 2008), however they can still have lateralizing value as suggested by our data. Similar to several previous studies, we found interictal and ictal EEG often falsely localized to the ipsilateral temporal region, although it is interesting to note that bifrontal discharges and onset were not seen in our group (Chang, et al. 1991, Jobst & Williamson 2005, Ludwig, et al. 1975). False localization to the temporal region occurs probably due to the close bidirectional connections between the temporal and OF region, as demonstrated by a classic study in 1958 using strychnine neuronography, in which the authors found that spikes generated in the OF region can quickly propagate to the ipsilateral temporal cortex and vice versa (Kendrick & Gibbs 1958).
Intracranial EEG remains the corner stone of orbitofrontal epilepsy evaluation. It is especially illustrative once a reasonable implantation hypothesis has been made based on the non-invasive evaluation. Intracranial EEG studies in all our patients sufficiently covered the OF region and exclusively pointed ictal onset to the OF region in all patients. The basal frontal region is usually sampled by a four-by-four subdural electrode array (shown in Figure 1, P2, P3 and Figure 2, P5), while stereotactic EEG can be especially useful to explore the generators located in the mesial and lateral aspects of the OF region (Figure 1, P1, P4, and Figure 3, P6) (Munari & Bancaud 1992). Due to the widespread connection from the OF area, coverage of the anterior and mesial temporal regions, insula, operculum, and cingulate gyrus is recommended to improve the investigators’ ability to differentiate seizure onset and propagation (Alexopoulos & Tandon 2008).
Although FDG-PET has some diagnostic value in non-lesional frontal lobe epilepsies as a whole (Lee, et al. 2005, Salamon, et al. 2008), there is no previous literature specifically studying its effectiveness in OF epilepsies. In all patients included in this study, PET was negative, multilobar, or falsely localizing to the temporal lobe. Only on retrospective review can one find concordance between hypometabolism on PET and the MAP+ foci / ICEEG onset. Several reasons could contribute to the relative low yield of PET to OF epilepsies, including complicated gyration pattern in the OF region which may lead to partial volume effect smearing the image resolution, and the fact that PET was visually inspected after coregistration with the MRI, without further quantitative analysis. Alternatively, perhaps epileptic activities originating from the OF region just spreads more quickly. As illustrated in previous studies, fast propagation of epileptic activities can be associated with widespread hypometabolism, or hypometabolism remote to the ictal-onset zone (Wong, et al. 2010, Wong, et al. 2012).
No previous studies specifically examined the effectiveness of SPECT SISCOM in localizing seizures from the OF region. In our cohort, SPECT was recorded in 4 of the 6 patients, but none localized exclusively to the OF region despite early injection. The hyperperfusion always existed in multiple adjacent areas. The low efficacy of SPECT in this cohort can be explained by rapid propagation of seizures to regions closely connected with OF area (Noachtar, et al. 1998).
The gold standard for accurate localization of the epileptogenic zone is invasive EEG recording over the seizure onset zone, with post resective seizure-freedom (Engel, et al. 1981, Kellinghaus & Luders 2004, Rosenow & Luders 2001). Patients with OF epilepsy carrying the strongest proof should not only have ictal onset exclusively from the OF region, but should also be rendered seizure-free after exclusive resection of the OF cortex. However in practice the OF cortex is rarely the only area of resection. Overall, multiple concordant modalities generally lead to a more confined resection. It is our hope that this study, with the addition of MRI post-processing findings, could shed light on future refinement of surgical strategies of orbitofrontal epilepsies.
Another potential benefit of our study is the contribution of MRI post-processing in the identification of a possibly epileptogenic lesion in an area of the brain that is rarely suspected as an anatomic location of frontal lobe epilepsy and frequently mislocalized to other neighboring regions. This hypothesis can be tested through MAP analyses of patients who failed previous frontal or temporal lobe resections, and verification of seizure-free status with subsequent resection of MAP+ areas (e.g. P1).
In summary, our study highlights the importance of MRI post-processing as a promising tool to complement conventional visual inspection of the MRI in terms of identifying potentially epileptogenic but subtle cortical abnormalities. This tool, when used in conjunction with other noninvasive modalities, has the potential to assist the planning of invasive electrode implantation and surgical resection in patients with MRI-negative pharmacoresistant orbitofrontal lobe epilepsy.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgements
The authors would like to acknowledge Professor Hans-Jürgen Huppertz for his gracious support to our research. The authors would also like to acknowledge the reviewers for their constructive critiques to improve this manuscript.
Funding
This research was supported by the Cleveland Clinic Epilepsy Center Fund, the Epilepsy Foundation Post-doctoral Fellowship Grant and NIH Grant R01-NS074980.
Footnotes
Disclosures
Andreas V. Alexopoulos serves on the editorial board of Epileptic Disorders, and has received research support from UCB, Pfizer Inc, and from the American Epilepsy Society. Imad M. Najm is on the Speakers’ bureau of UCB Inc and receives research funding from the US Department of Defense. Stephen E. Jones and Jorge A Gonzalez-Martinez have received research support from Citizens United for Research in Epilepsy (CURE). Shuang Wang has received research support from the Chinese National Natural Science Foundation.
Contributor Information
Z. Irene Wang, Cleveland Clinic Epilepsy Center.
Aleksandar J. Ristic, Epilepsy Center Neurology Clinic, Clinical Center of Serbia Dr Subotica 6, 11000 Belgrade, Serbia
Chong H. Wong, Department of Neurology, Westmead Hospital, Sydney, Australia
Stephen E. Jones, Diagnostic Radiology, Mellen Center, Cleveland Clinic
Imad M. Najm, Cleveland Clinic Epilepsy Center
Felix Schneider, Department of Neurology, Epilepsy Center, University of Greifswald, Greifswald, Germany.
Shuang Wang, Epilepsy Center, Second Affiliated Hospital, Zhejiang University, Hangzhou, China.
Jorge A. Gonzalez-Martinez, Cleveland Clinic Epilepsy Center
W Bingaman, Cleveland Clinic Epilepsy Center.
Andreas V. Alexopoulos, Cleveland Clinic Epilepsy Center
References
- Alexopoulos AV, Tandon N. Basal frontal lobe epilepsy. In: Lüders HO, editor. Textbook of Epilepsy Surgery. Informa HealthCare; 2008. pp. 285–313. [Google Scholar]
- Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009;66:1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- Burgess RC, Iwasaki M, Nair D. Localization and field determination in electroencephalography and magnetoencephalography. In: Wyllie E, Gupta A, Lachhwani DK, editors. The Treatment of Epilepsy: Principles & Practice. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 141–167. [Google Scholar]
- Cavada C, Schultz W. The mysterious orbitofrontal cortex. foreword. Cereb Cortex. 2000;10:205. doi: 10.1093/cercor/10.3.205. [DOI] [PubMed] [Google Scholar]
- Chang CN, Ojemann LM, Ojemann GA, Lettich E. Seizures of fronto-orbital origin: a proven case. Epilepsia. 1991;32:487–491. doi: 10.1111/j.1528-1157.1991.tb04681.x. [DOI] [PubMed] [Google Scholar]
- Colliot O, Bernasconi N, Khalili N, Antel SB, Naessens V, Bernasconi A. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006;29:162–171. doi: 10.1016/j.neuroimage.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Ebner A. Epileptic syndromes in adults. In: Lüders HO, editor. Comprehensive Review and Case Discussions. Martin Dunitz Ltd; London: 2001. pp. 169–179. [Google Scholar]
- Engel J, Jr., Rausch R, Lieb JP, Kuhl DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patients considered for surgical therapy of epilepsy. Ann Neurol. 1981;9:215–224. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Van NP, Rasmussen TB, Ojemann LM. Surgical treatment of the epilepsies. Raven Press; New York: 1993. Outcome with respect to epileptic seizures. [Google Scholar]
- Fauser S, Sisodiya SM, Martinian L, Thom M, Gumbinger C, Huppertz HJ, Hader C, Strobl K, Steinhoff BJ, Prinz M, Zentner J, Schulze-Bonhage A. Multi-focal occurrence of cortical dysplasia in epilepsy patients. Brain. 2009;132:2079–2090. doi: 10.1093/brain/awp145. [DOI] [PubMed] [Google Scholar]
- Focke NK, Symms MR, Burdett JL, Duncan JS. Voxel-based analysis of whole brain FLAIR at 3T detects focal cortical dysplasia. Epilepsia. 2008;49:786–793. doi: 10.1111/j.1528-1167.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Grimm C, Fauser S, Kassubek J, Mader I, Hochmuth A, Spreer J, Schulze-Bonhage A. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005;67:35–50. doi: 10.1016/j.eplepsyres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Kroll-Seger J, Kloppel S, Ganz RE, Kassubek J. Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage. 2010;49:2216–2224. doi: 10.1016/j.neuroimage.2009.10.066. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Wellmer J, Staack AM, Altenmuller DM, Urbach H, Kroll J. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia. 2008;49:772–785. doi: 10.1111/j.1528-1167.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- Jobst BC, Williamson PD. Frontal lobe seizures. Psychiatr Clin North Am. 2005;28:635–651. 648–639. doi: 10.1016/j.psc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Kellinghaus C, Luders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6:223–239. [PubMed] [Google Scholar]
- Kendrick JF, Gibbs FA. Interrelations of mesial temporal and orbital frontal areas of man revealed by strychnine spikes. AMA Arch Neurol Psychiatry. 1958;79:518–524. doi: 10.1001/archneurpsyc.1958.02340050046005. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, Burneo JG, Ver Hoef L, Paige L, Faught E, Kankirawatana P, Riley K, Kuzniecky R. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008;64:25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- Kriegel MF, Roberts DW, Jobst BC. Orbitofrontal and insular epilepsy. J Clin Neurophysiol. 2012;29:385–391. doi: 10.1097/WNP.0b013e31826bd82e. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, Rey G, Morrison G, Ragheb J, Vinters HV, Resnick T, Duchowny M. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008;63:758–769. doi: 10.1002/ana.21398. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann.Neurol. 2005;58:525–532. doi: 10.1002/ana.20569. [DOI] [PubMed] [Google Scholar]
- Lorenzo NY, Parisi JE, Cascino GD, Jack CR, Jr., Marsh WR, Hirschorn KA. Intractable frontal lobe epilepsy: pathological and MRI features. Epilepsy Res. 1995;20:171–178. doi: 10.1016/0920-1211(94)00072-5. [DOI] [PubMed] [Google Scholar]
- Luders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, Dinner DS, Ebner A, Foldvary N, Geller E, Hamer H, Holthausen H, Kotagal P, Morris H, Meencke HJ, Noachtar S, Rosenow F, Sakamoto A, Steinhoff BJ, Tuxhorn I, Wyllie E. Semiological seizure classification. Epilepsia. 1998;39:1006–1013. doi: 10.1111/j.1528-1157.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Ludwig B, Marsan CA, Van Buren J. Cerebral seizures of probable orbitofrontal origin. Epilepsia. 1975;16:141–158. doi: 10.1111/j.1528-1157.1975.tb04731.x. [DOI] [PubMed] [Google Scholar]
- Munari C, Bancaud J. Electroclinical symptomatology of partial seizures of orbital frontal origin. Adv Neurol. 1992;57:257–265. [PubMed] [Google Scholar]
- Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013 doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- Noachtar S, Arnold S, Yousry TA, Bartenstein P, Werhahn KJ, Tatsch K. Ictal technetium-99m ethyl cysteinate dimer single-photon emission tomographic findings and propagation of epileptic seizure activity in patients with extratemporal epilepsies. Eur J Nucl Med. 1998;25:166–172. doi: 10.1007/s002590050210. [DOI] [PubMed] [Google Scholar]
- Noe K, Sulc V, Wong-Kisiel L, Wirrell E, Van Gompel JJ, Wetjen N, Britton J, So E, Cascino GD, Marsh WR, Meyer F, Horinek D, Giannini C, Watson R, Brinkmann BH, Stead M, Worrell GA. Long-term Outcomes After Nonlesional Extratemporal Lobe Epilepsy Surgery. JAMA Neurol. 2013:1–6. doi: 10.1001/jamaneurol.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TJ, O'Connor MK, Mullan BP, Brinkmann BH, Hanson D, Jack CR, So EL. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998;19:31–45. doi: 10.1097/00006231-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Pedley TA, Mendiratta A, Walczak TS. Seizures and epilepsy. In: Ebersole JS, editor. Current Practice of Clinical Electroencephalography. Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 506–587. [Google Scholar]
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Thom M, Harkness W, Duncan JS. Diffusion tensor imaging in refractory epilepsy. Lancet. 2002;359:1748–1751. doi: 10.1016/S0140-6736(02)08615-4. [DOI] [PubMed] [Google Scholar]
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, Lerner JT, Sankar R, Shields WD, Engel J, Jr., Fried I, Miyata H, Yong WH, Vinters HV, Mathern GW. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594–1601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmenpera TM, Symms MR, Rugg-Gunn FJ, Boulby PA, Free SL, Barker GJ, Yousry TA, Duncan JS. Evaluation of quantitative magnetic resonance imaging contrasts in MRI-negative refractory focal epilepsy. Epilepsia. 2007;48:229–237. doi: 10.1111/j.1528-1167.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- See SJ, Jehi LE, Vadera S, Bulacio J, Najm I, Bingaman W. Surgical outcomes in patients with extratemporal epilepsy and subtle or normal magnetic resonance imaging findings. Neurosurgery. 2013;73:68–77. doi: 10.1227/01.neu.0000429839.76460.b7. [DOI] [PubMed] [Google Scholar]
- Smith JR, Sillay K, Winkler P, King DW, Loring DW. Orbitofrontal epilepsy: electroclinical analysis of surgical cases and literature review. Stereotact Funct Neurosurg. 2004;82:20–25. doi: 10.1159/000076656. [DOI] [PubMed] [Google Scholar]
- Wagner J, Weber B, Urbach H, Elger C, Huppertz H. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134:2844–2854. doi: 10.1093/brain/awr204. [DOI] [PubMed] [Google Scholar]
- Wang ZI, Jones SE, Ristic AJ, Wong C, Kakisaka Y, Jin K, Schneider F, Gonzalez-Martinez JA, Mosher JC, Nair D, Burgess RC, Najm IM, Alexopoulos AV. Voxel-based morphometric MRI post-processing in MRI-negative focal cortical dysplasia followed by simultaneously recorded MEG and stereo-EEG. Epilepsy Res. 2012 doi: 10.1016/j.eplepsyres.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, Somerville E, Mohamed A. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia. 2010;51:1365–1373. doi: 10.1111/j.1528-1167.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, Somerville E, Mohamed A. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia. 2012;53:1333–1340. doi: 10.1111/j.1528-1167.2012.03547.x. [DOI] [PubMed] [Google Scholar]
- Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy. Expert Rev Neurother. 2010;10:975–984. doi: 10.1586/ern.10.63. [DOI] [PubMed] [Google Scholar]