Abstract
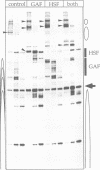
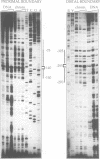
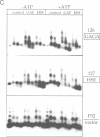
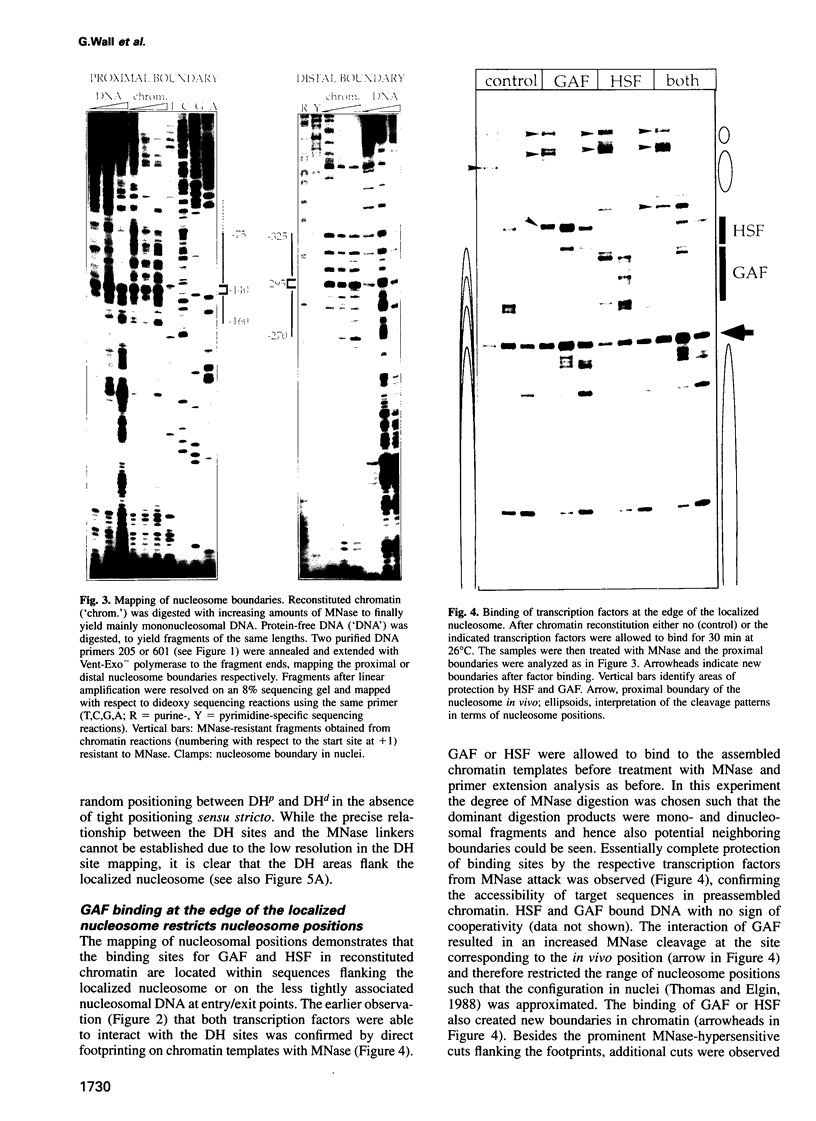
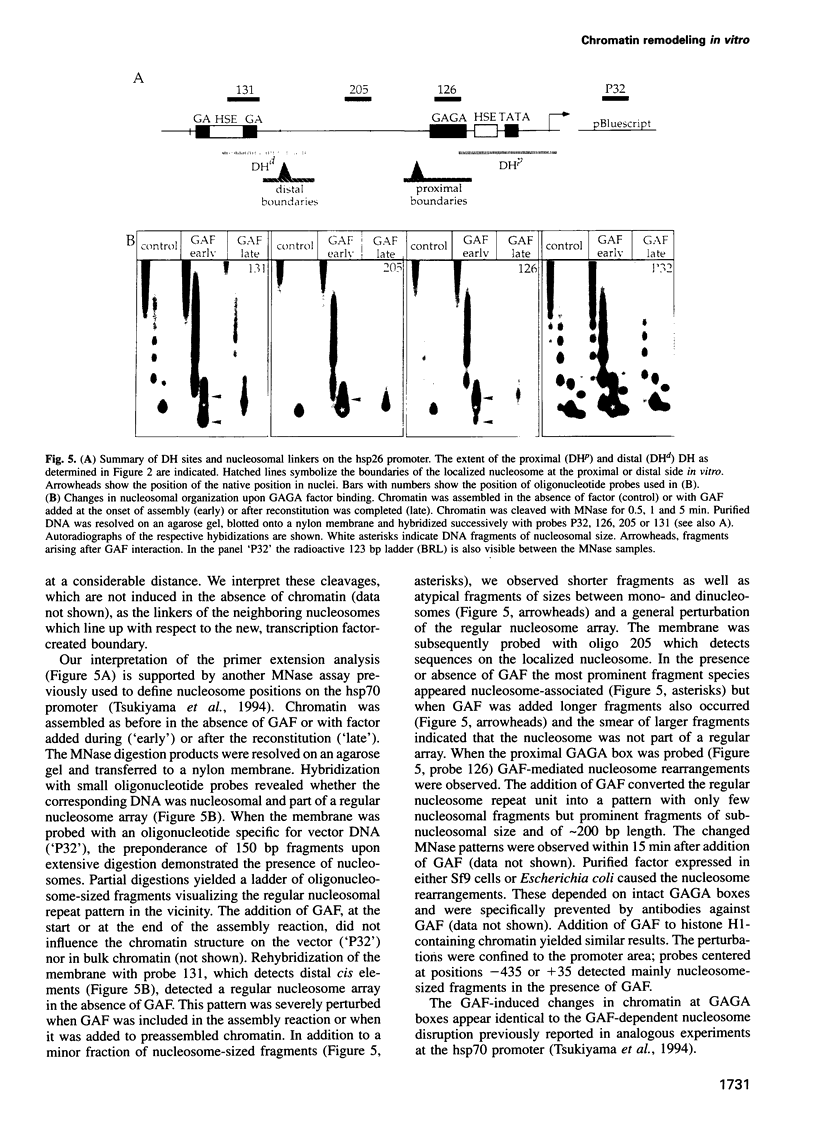
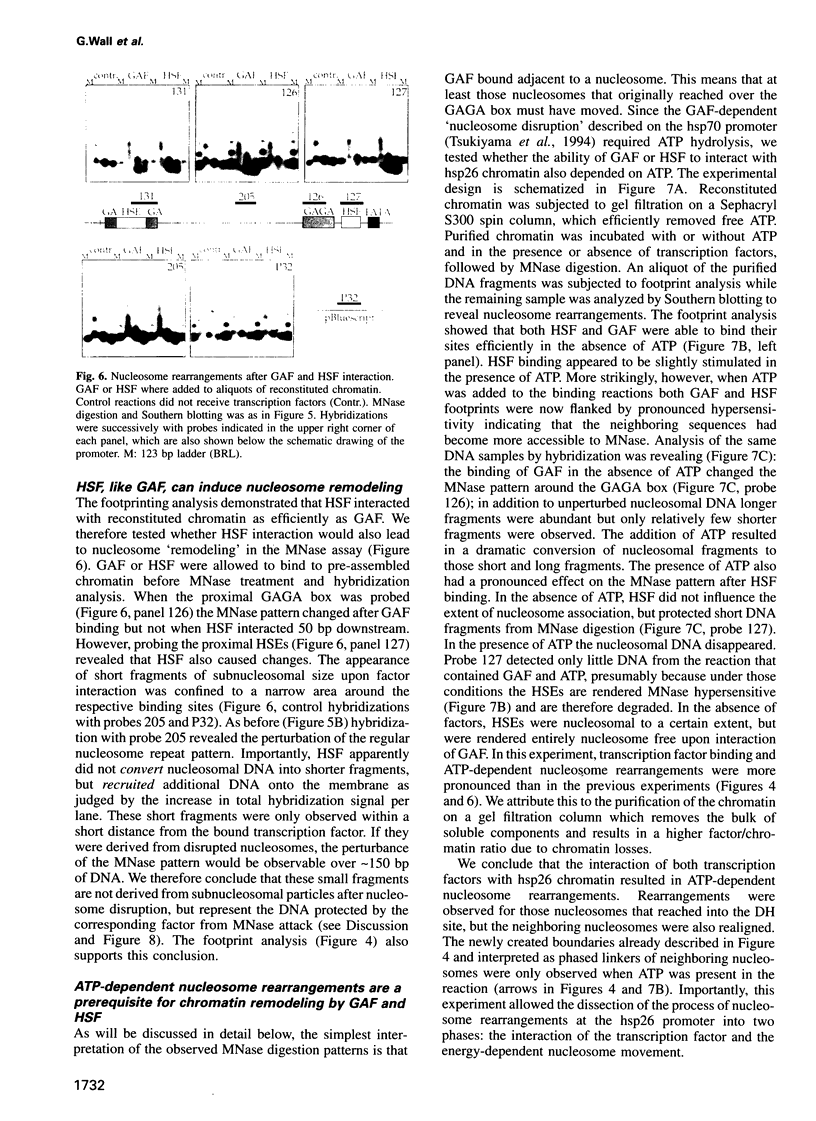
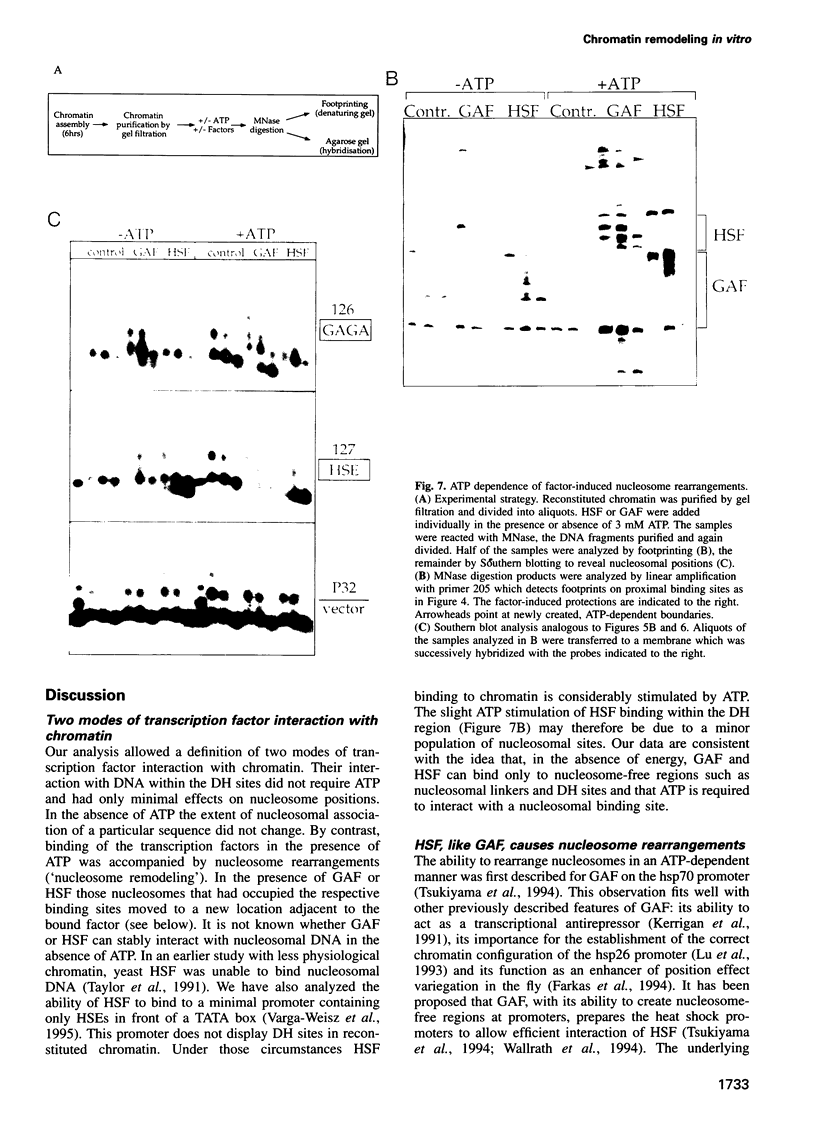
The chromatin structure at the Drosophila hsp26 promoter in vivo is characterized by two DNase I-hypersensitive (DH) sites harboring regulatory elements. Proximal and distal DH sites are separated by a positioned nucleosome. To study the contribution of transcription factors to the establishment of this specific chromatin configuration we assembled nucleosomes on the hsp26 promoter using a cell-free reconstitution system derived from fly embryos. Both DH sites were readily reconstituted from extract components. They were separated by a nucleosome which was less strictly positioned than its in vivo counterpart. The interactions of GAGA factor and heat shock factor with their binding sites in chromatin occurred in two modes. Their interaction with binding sites in the nucleosome-free regions did not require ATP. In the presence of ATP both factors interacted also with nucleosomal binding sites, causing nucleosome rearrangements and a refinement of nucleosome positions. While chromatin remodeling upon transcription factor interaction has previously been interpreted to involve nucleosome disruption, the data suggest energy-dependent nucleosome sliding as main principle of chromatin reorganization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. B. The establishment of active promoters in chromatin. Bioessays. 1994 Aug;16(8):541–547. doi: 10.1002/bies.950160807. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Tsukiyama T., Wu C. Chromatin assembly extracts from Drosophila embryos. Methods Cell Biol. 1994;44:207–223. doi: 10.1016/s0091-679x(08)60915-2. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Wu C. Cell-free system for assembly of transcriptionally repressed chromatin from Drosophila embryos. Mol Cell Biol. 1992 May;12(5):2241–2249. doi: 10.1128/mcb.12.5.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttinelli M., Di Mauro E., Negri R. Multiple nucleosome positioning with unique rotational setting for the Saccharomyces cerevisiae 5S rRNA gene in vitro and in vivo. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9315–9319. doi: 10.1073/pnas.90.20.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. L., Elgin S. C. Nucleosomal instability and induction of new upstream protein-DNA associations accompany activation of four small heat shock protein genes in Drosophila melanogaster. Mol Cell Biol. 1986 Mar;6(3):779–791. doi: 10.1128/mcb.6.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos J., Westwood J. T., Becker P. B., Wilson S., Lambert K., Wu C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell. 1990 Nov 30;63(5):1085–1097. doi: 10.1016/0092-8674(90)90511-c. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Separate regulatory elements for the heat-inducible and ovarian expression of the Drosophila hsp26 gene. Cell. 1985 Dec;43(3 Pt 2):737–746. doi: 10.1016/0092-8674(85)90247-8. [DOI] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J. Four Drosophila heat shock genes at 67B: characterization of recombinant plasmids. Nucleic Acids Res. 1980 Oct 10;8(19):4441–4457. doi: 10.1093/nar/8.19.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C., Granok H., Lu Q., Wallrath L. L. Role of chromatin structure in regulating gene expression: the hsp26 gene of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1993;58:83–96. doi: 10.1101/sqb.1993.058.01.012. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Farkas G., Gausz J., Galloni M., Reuter G., Gyurkovics H., Karch F. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature. 1994 Oct 27;371(6500):806–808. doi: 10.1038/371806a0. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Lue N. F., Kornberg R. D. Statistical positioning of nucleosomes by specific protein-binding to an upstream activating sequence in yeast. J Mol Biol. 1988 Nov 5;204(1):109–127. doi: 10.1016/0022-2836(88)90603-1. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Thomas G. H., Elgin S. C. Drosophila nuclear proteins bind to regions of alternating C and T residues in gene promoters. Science. 1989 Sep 29;245(4925):1487–1490. doi: 10.1126/science.2781290. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Lis J. T. Multiple, compensatory regulatory elements specify spermatocyte-specific expression of the Drosophila melanogaster hsp26 gene. Mol Cell Biol. 1990 Jan;10(1):131–137. doi: 10.1128/mcb.10.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. L., Thomas G. H., Siegfried E., Elgin S. C., Lis J. T. Optimal heat-induced expression of the Drosophila hsp26 gene requires a promoter sequence containing (CT)n.(GA)n repeats. J Mol Biol. 1990 Feb 20;211(4):751–761. doi: 10.1016/0022-2836(90)90075-W. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Wolfner M. F., Lis J. T. Spatial and temporal pattern of hsp26 expression during normal development. EMBO J. 1986 Apr;5(4):747–754. doi: 10.1002/j.1460-2075.1986.tb04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. J., Wolffe A. P. The interaction of transcription factors with nucleosomal DNA. Bioessays. 1992 Sep;14(9):597–603. doi: 10.1002/bies.950140905. [DOI] [PubMed] [Google Scholar]
- Kerrigan L. A., Croston G. E., Lira L. M., Kadonaga J. T. Sequence-specific transcriptional antirepression of the Drosophila Krüppel gene by the GAGA factor. J Biol Chem. 1991 Jan 5;266(1):574–582. [PubMed] [Google Scholar]
- Lee H., Kraus K. W., Wolfner M. F., Lis J. T. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 1992 Feb;6(2):284–295. doi: 10.1101/gad.6.2.284. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Cartwright I. L., Keene M. A., Zimmerman J. L., Elgin S. C. Chromatin structure in pre- and postblastula embryos of Drosophila. Dev Biol. 1983 Sep;99(1):194–201. doi: 10.1016/0012-1606(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wallrath L. L., Allan B. D., Glaser R. L., Lis J. T., Elgin S. C. Promoter sequence containing (CT)n.(GA)n repeats is critical for the formation of the DNase I hypersensitive sites in the Drosophila hsp26 gene. J Mol Biol. 1992 Jun 20;225(4):985–998. doi: 10.1016/0022-2836(92)90099-6. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wallrath L. L., Granok H., Elgin S. C. (CT)n (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene. Mol Cell Biol. 1993 May;13(5):2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meersseman G., Pennings S., Bradbury E. M. Mobile nucleosomes--a general behavior. EMBO J. 1992 Aug;11(8):2951–2959. doi: 10.1002/j.1460-2075.1992.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape S. M., Kamakaka R. T., Kadonaga J. T. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. Postinitiation transcriptional control in Drosophila melanogaster. Mol Cell Biol. 1990 Nov;10(11):6041–6045. doi: 10.1128/mcb.10.11.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988 Sep 9;54(6):795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- Sandaltzopoulos R., Blank T., Becker P. B. Transcriptional repression by nucleosomes but not H1 in reconstituted preblastoderm Drosophila chromatin. EMBO J. 1994 Jan 15;13(2):373–379. doi: 10.1002/j.1460-2075.1994.tb06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Oh C. E., Kornberg T. B. Isolation of cDNAs encoding the Drosophila GAGA transcription factor. Mol Cell Biol. 1993 Dec;13(12):7961–7970. doi: 10.1128/mcb.13.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Thomas G. H., Elgin S. C. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 1988 Jul;7(7):2191–2201. doi: 10.1002/j.1460-2075.1988.tb03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Becker P. B., Wu C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature. 1994 Feb 10;367(6463):525–532. doi: 10.1038/367525a0. [DOI] [PubMed] [Google Scholar]
- Wallrath L. L., Lu Q., Granok H., Elgin S. C. Architectural variations of inducible eukaryotic promoters: preset and remodeling chromatin structures. Bioessays. 1994 Mar;16(3):165–170. doi: 10.1002/bies.950160306. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Dimitrov S. Histone-modulated gene activity: developmental implications. Crit Rev Eukaryot Gene Expr. 1993;3(3):167–191. [PubMed] [Google Scholar]
- Workman J. L., Buchman A. R. Multiple functions of nucleosomes and regulatory factors in transcription. Trends Biochem Sci. 1993 Mar;18(3):90–95. doi: 10.1016/0968-0004(93)90160-o. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]