Abstract
Genetic conflict may have played an important role in the evolution of novel genetic systems. The ancestral system of eumendelian genetics is highly symmetrical. Those derived from it (e.g. thelytokous parthenogenesis, haplodiploidy and parent-specific allele expression) are more asymmetrical in the genetic role played by maternal versus paternal alleles. These asymmetries may have arisen from maternal–paternal genetic conflict, or cytonuclear conflict, or from an interaction between them. Asymmetric genetic systems are much more common in terrestrial and freshwater taxa than in marine taxa. We suggest three reasons for this, based on the relative inhospitability of terrestrial environments to three types of organism: (i) pathogens—departure from the marine realm meant escape from many pathogens and parasites, reducing the need for sexual reproduction; (ii) symbionts—symbionts are no more important in the terrestrial realm than the marine realm but are more likely to be obligately intracellular and vertically transmitted, making them more likely to disrupt their host's genetic systems; (iii) Gametes and embryos—because neither gametes nor embryos can be shed into air as easily as into seawater, the mother's body is a more important environment for both types of organisms in the terrestrial realm than in the marine realm. This environment of asymmetric kinship (with neighbours more closely related by maternal alleles than by paternal alleles) may have helped to drive asymmetries in expression and transmission.
Keywords: endosymbiont, Wolbachia, genetic system, haplodiploidy, parthenogenesis
1. Introduction
Meiosis is a fair lottery: each allele in a diploid genome has the same probability of being passed on to the next generation. Asymmetry in transmission probability between alleles at a locus—meiotic drive—evolves frequently in some taxa but in most cases is quickly suppressed [1,2]. But one kind of genetic asymmetry can be persistent, and that is asymmetry between males and females, which is seen in unusual genetic systems such as haplodiploidy and parthenogenesis (figure 1). The origin and distribution of these asymmetric genetic systems remains poorly understood. In this review, we discuss conflicts between maternal and paternal alleles, as well as between nuclear and cytoplasmic genes, and consider the extent to which each of these might have been important in the evolution of asymmetric genetic systems. We also consider interactions among relatives, and how indirect fitness may have affected the evolution of asymmetric reproductive systems. Finally, we consider the distribution of these systems across habitats and consider the question of why asymmetric genetic systems occur mainly in terrestrial and freshwater rather than marine habitats.
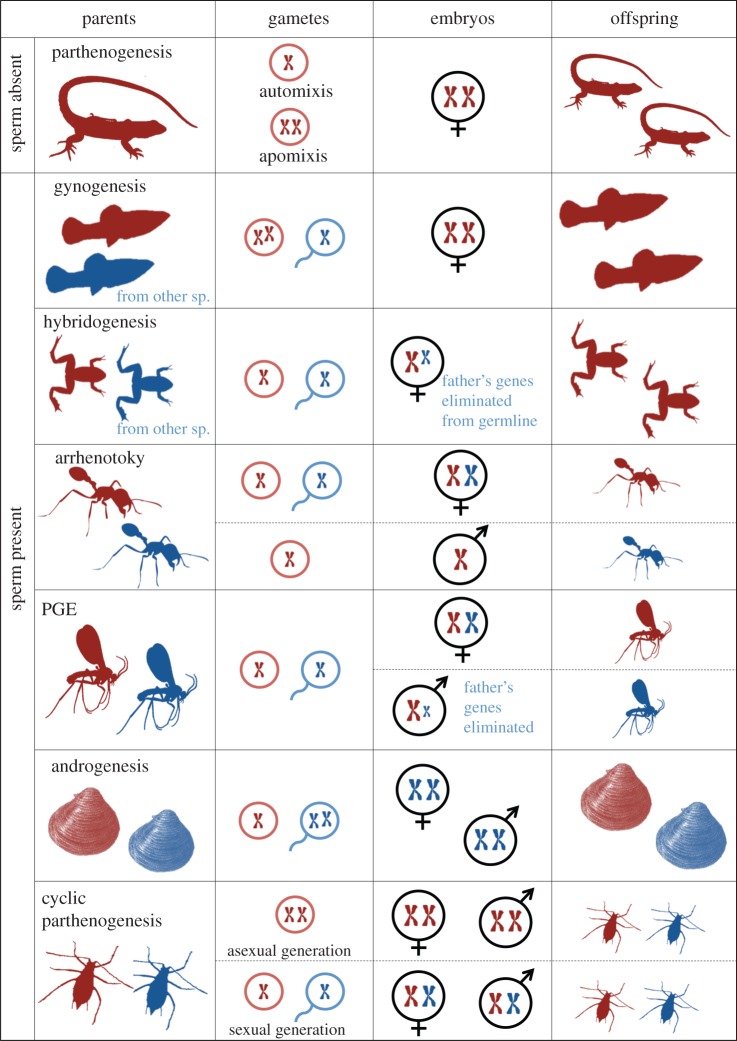
Figure 1.
Asymmetric genetic systems, where the sperm and egg genome have different roles or fates [3–11]. Each row of this figure describes a different asymmetric genetic system. The first column of each row shows the adult generation, the second column the gametes produced, the third column the embryos shortly after fertilization and the last column the offspring generation. The animal symbols represent males (in blue) and females (in red). Haploid individuals and gametes are shown with one chromosome. Diploid individuals and gametes are shown with two chromosomes. From top to bottom: Parthenogenesis = obligate thelytokous parthenogenesis, development of females from unfertilized eggs (including apomixis = egg production by mitosis and automixis = egg production involving meiosis). A few thousand eukaryote species, especially freshwater and terrestrial animals. Gynogenesis = development of females from fertilized eggs, but with only the mother's genome. Many groups including salamanders, fish, insects and angiosperms. Hybridogenesis = gynogenesis, except that the paternal genome is incorporated and expressed in the offspring but later eliminated from the offspring's germline. Several species of fish and frogs. Arrhenotoky = arrhenotokous haplodiploidy or haplodiploidy in the strict sense, development of males from unfertilized eggs. Males are haploid and never have a paternal genome at all. Several groups of insects and mites, oxyurid nematodes and monogonont rotifers. PGE = paternal genome elimination, development of males from fertilized eggs in which males have a paternal genome but do not transmit it. Males may lose their paternal genome either early in development, becoming haploid (early PGE) or retain their paternal genome without transmitting it (late PGE). A few groups of insects, mites and springtails. Androgenesis = development of either sex from fertilized eggs in which the sperm genome supplants egg genome. Offspring have the father's nuclear genome. A few clam species and Saharan cypress. Cyclic parthenogenesis = system in which one or more parthenogenetic generations regularly alternates with a sexual generation. Several groups including cladocera, monogonont rotifers, some loriciferans and several groups of insects.
2. Ancestral symmetry, novel asymmetry
Most eukaryotes reproduce sexually, with two gamete types: egg and sperm. Although there is a large size asymmetry between egg and sperm, in most eukaryotes they play symmetrical genetic roles. In the ancestral ‘eumendelian’ genetic system of eukaryotes, an allele contributed by a sperm cell has the same fate as one contributed by an ovum: an identical chance of transmission to offspring, as mediated by the meiotic lottery. But this ancestral system is not universal. Less-symmetrical genetic systems have arisen many times in the history of life (figure 1 and table 1). Novel genetic systems disrupt early embryonic development and are likely to impose initial viability costs on embryos [15]. This has been shown empirically in insects, where many species are able to lay occasional parthenogenetic eggs, but where there is often a significantly reduced fitness for embryos developing from unfertilized eggs compared with those developing from fertilized eggs [16,17]. So, if transitions to new genetic systems reduce organismal fitness, at least initially, then why do they occur?
Table 1.
Asymmetric genetic systems across animal phyla. Information from Jarne & Auld [12], Hughes [13] and Bell [5]. Non-marine taxa from each order are in italics, while marine taxa are described in roman font.
| phylum | marine/non-marine | parthenogenesis | other asymmetric reproduction |
|---|---|---|---|
| Annelida | marine | − | none |
| Annelida | non-marine | + | sperm-dependent parthenogenesis |
| Arthropoda | marine | − | none |
| Arthropoda | non-marine | + | parent-specific allele expression, gynogenesis, hybridogenesis, arrhenotoky, PGE, cyclic parthenogenesis |
| Brachiopoda | marine | − | none |
| Cephalochordata | marine | − | none |
| Chaetognatha | marine | − | none |
| Cnidaria | marine | rare | none |
| Ctenophora | marine | − | none |
| Cycliophora | marine | − | none |
| Echinodermata | marine | rare | none |
| Ectoprocta (Bryozoa) | marine | − | none |
| Ectoprocta (Bryozoa) | non-marine | − | none |
| Entoprocta | marine | − | none |
| Gastrotricha | marine | rare | none |
| Gastrotricha | non-marine | + | none |
| Gnathostomulida | marine | − | none |
| Hemichordata | marine | − | none |
| Kinorhyncha | marine | − | none |
| Loricifera | marine | − | cyclic parthenogenesis |
| Mesozoa | marine | − | none |
| Mollusca | marine | rare | sperm-dependent parthenogenesisa |
| Mollusca | non-marine | + | androgenesisa |
| Nematoda | marine | rare | none |
| Nematoda | non-marine | + | sperm-dependent parthenogenesis, cyclic parthenogenesis, arrhenotoky |
| Nematomorpha | non-marine | + | none |
| Nemertea | marine | − | none |
| Nemertea | non-marine | − | none |
| Onychophora | non-marine | rare | none |
| Pentastomida | non-marine | − | none |
| Phoronida | marine | − | none |
| Phoronida | marine | − | none |
| Platyhelminths | marine | − | none |
| Platyhelminths | non-marine | + | sperm-dependent parthenogenesis |
| Porifera | marine | − | none |
| Priapulida | marine | − | none |
| Rotifera | non-marine | + | cyclic parthenogenesis, arrhenotoky |
| Rotifera | marine | − | none |
| Sipuncula | marine | rare | none |
| Tardigrada | marine | − | none |
| Tardigrada | non-marine | + | none |
| Urochordata | marine | − | none |
| Vertebrata | marine | − | none |
| Vertebrata | non-marine | + | parent-specific allele expression, gynogenesis, hybridogenesis |
aAn unusual inheritance pattern of mitochondria has evolved multiple times both in marine and freshwater molluscs. Here, females transmit their mitochondria to both their male and female offspring, whereas males transmit their mitochondria only through sons [14].
3. Genetic conflict in the nucleus: maternal versus paternal alleles
Selfish genetic elements favour their own direct or indirect fitness while sacrificing the fitness of other genetic elements residing in the same genome. A selfish genetic element might mediate a transition to a novel genetic system that favours the element, even at a cost to organismal fitness. Consider the haplodiploid systems, in the broad sense: those in which males transmit only the alleles they received from their mothers (figure 1). Most scale insects have a system of late paternal genome elimination [18] in which males are diploid but the chromosomes that a male received from his father are heterochromatic. When sperm are formed, the maternal chromosomes are packaged into the sperm while the paternal chromosomes are left behind [19,20]. S. W. Brown, observing this system 50 years ago, noted its similarity to meiotic drive [21], and showed that a gene expressed in females that causes paternal chromosome elimination would spread rapidly through a population. Arrhenotoky—parthenogenetic production of haploid male offspring (figure 1)—evokes conflict less vividly than does the physical destruction of paternal chromosomes, but it has identical transmission genetics and confers an identical advantage if caused by a gene expressed in females [15,22]. Even if haploid males have relatively low viability compared with diploid males, an allele causing haplodiploidy can still invade (as long as haploid male viability is more than 50% diploid male viability). Ever since Brown, most models of haplodiploidy have followed his in invoking a transmission advantage for maternal alleles at the expense of paternal alleles, essentially viewing haplodiploidy as an outcome of maternal-allele victory in a conflict with paternal alleles over transmission by males [3,15,23–28].
Usually, asymmetric genetic systems are those in which the role of the male is reduced. Either males are excluded from parentage of sons (haplodiploidy), or they are excluded from some generations (cyclic parthenogenesis) or they are simply eliminated altogether (thelytokous parthenogenesis; figure 1). Perhaps Brown's insight can be generalized and we can view the origins of such systems as instances of maternal victory in a maternal–paternal conflict over transmission. It is not entirely clear why females are typically the victors in these conflicts, but it is plausible that, in crude terms, when the sperm and egg fight the egg usually wins because it is bigger [29]. Females deposit many more gene products that males into the zygote and do so earlier, and therefore may be at an advantage in affecting the outcome of the interaction. Indeed most such systems feature parthenogenesis, in which sperm are excluded from the ovum altogether.
In a few cases, the asymmetry runs the other way, with an expanded role for males and a reduced role for females. The most dramatic case of this is androgenesis (figure 1), in which the nuclear genome of the sperm cell entirely supplants that of the egg, resulting in clonal reproduction via sperm [4]. Another system in which males may strongly influence outcomes is parent-specific allele expression—or ‘genomic imprinting’ as it is often simply called, though genomic imprinting also underlies paternal genome elimination. Parent-specific allele expression in mammals and angiosperms affects only the expression of genes and does not directly affect their transmission, although by affecting maternal and offspring survivorship and reproduction it indirectly influences transmission. In placental mammals, the paternal allele in an embryo often has a pattern of expression that results in a relatively high rate of nutrient transfer from mother to embryo, while the maternal allele often has a pattern of expression that results in a lower rate of nutrient transfer [30]—paternal alleles are ‘greedier’ for maternal resources, as they are less related to the mother and to her other offspring.
4. Cytonuclear conflict and inclusive fitness of intracellular bacteria
Although the genetics of the nucleus is usually highly symmetrical, there is typically an enormous asymmetry between the two sexes in cytoplasmic inheritance. The egg has a much greater volume of cytoplasm than the sperm and usually includes cytoplasmic elements that have their own genome. As a result, these cytoplasmic genomes are only transmitted through females. Some authors draw a hard distinction between cytonuclear genetic conflict involving organelles, on the one hand, and host–symbiont conflict involving intracellular bacteria, on the other [3]. But in our view, this dichotomy obscures more than it illuminates. Many insects are utterly dependent upon their primary bacterial endosymbionts, some of which have been vertically transmitted within their hosts for hundreds of millions of years, losing a large majority of their genes and becoming effectively organelle-like [31]. Compared with them, the plant mitochondrial genome often has a much larger genome size and much higher rates of gene rearrangement, and indeed, the plant mitochondrial genome often causes cytoplasmic male sterility, analogous to the male-killing engaged in by intracellular bacteria [32]. Instead of a dichotomy, we see a diverse continuum, with animal mitochondria and ancient reduced-genome symbionts at one (well-behaved mutualist) end of the spectrum, and frequently swapped reproduction manipulators like Cardinium and Wolbachia at the other (parasitic) end, with plant mitochondria and an enormous variety of other bacteria in between [31,33–38]. The aggregate of all the maternally transmitted genomes in a cell can be viewed as a single ‘cytogenome’ with near-complete genetic linkage and a unified interest in skewing the sex ratio towards females. Here, we treat the sex ratio conflict between the nucleus and the entire cytogenome as a form of genetic conflict.
Bacteria that invade the cytoplasm of multicellular organisms acquire a population structure that mirrors that of the cells they have invaded: differentiation of the host's cells into germline and soma often imposes something very much like a germline/soma distinction upon the resident bacterial lineage. It is a remarkable natural experiment that has been repeated many times: when you impose multicellularity and germline sequestration on a unicellular organism, do its sterile members—its potential ‘soma’—evolve somatic functions that enhance the fitness of its germline? In most respects, the predicted optimal phenotype of both bacterial and host genomes is the same—maximal fitness of infected hosts—leading to tight integration of host and bacterial physiology and few insights into bacterium-specific selection. But there is one major source of conflict between the nucleus and the cytogenome: males. Male offspring represent half of the nucleus's direct fitness, but cytoplasmic elements in males have no direct fitness. This creates a permanent conflict of interest over male fitness—the nucleus is under selection to maximize the fitness of both males and females, whereas the cytogenome is under selection to maximize the fitness of females only.
Accordingly, elements within the cytogenome have evolved various means of sacrificing male fitness in favour of female fitness. According to almost all accounts, there are four such means: feminization, parthenogenesis-induction, male killing and cytoplasmic incompatibility (CI) [33,39–41]. This standard four-part taxonomy obscures a more fundamental dichotomy between selfish versus cooperative phenotypes of cytoplasmic elements (figure 2). For a maternally transmitted bacterium in a male host, there are two different ways it can enhance its fitness: it can gain direct fitness by regaining access to a female germline or it can gain indirect fitness by enhancing the reproductive success of its clone-mates housed in the female germline. Bacterial lineages that take the first route—regain of direct fitness—benefit themselves as individual cells. They remain unicellular organisms in terms of the level at which selection acts on them. But bacteria that take the second route—gain of indirect fitness—become essentially multicellular organisms with a germline and an adaptively functional soma.
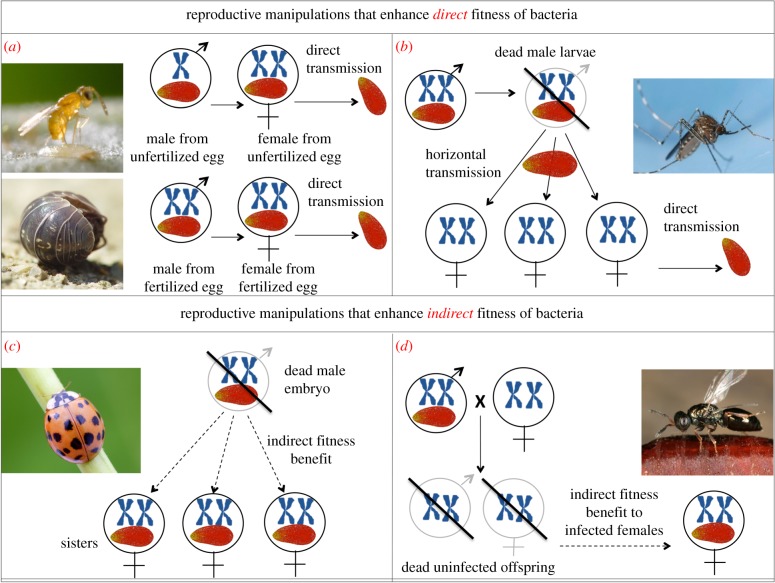
Figure 2.
Adaptations of intracellular bacteria to increase their direct or indirect fitness by manipulating the reproduction of their host. Direct effects of endosymbiont (including reproductive manipulation and transmission) are indicated with solid arrows, while indirect effects that benefit related endosymbionts in different hosts are indicated with dashed arrows. Diploid individuals are represented by male or female symbols with two chromosomes. Haploid males are represented by a male symbol with one chromosome. The infection status of an individual is indicated by the presence of the red Wolbachia icon. (a) Feminization, conversion of males into females, here including feminization of parthenogenetically produced males (often referred to as ‘parthenogenesis-induction’; example Encarsia pergandiella, photo by Alex Wild), as well as feminization of sexually produced males (example Armadillidium vulgare, photo by Franco Folini). (b) Late male-killing, killing of males late in development, such that dead males can serve as a source of infection of nearby females (example Culex tarsalis, photo by Joseph Berger, Bugwood.org). (c) Early male-killing (EMK), killing of male embryos. When there is competition between host siblings, EMK enhances fitness of the bacterial clone inhabiting a single host individual (example Harmonia axyridis, photo by Alex Wild). (d) CI, modification of sperm to kill uninfected eggs. Infected eggs are rescued by an antitoxin. CI enhances the fitness of the bacterial clone(s) encoding compatible antitoxins (example Nasonia vitripennis, photo by Gernot Kunz).
For obligately intracellular bacteria that need to be transmitted vertically, there appears to be only one means of regaining direct fitness in the host's male germline: feminization of the host. There is a large literature devoted to ‘parthenogenesis-induction’ by intracellular bacteria as a phenomenon different from feminization, but this is a misnomer. ‘Parthenogenesis-induction’ has been reliably demonstrated only in groups that have parthenogenetically produced males, and it consists of the conversion of parthenogenetically produced males into females [42–45]. Thus, it is simply feminization. Of course, feminization has very different long-term consequences when it occurs in a population in which males are produced parthenogenetically compared to one in which all individuals are produced sexually: in populations with parthenogenetically produced males, feminizers can confer short-term fitness benefits and can be found at 100% frequency; in populations with sexually produced males, feminizers impose bigger fitness costs and cannot reach 100% frequency without causing local population extinction. There is also usually a mechanistic difference. Feminizing bacteria in sexually produced male zygotes induce development of a female phenotype, without changing the genotype [46,47]. In parthenogenetically produced males, on the other hand, the most commonly employed means of feminization is diploidization of the haploid zygote, resulting in an individual that is both genetically and phenotypically female [48]. But exceptions are known, highlighting the falseness of the distinction between feminization and ‘parthenogenesis-induction’: in the mite genus Brevipalpus, Cardinium bacteria cause parthenogenetically produced males to develop as females, though they remain haploid and thus genetically male [49,50].
For bacteria that can be either vertically or horizontally transmitted, bacteria in males may be able to reproduce if they can be transmitted horizontally. Such bacteria rarely affect host sex ratio, but a few are known to induce late male-killing: proliferation of bacteria in males after they reach a large body size, with the dead male then serving as a source of horizontal transmission [51] (figure 2).
Bacteria that can induce feminization or late male-killing gain a direct-fitness benefit. But other adaptations of intracellular bacteria to the male germline have evolved in spite of the fact that they do not enhance the direct fitness of the bacteria, only their indirect fitness. One such adaptation is early male-killing (EMK), which might also be called immediate suicide. At a very early stage in embryogenesis—plausibly, as soon as the bacteria detect a cue indicating that they are in a male rather than a female—bacteria in male embryos kill themselves and their host. Male-killing evolves in bacteria inhabiting hosts with gregarious broods, often with inter-sibling cannibalism, such that the death of one sibling benefits surviving siblings [51]. Under these circumstances, suicidal male-killing bacteria benefit their clone-mates residing in the males’ sisters and thus enhances the indirect fitness of the male-killers [51,52].
In some lineages of intracellular bacteria, cells inhabiting the host's male germline have taken on a much more elaborate somatic function: cytoplasmic incompatibility (CI) [53]. The precise mechanisms of CI are not well known, but its effects can be intuitively understood by imagining that bacteria in the male germline secrete a poison that is incorporated into sperm, while their clone-mates in the female germline secrete an antidote that is incorporated into eggs [54]. Sperm deliver the poison when fertilizing an egg, and zygotes containing the poison but not the antidote do not survive. Thus, infected males destroy uninfected eggs, while successfully fertilizing infected eggs. An infected male also destroys eggs infected with other strains of bacteria, if they are incompatible with the strain that he carries. Whereas male-killing benefits very close clone-mates (derived from the same host female a single host generation ago), CI benefits a much broader pool of clone-mates across the population. Indeed in the case of the killing of uninfected eggs, the CI phenotype benefits the entire guild of intracellular bacteria. Inter-clone battles emerge when CI sperm destroy eggs infected by incompatible bacterial clones, but within-clone compatibility persists for many generations in laboratory culture, indicating that the service provided by bacteria in CI males benefits quite distant kin.
5. Intracellular bacteria and the origins of asymmetric genetic systems
We have discussed conflict between the nucleus and the cytogenome, and how elements within the cytogenome have evolved a number of ways to manipulate reproduction. But the question remains: does this reproductive manipulation lead to the origin of novel genetic systems?
(a). Parthenogenesis
The clearest case of genetic conflict leading to the evolution of a novel genetic system is that of feminizing bacteria converting arrhenotoky (figure 1) to thelytokous parthenogenesis (parthenogenetic production of females), which has been documented in several species of wasps and thrips [42–45,55]. Origins of obligate thelytokous parthenogenesis are probably the most frequently occurring evolutionary transition between genetic systems in nature. Are cytoplasmic elements often the instigators of these transitions? At this point, there is little evidence that they are. Well-documented cases are limited to parasitic wasps and thrips, and there are many parthenogenetic lineages in which no intracellular bacteria have been detected [56,57]. There are some cases in groups other than parasitic wasps in which parthenogenetic lineages are Wolbachia or Cardinium infected while related sexual lineages are not [58,59]. These are sometimes seen as apparent cases of parthenogenesis-induction, but it is also possible that parthenogenesis in these cases is a host-mediated response to a shortage of males resulting from bacterium-induced male-killing or feminization.
More broadly, there are several ways in which genetic conflict could lead to origins of parthenogenesis. Genetic conflict elevates the cost of sex [60], which may contribute to enabling parthenogenetic lineages to outcompete sexual ones [29]. In aquatic invertebrates, parthenogenesis arises more frequently in groups that brood their young [61]. It has been suggested that the viability costs of the origin of a new genetic system are reduced in brooders, because resources that would have been used by aborted embryos can be used by their siblings instead [61]. This same phenomenon—resource competition among siblings—can increase the intensity of genetic conflict, as seen in the emergence of male-killing in intracellular bacteria inhabiting hosts with sibling competition, and hence increase the cost of sex. On the whole, the evidence that genetic conflict is an important cause of the origins of parthenogenetic lineages is weak. The many other costs of sex ensure that there is a broad parameter space in which newly arising parthenogenetic lineages can thrive, at least in the short term [5,60]. Genetic conflict may have been more important in the origins of the other, more obviously asymmetric genetic systems, in which there is an ongoing, unequal role for males.
(b). Haplodiploidy
The hypothesis that conflict between maternal and paternal alleles has led to haplodiploidy runs into the difficulty of explaining why haplodiploidy has arisen in only certain groups and not others. Hamilton noted that haplodiploidy arises in groups that have gregarious broods [62,63] and maternally transmitted endosymbionts [64]. Normark [28] pointed out that this combination of features leads to male-killing endosymbionts and speculated that male haploidy may have arisen as a male-killing endosymbiont phenotype that was later coopted by the maternal genome in the host. Engelstädter & Hurst [65] pointed out that CI bacteria often effectively haploidize embryos and that these might have played a role in the origins of viable haploid males. Jack Werren has suggested an alternative route for the origin of arrhenotokous haplodiploidy from an ancestral population beset by male-killers. When male-killers are at high frequency, there is strong selection to produce offspring parthenogenetically (as many females go unmated) and to produce male offspring (as these are rare and have extraordinary mating opportunities). Thus, the parthenogenetic production of male offspring would be strongly favoured by selection in a population in which male-killers are at high frequency, and this might have led to the direct origin of arrhenotoky in the uninfected segment of the population (J. H. Werren 2004, personal communication). To date, there is no direct empirical support for a role of endosymbionts in the evolution of haplodiploidy, although the presence of endosymbionts is correlated with haplodiploidy in scale insects [66].
6. Interactions among relatives, indirect fitness and origins of novel genetic systems
Although better comparative evidence is still needed, it appears that species with brood chambers in which maternal kin interact are more likely to give rise to novel asymmetric genetic systems. This is apparently true of parthenogenesis [61], haplodiploidy [28] and parent-specific allele expression [67]. There are at least two possible reasons why this might be so. One is that the death of one embryo in a brood chamber can benefit its siblings. This reduces the cost of viability-lowering innovations, for example novel genetic systems [61]. It also makes minimum male viability the adaptive optimum for the cytogenome, creating a cytonuclear conflict over male viability that can drive the evolution of novelties relating to sex allocation and sex determination [28]. The second reason why brood chambers may lead to novel genetic systems is asymmetric relatedness. If the mother is the parent that establishes the brood chamber, all the offspring in the brood are maternally related, though they may not all be paternally related. An additional layer of asymmetric relatedness holds if the brood chamber is the mother's body, as all of the offspring are related to her through their maternal genes, though none (under outcrossing) are related to her by their paternal genes. This relatedness asymmetry has apparently led to the evolution of parent-specific allele expression in mammals [67]. In mammals, paternally expressed alleles in the embryo typically have a greedy phenotype, benefitting the embryo but imposing fitness costs on maternal kin. Parent-specific allele expression with greedy paternally imprinted phenotypes may have arisen in other taxa as well, and asymmetric genetic systems that limit the role of the paternal genome may evolved in response to this [29].
Thus, we can identify three effects of indirect fitness that may have played a role in the origins of novel genetic systems. First, novel genetic systems have often arisen in situations in which the direct-fitness costs of early embryo mortality are compensated in part by indirect-fitness benefits. Second, novel genetic systems have often arisen under conditions of asymmetric relatedness, such that indirect-fitness costs and benefits are disproportionately due to effects on maternal rather than paternal kin. This may have led to a divergence in optimal phenotype between maternally versus paternally inherited alleles, which may have led in turn to differentiation in the roles played by maternal and paternal alleles in the genetic system. Finally, novel genetic systems have often arisen in animals whose cytoplasm contains maternally inherited bacteria that are deprived of direct fitness in male hosts. Some of these bacteria may have evolved to enhance their indirect fitness via ‘somatic’ phenotypes, for example male-killing, and these may have played a role in altering the role of males in the host's genetic system.
7. Ecology of asymmetric kin and asymmetric genetics: the big picture
Asymmetric genetic systems occur in terrestrial and freshwater environments. They appear to be exceedingly rare in marine environments (table 1). This pattern has probably been exaggerated by observational bias, as terrestrial and freshwater organisms are often more amenable to study than marine organisms. But enough is known about marine organisms that the pattern is unlikely to be entirely artefactual [68,69]. The pattern is a striking feature of the distribution of genetic systems on earth, albeit one that has rarely been remarked upon. The marine environment is presumably the one in which sex and symmetrical genetics evolved, and they are apparently all but indispensable in that environment. The repeated invasion of the novel environments of land and freshwater repeatedly gave rise to novel, less-symmetrical genetic systems. Why should this be so? Marine organisms dwell within the medium in which life evolved and to which all life, until relatively recently, was adapted. We propose that the distribution of genetic systems has been shaped by seawater's suitability for four classes of organisms in particular: pathogens, symbionts, gametes and embryos. Air, subjected to constant UV radiation, is a persistently challenging medium for the survival and dispersal of any of them. Freshwater, too, is constantly being distilled by the hydrologic cycle and subjected to greater thermal fluctuations and other abiotic variations than marine habitats, making it, too, a more challenging medium than seawater.
(a). Pathogens: the Red Queen rules the waves
Viruses are an abundant component of seawater [70]. This may help to explain one aspect of the distribution of genetic systems: the near-absence of obligate parthenogenesis from marine environments, in comparison to reasonably high frequency in some terrestrial and freshwater environments (table 1). This observation may be understandable in terms of the Parasite Red Queen hypothesis for the adaptive significance of sex [71,72]. When we left the ocean, we may have left most of our pathogens and parasites behind and thus are less reliant on sex than marine organisms are.
(b). Symbionts: the Trojan microbiota
Symbiosis between animals and microorganisms is probably at least as important in marine environments as in terrestrial and freshwater environments, as illustrated dramatically by corals [73,74]. But in marine environments, microbial symbionts are more often transmitted horizontally and acquired from the environment by each generation of animals [75]. In terrestrial environments in particular, vertical transmission of microbial endosymbionts is much more common, possibly because air is such a poor medium for symbiont transmission, such that a large percentage of terrestrial species carry maternally transmitted endosymbionts [31,36,76]. Such symbionts may engage in conflicts with their host's genome over sex determination and male viability, de-stabilizing their hosts’ genetic systems. A few groups of marine invertebrates buck this trend and have vertically transmitted symbionts: gutless oligochaetes, and solemyid and vesicomyid clams [75]. These are promising systems to test the effects of endosymbionts on host reproduction in a marine environment.
(c). Gametes and embryos: both blood and water are thicker than air
Shedding gametes into the water column is a successful reproductive strategy for many completely sessile marine organisms. No comparable strategy is available to terrestrial organisms, as air is a poor medium for the propagation of gametes. Embryos, likewise, can be shed into seawater but cannot as easily be shed into air. Consequently, multicellular terrestrial organisms are largely dependent upon internal fertilization, and embryos often complete much of their development within a medium supplied by the mother, sometimes within the mother's own body. The mother, and maternal kin, thus form a much more important part of the environment for terrestrial organisms than for marine organisms. This asymmetric relatedness to neighbours produces asymmetric selection on different components of the genome in terrestrial organisms—for instance, favouring male-killing cytoplasmic elements and greedy paternal alleles—which may play a role in the origin of asymmetric genetic systems. It might appear that terrestrial plants follow a marine-like strategy of shedding gametes and embryos into the air, but this is not really the case. Plants do not shed female gametophytes, only male gametophytes, so fertilization is still effectively internal within the seed parent. Many plants shed fruits containing multiple maternally related embryos [77], and even a lone plant seed is far from being a naked embryo: it usually consists primarily of endosperm, which often carries a double dose of the maternal genome and single dose of the paternal genome, along with a maternally derived integument. Thus plants, like terrestrial animals, have a maternal/paternal asymmetry in relatedness to neighbouring tissues during early development, and, like terrestrial animals, have strong cytonuclear conflicts (e.g. origins of cytoplasmic male sterility) and have frequently originated asymmetric genetic systems including apomixis, gynogenesis (pseudogamy) and parent-specific allele expression.
8. Conclusion
Symmetrical, eumendelian genetics is the genetics of fully independent individuals. It is a system agnostic about kin, in which information about the parental origin of each allele is destroyed in each generation, with both parents’ alleles treated identically. This was the ancestral eukaryotic sexual system that presumably arose in an isogamous unicellular lineage and that still characterizes the great majority of eukaryotes. Novel, asymmetric genetic systems derived from this ancestral system have arisen many times, especially in multicellular organisms inhabiting terrestrial and freshwater environments. The reasons for this are not clear, but one important reason may be the greater role played by maternal kin in environments relatively hostile to gametes and embryos. Extended contact between mother and offspring may facilitate all kinds of evolutionary novelties, by reducing the cost of early abortion of a proportion of embryos [61]. In particular, extended contact between maternal kin means that the inclusive-fitness effects of maternal alleles will differ from those of paternal alleles, and this may have led to the evolution of different roles and fates for maternal versus paternal alleles [29,78]. The importance of maternal kin is amplified further in environments hostile to unicellular symbionts, with the evolution of maternal channels of inheritance for symbionts, which can then become highly disruptive of their hosts’ sex determination systems.
Acknowledgements
For inspiration and encouragement, we thank Jon Seger, Robert Trivers, Jack Werren, David Haig and Andy Gardner. For comments on a previous draft that improved the manuscript, we thank Stuart West and two anonymous reviewers.
Funding statement
Our collaboration benefited from travel funding provided by the Royal Entomological Society, the National Evolutionary Synthesis Center (NESCent) and NSF (DEB-1258001 to B.B.N.). L.R. is financially supported by NERC.
References
- 1.Leigh EG., Jr 1971. Adaptation and diversity. San Francisco, CA: Freeman, Cooper. [Google Scholar]
- 2.McDermott SR, Noor MA. 2010. The role of meiotic drive in hybrid male sterility. Phil. Trans. R. Soc. B 365, 1265–1272. ( 10.1098/rstb.2009.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt A, Trivers R. 2006. Genes in conflict: the biology of selfish genetic elements. Cambridge, MA: Harvard University Press. [Google Scholar]
- 4.McKone MJ, Halpern SL. 2003. The evolution of androgenesis. Am. Nat. 161, 641–656. ( 10.1086/368291) [DOI] [PubMed] [Google Scholar]
- 5.Bell G. 1982. The masterpiece of nature. Berkeley, CA: University of California Press. [Google Scholar]
- 6.Bull JJ. 1983. Evolution of sex determining mechanisms. Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- 7.Hebert PDN. 1987. Genotypic characteristics of cyclic parthenogens and their obligately asexual derivatives. In The evolution of sex and its consequences (ed. Stearns SJ.), pp. 175–195. Basel, Switzerland: Birhäuser Verlag. [DOI] [PubMed] [Google Scholar]
- 8.Beukeboom LW, Vrijenhoek RC. 1998. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J. Evol. Biol. 11, 755–782. ( 10.1046/j.1420-9101.1998.11060755.x) [DOI] [Google Scholar]
- 9.Normark BB. 2003. The evolution of alternative genetic systems in insects. Annu. Rev. Entomol. 48, 397–423. ( 10.1146/annurev.ento.48.091801.112703) [DOI] [PubMed] [Google Scholar]
- 10.Fournier D, Estoup A, Orivel J, Foucaud J, Jourdan H, Breton JL, Keller L. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435, 1230–1234. ( 10.1038/nature03705) [DOI] [PubMed] [Google Scholar]
- 11.Schlupp I. 2005. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Syst. 36, 399–417. ( 10.1146/annurev.ecolsys.36.102003.152629) [DOI] [Google Scholar]
- 12.Jarne P, Auld JR. 2006. Animals mix it up too: the distribution of self-fertilization among hermaphroditic animals. Evolution 60, 1816–1824. ( 10.1111/j.0014-3820.2006.tb00525.x) [DOI] [PubMed] [Google Scholar]
- 13.Hughes RN. 1989. A functional biology of clonal animals. Functional biology series. London, UK: Chapman and Hall. [Google Scholar]
- 14.Hoeh WR, Stewart DT, Sutherland BW, Zouros E. 1996. Multiple origins of gender-associated mitochondrial DNA lineages in bivalves (Mollusca: Bivalvia). Evolution 50, 2276–2286. ( 10.2307/2410697) [DOI] [PubMed] [Google Scholar]
- 15.Bull JJ. 1979. An advantage for the evolution of male haploidy and systems with similar genetic transmission. Heredity 43, 361–381. ( 10.1038/hdy.1979.88) [DOI] [Google Scholar]
- 16.Corley LS, Moore AJ. 1999. Fitness of alternative modes of reproduction: developmental constraints and the evolutionary maintenance of sex. Proc. R. Soc. Lond. B 266, 471–476. ( 10.1098/rspb.1999.0661) [DOI] [Google Scholar]
- 17.Engelstadter J. 2008. Constraints on the evolution of asexual reproduction. Bioessays 30, 1138–1150. ( 10.1002/bies.20833) [DOI] [PubMed] [Google Scholar]
- 18.Ross L, Pen I, Shuker DM. 2010. Genomic conflict in scale insects: the causes and consequences of bizarre genetic systems. Biol. Rev. 85, 807–828. ( 10.1111/j.1469-185X.2010.00127.x) [DOI] [PubMed] [Google Scholar]
- 19.Brown SW, Nelson-Rees WA. 1961. Radiation analysis of a lecanoid genetic system. Genetics 46, 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitchin RM. 1970. A radiation analysis of a comstockiella chromosome system: destruction of heterochromatic chromosomes during spermatogenesis in Parlatoria oleae (Coccoidea: Diaspididae). Chromosoma 31, 165–197. ( 10.1007/BF00285146) [DOI] [PubMed] [Google Scholar]
- 21.Brown SW. 1964. Automatic frequency response in the evolution of male haploidy and other coccid chromosome systems. Genetics 49, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartl DL, Brown SW. 1970. The origin of male haploid genetic systems and their expected sex ratio. Theor. Popul. Biol. 1, 165–190. ( 10.1016/0040-5809(70)90033-X) [DOI] [PubMed] [Google Scholar]
- 23.Borgia G. 1980. Evolution of haplodiploidy: models for inbred and outbred systems. Theor. Popul. Biol. 17, 103–128. ( 10.1016/0040-5809(80)90001-5) [DOI] [PubMed] [Google Scholar]
- 24.Haig D. 1993. The evolution of unusual chromosomal systems in coccoids: extraordinary sex ratios revisited. J. Evol. Biol. 6, 69–77. ( 10.1046/j.1420-9101.1993.6010069.x) [DOI] [Google Scholar]
- 25.Haig D. 1993. The evolution of unusual chromosomal systems in sciarid flies: intragenomic conflict and the sex raio. J. Evol. Biol. 6, 249–261. ( 10.1046/j.1420-9101.1993.6020249.x) [DOI] [Google Scholar]
- 26.Herrick G, Seger J. 1999. Imprinting and paternal genome elimination in insects. In Genomic imprinting: an interdisciplinary approach, vol. 25 (ed. Ohlsson R.), pp. 41–71. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 27.Úbeda F, Normark BB. 2006. Male killers and the origins of paternal genome elimination. Theor. Popul. Biol. 70, 511–526. ( 10.1016/j.tpb.2006.06.011) [DOI] [PubMed] [Google Scholar]
- 28.Normark BB. 2004. Haplodiploidy as an outcome of coevolution between male-killing cytoplasmic elements and their hosts. Evolution 58, 790–798. ( 10.1111/j.0014-3820.2004.tb00412.x) [DOI] [PubMed] [Google Scholar]
- 29.Normark BB. 2006. Perspective: maternal kin groups and the evolution of asymmetric genetic systems: genomic imprinting, haplodiploidy, thelytoky. Evolution 60, 631–642. ( 10.1111/j.0014-3820.2006.tb01145.x) [DOI] [PubMed] [Google Scholar]
- 30.Haig D. 2004. Genomic imprinting and kinship: how good is the evidence? Annu. Rev. Genet. 38, 553–585. ( 10.1146/annurev.genet.37.110801.142741) [DOI] [PubMed] [Google Scholar]
- 31.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190. ( 10.1146/annurev.genet.41.110306.130119) [DOI] [PubMed] [Google Scholar]
- 32.Saumitou-Laprade P, Cuguen J, Vernet P. 1994. Cytoplasmic male sterility in plants: molecular evidence and the nucleocytoplasmic conflict. Trends Ecol. Evol. 9, 431–435. ( 10.1016/0169-5347(94)90126-0) [DOI] [PubMed] [Google Scholar]
- 33.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 34.Perotti MA, Allen JM, Reed DL, Braig HR. 2007. Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J. 21, 1058–1066. ( 10.1096/fj.06-6808com) [DOI] [PubMed] [Google Scholar]
- 35.Perlman SJ, Hunter MS, Zchori-Fein E. 2006. The emerging diversity of Rickettsia. Proc. R. Soc. B 273, 2097–2106. ( 10.1098/rspb.2006.3541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zchori-Fein E, Perlman SJ. 2004. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 13, 2009–2016. ( 10.1111/j.1365-294X.2004.02203.x) [DOI] [PubMed] [Google Scholar]
- 37.Anbutsu H, Fukatsu T. 2011. Spiroplasma as a model insect endosymbiont. Environ. Microbiol. Rep. 3, 144–153. ( 10.1111/j.1758-2229.2010.00240.x) [DOI] [PubMed] [Google Scholar]
- 38.Thao MLL, Baumann P. 2004. Evidence for multiple acquisition of Arsephonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 48, 140–144. ( 10.1007/s00284-003-4157-7) [DOI] [PubMed] [Google Scholar]
- 39.Bandi C, Dunn AM, Hurst GD, Rigaud T. 2001. Inherited microorganisms, sex-specific virulence and reproductive parasitism. Trends Parasitol. 17, 88–94. ( 10.1016/S1471-4922(00)01812-2) [DOI] [PubMed] [Google Scholar]
- 40.Veneti Z, et al. 2012. Loss of reproductive parasitism following transfer of male-killing Wolbachia to Drosophila melanogaster and Drosophila simulans. Heredity 109, 306–312. ( 10.1038/hdy.2012.43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanada-Morimura S, Matsumura M, Noda H. 2013. Male killing caused by a Spiroplasma symbiont in the Small Brown Planthopper, Laodelphax striatellus. J. Hered. 104, 821–829. ( 10.1093/jhered/est052) [DOI] [PubMed] [Google Scholar]
- 42.Stouthamer R, Luck RF, Hamilton WD. 1990. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc. Natl Acad. Sci. USA 87, 2424–2427. ( 10.1073/pnas.87.7.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell JE, Stouthamer R. 2011. The genetics and evolution of obligate reproductive parasitism in Trichogramma pretiosum infected with parthenogenesis-inducing Wolbachia. Heredity 106, 58–67. ( 10.1038/hdy.2010.48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraaijeveld K, Reumer BM, Mouton L, Kremer N, Vavre F, Alphen JJM. 2011. Does a parthenogenesis-inducing Wolbachia induce vestigial cytoplasmic incompatibility? Naturwissenschaften 98, 175–180. ( 10.1007/s00114-010-0756-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pannebakker BA, Schidlo NS, Boskamp GJF, Dekker L, Dooren TJM, Beukeboom LW, Zwaan BJ, Brakefield PM, Alphen JJM. 2005. Sexual functionality of Leptopilina clavipes (Hymenoptera: Figitidae) after reversing Wolbachia-induced parthenogenesis. J. Evol. Biol. 18, 1019–1028. ( 10.1111/j.1420-9101.2005.00898.x) [DOI] [PubMed] [Google Scholar]
- 46.Negri I, Pellecchia M, Mazzoglio PJ, Patetta A, Alma A. 2006. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. R. Soc. B 273, 2409–2416. ( 10.1098/rspb.2006.3592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouchon D, Cordaux R, Grève P. 2009. Feminizing Wolbachia and the evolution of sex determination in isopods. In Insect symbiosis, vol. 3 (eds Bourtzis K, Miller TA.), pp. 273–294. Boca Raton, FL: CRC Press. [Google Scholar]
- 48.Cordaux R, Bouchon D, Greve P. 2011. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 27, 332–341. ( 10.1016/j.tig.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 49.Weeks AR, Marec F, Breeuwer JAJ. 2001. A mite species that consists entirely of haploid females. Science 292, 2479–2482. ( 10.1126/science.1060411) [DOI] [PubMed] [Google Scholar]
- 50.Groot TVM, Breeuwer JAJ. 2006. Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Exp. Appl. Acarol. 39, 257–271. ( 10.1007/s10493-006-9019-0) [DOI] [PubMed] [Google Scholar]
- 51.Hurst LD. 1991. The incidences and evolution of cytoplasmic male-killers. Proc. R. Soc. Lond. B 244, 91–99. ( 10.1098/rspb.1991.0056) [DOI] [Google Scholar]
- 52.Hurst GD, Jiggins FM. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336. ( 10.3201/eid0604.000402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelstadter J, Telschow A. 2009. Cytoplasmic incompatibility and host population structure. Heredity 103, 196–207. ( 10.1038/hdy.2009.53) [DOI] [PubMed] [Google Scholar]
- 54.Nor I, Engelstädter J, Duron O, Reuter M, Sagot M-F, Charlat S. 2013. On the genetic architecture of cytoplasmic incompatibility: inference from phenotypic data. Am. Nat. 182, E15–E24. ( 10.1086/670612) [DOI] [PubMed] [Google Scholar]
- 55.Arakaki N, Miyoshi T, Noda H. 2001. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis (Thysanoptera: Insecta). Proc. R. Soc. Lond. B 268, 1011–1016. ( 10.1098/rspb.2001.1628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabeling C, Kronauer DJ. 2013. Thelytokous parthenogenesis in eusocial Hymenoptera. Annu. Rev. Entomol. 58, 273–292. ( 10.1146/annurev-ento-120811-153710) [DOI] [PubMed] [Google Scholar]
- 57.Choleva L, Janko K. 2013. Rise and persistence of animal polyploidy: evolutionary constraints and potential. Cytogenet. Genome Res. 140, 151–170. ( 10.1159/000353464) [DOI] [PubMed] [Google Scholar]
- 58.Koivisto RKK, Braig HR. 2003. Microorganisms and parthenogenesis. Biol. J. Linn. Soc. 79, 43–58. ( 10.1046/j.1095-8312.2003.00185.x) [DOI] [Google Scholar]
- 59.Provencher LM, Morse GE, Weeks AR, Normark BB. 2005. Parthenogenesis in the Aspidiotus nerii complex (Hemiptera: Diaspididae): a single origin of a worldwide, polyphagous lineage associated with Cardinium bacteria. Ann. Entomol. Soc. Am. 98, 629–635. ( 10.1603/0013-8746(2005)098[0629:PITANC]2.0.CO;2) [DOI] [Google Scholar]
- 60.Lehtonen J, Jennions MD, Kokko H. 2012. The many costs of sex. Trends Ecol. Evol. 27, 172–178. ( 10.1016/j.tree.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 61.Lively CM, Johnson SG. 1994. Brooding and the evolution of parthenogenesis: strategy models and evidence from aquatic invertebrates. Proc. R. Soc. Lond. B 256, 89–95. ( 10.1098/rspb.1994.0054) [DOI] [PubMed] [Google Scholar]
- 62.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 63.Hamilton WD. 1978. Evolution and diversity under bark. In Diversity of insect faunas (eds Mound LA, Waloff N.), pp. 154–175. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 64.Hamilton WD. 1993. Inbreeding in Egypt and in this book: a childish perspective. In The natural history of inbreeding and outbreeding: theoretical and empirical perspectives (ed. Thornhill NW.), pp. 429–450. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 65.Engelstädter J, Hurst GDD. 2006. Can maternally transmitted endosymbionts facilitate the evolution of haplodiploidy? J. Evol. Biol. 19, 194–202. ( 10.1111/j.1420-9101.2005.00974.x) [DOI] [PubMed] [Google Scholar]
- 66.Ross L, Shuker DM, Normark BB, Pen I. 2012. The role of endosymbionts in the evolution of haploid-male genetic systems in scale insects (Coccoidea). Ecol. Evol. 2, 1071–1081. ( 10.1002/ece3.222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkins JF, Haig D. 2003. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 4, 359–368. ( 10.1038/nrg1062) [DOI] [PubMed] [Google Scholar]
- 68.Nielsen EE, Hemmer-Hansen J, Larsen PF, Bekkevold D. 2009. Population genomics of marine fishes: identifying adaptive variation in space and time. Mol. Ecol. 18, 3128–3150. ( 10.1111/j.1365-294X.2009.04272.x) [DOI] [PubMed] [Google Scholar]
- 69.Weersing K, Toonen RJ. 2009. Population genetics, larval dispersal, and connectivity in marine systems. Mar. Ecol. Prog. Ser. 393, 1–12. ( 10.3354/meps08287) [DOI] [Google Scholar]
- 70.Suttle CA. 2005. Viruses in the sea. Nature 437, 356–361. ( 10.1038/nature04160) [DOI] [PubMed] [Google Scholar]
- 71.Hamilton WD, Axelrod R, Tanese R. 1990. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 87, 3566–3573. ( 10.1073/pnas.87.9.3566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mostowy R, Engelstadter J. 2012. Host–parasite coevolution induces selection for condition-dependent sex. J. Evol. Biol. 25, 2033–2046. ( 10.1111/j.1420-9101.2012.02584.x) [DOI] [PubMed] [Google Scholar]
- 73.Baker AC. 2003. Flexibility and specificity in coral–algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu . Rev. Ecol. Syst. 34, 661–689. ( 10.1146/annurev.ecolsys.34.011802.132417) [DOI] [Google Scholar]
- 74.Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 71, 295–347. ( 10.1128/MMBR.00040-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubilier N, Bergin C, Lott C. 2008. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat. Rev. Microbiol. 6, 725–740. ( 10.1038/nrmicro1992) [DOI] [PubMed] [Google Scholar]
- 76.Weeks AR, Velten R, Stouthamer R. 2003. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. B 270, 1857–1865. ( 10.1098/rspb.2003.2425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haig D. 1992. Brood reduction in gymnosperms. In Cannibalism: ecology and evolution among diverse taxa (eds Elgar M, Crespi B.), pp. 63–84. Oxford, UK: Oxford University Press. [Google Scholar]
- 78.Haig D. 2000. The kinship theory of genomic imprinting. Annu. Rev. Ecol. Syst. 31, 9–32. ( 10.1146/annurev.ecolsys.31.1.9) [DOI] [Google Scholar]