Abstract
Previous studies have suggested that the rostrocaudal patterning of branchial arches in the vertebrate embryo derives from a coordinate segmental specification of gene expression in rhombomeres (r) and neural crest. However, expression of the Krox-20 gene is restricted to neural crest cells migrating to the third branchial arch, apparently from r5, whereas this rhombomere contributes cells to both the second and third arches. We examined in the chick embryo how this spatially restricted expression is established. Expression occurs in precursors in both r5 and r6, and we show by cell labelling that both rhombomeres contribute to Krox-20-expressing neural crest, emigration occurring first from r6 and later caudally from r5. Krox-20 transcripts are not detected in some precursors in rostral r5, presaging the lack of expression in cells migrating rostrally from this rhombomere. After transposition of r6 to the position of r4 or r5, many Krox-20-expressing cells migrate rostral to the otic vesicle, whereas when r5 is transplanted to the position of r4, only a small number of migrating cells express Krox-20. These results indicate that, in the chick, Krox-20 expression in branchial neural crest does not correlate with rhombomeric segmentation, and that there may be intrinsic differences in regulation between the r5 and r6 Krox-20-expressing populations.
Full text
PDF

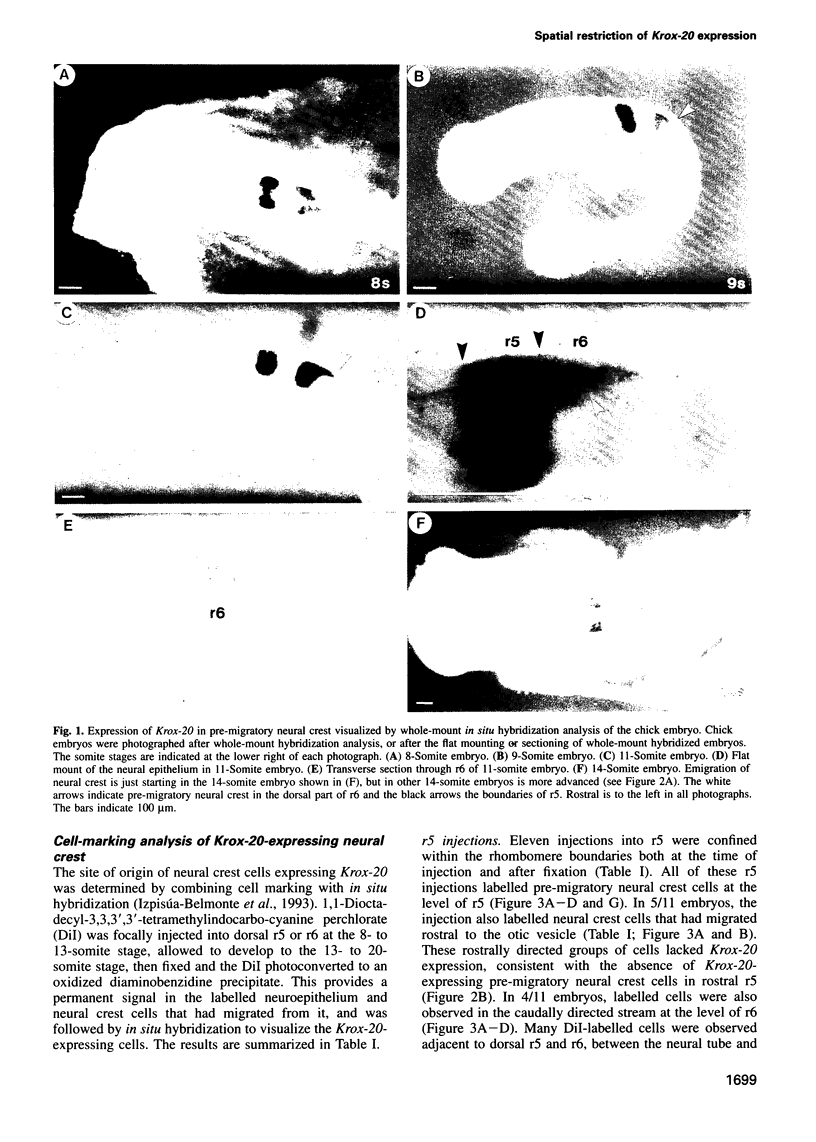








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. B., Meier S. The influence of the metameric pattern in the mesoderm on migration of cranial neural crest cells in the chick embryo. Dev Biol. 1981 Jul 30;85(2):385–402. doi: 10.1016/0012-1606(81)90270-0. [DOI] [PubMed] [Google Scholar]
- Ayer-Le Lievre C. S., Le Douarin N. M. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982 Dec;94(2):291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Birgbauer E., Fraser S. E. Violation of cell lineage restriction compartments in the chick hindbrain. Development. 1994 Jun;120(6):1347–1356. doi: 10.1242/dev.120.6.1347. [DOI] [PubMed] [Google Scholar]
- Bradley L. C., Snape A., Bhatt S., Wilkinson D. G. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech Dev. 1993 Jan;40(1-2):73–84. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A., Noden D. M. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983 Apr;166(4):445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990 Mar 29;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M., Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993 Dec 31;75(7):1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Graham A., Heyman I., Lumsden A. Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development. 1993 Sep;119(1):233–245. doi: 10.1242/dev.119.1.233. [DOI] [PubMed] [Google Scholar]
- Hunt P., Gulisano M., Cook M., Sham M. H., Faiella A., Wilkinson D., Boncinelli E., Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991 Oct 31;353(6347):861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., De Robertis E. M., Storey K. G., Stern C. D. The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell. 1993 Aug 27;74(4):645–659. doi: 10.1016/0092-8674(93)90512-o. [DOI] [PubMed] [Google Scholar]
- Jeffs P., Jaques K., Osmond M. Cell death in cranial neural crest development. Anat Embryol (Berl) 1992;185(6):583–588. doi: 10.1007/BF00185617. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Sprawson N., Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991 Dec;113(4):1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Bradley L. C., Wilkinson D. G. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Dev Suppl. 1991;Suppl 2:59–62. [PubMed] [Google Scholar]
- Nieto M. A., Gilardi-Hebenstreit P., Charnay P., Wilkinson D. G. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development. 1992 Dec;116(4):1137–1150. doi: 10.1242/dev.116.4.1137. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103 (Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Noden D. M. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983 Mar;96(1):144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993 Mar 11;21(5):1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince V., Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development. 1994 Apr;120(4):911–923. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Mark M., Lakkaraju S., Dierich A., Dollé P., Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993 Dec 31;75(7):1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sechrist J., Scherson T., Bronner-Fraser M. Rhombomere rotation reveals that multiple mechanisms contribute to the segmental pattern of hindbrain neural crest migration. Development. 1994 Jul;120(7):1777–1790. doi: 10.1242/dev.120.7.1777. [DOI] [PubMed] [Google Scholar]
- Sechrist J., Serbedzija G. N., Scherson T., Fraser S. E., Bronner-Fraser M. Segmental migration of the hindbrain neural crest does not arise from its segmental generation. Development. 1993 Jul;118(3):691–703. doi: 10.1242/dev.118.3.691. [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Bronner-Fraser M., Fraser S. E. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992 Oct;116(2):297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Morriss-Kay G. The development and distribution of the cranial neural crest in the rat embryo. Cell Tissue Res. 1985;240(2):403–416. doi: 10.1007/BF00222353. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989 Feb 2;337(6206):461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G. Molecular mechanisms of segmental patterning in the vertebrate hindbrain and neural crest. Bioessays. 1993 Aug;15(8):499–505. doi: 10.1002/bies.950150802. [DOI] [PubMed] [Google Scholar]