Abstract
Chromatin organization and structure are crucial for transcriptional regulation, DNA replication, and damage repair. Although initially characterized in remodeling cell wall glucans, the β-1,3-glucanosyltransferase Gas1 was recently discovered to regulate transcriptional silencing in a manner separable from its activity at the cell wall. However, the function of Gas1 in modulating chromatin remains largely unexplored. Our genetic characterization revealed that GAS1 had critical interactions with genes encoding the histone H3 lysine acetyltransferases Gcn5 and Sas3. Specifically, whereas the gas1gcn5 double mutant was synthetically lethal, deletion of both GAS1 and SAS3 restored silencing in Saccharomyces cerevisiae. The loss of GAS1 also led to broad DNA damage sensitivity with reduced Rad53 phosphorylation and defective cell cycle checkpoint activation following exposure to select genotoxins. Deletion of SAS3 in the gas1 background restored both Rad53 phosphorylation and checkpoint activation following exposure to genotoxins that trigger the DNA replication checkpoint. Our analysis thus uncovers previously unsuspected functions for both Gas1 and Sas3 in DNA damage response and cell cycle regulation.
Keywords: chromatin, acetyltransferase, DNA damage, cell cycle checkpoint, glucan
CHROMATIN packages DNA in the nucleus and regulates accessibility to the underlying genome. Tightly compacted chromatin, or heterochromatin, impedes nuclear processes including transcription, DNA replication, and DNA damage repair (reviewed in Li and Reinberg 2011; Papamichos-Chronakis and Peterson 2013). Thus, genes found within heterochromatic regions are repressed or silenced (reviewed in Rusche et al. 2003). However, the degree of chromatin compaction is highly dynamic, as cells must continuously alter transcriptional programs in response to environmental or metabolic demands while promoting replication and repair processes.
The basic unit of chromatin is the nucleosome, consisting of DNA wrapped around an octamer of conserved core histone proteins (Kornberg and Lorch 1999). Post-translational modification (PTM) of histones is a prime means for altering chromatin structure. These modifications are dynamic and tightly controlled as they regulate higher order chromatin structure and DNA accessibility by altering the interaction between DNA and histones in addition to recruiting chromatin-modifying enzymes (reviewed in Kouzarides 2007; Campos and Reinberg 2009). The localization of chromatin within the nucleus also plays a fundamental role in chromatin dynamics, such that localization to the nuclear periphery regulates processes including silencing and the DNA damage response (DDR) (reviewed in Bermejo et al. 2012; Taddei and Gasser 2012).
The β-1,3-glucanosyltransferase Gas1, a member of the Gas family of proteins, was initially characterized at the cell wall where it remodels chains of β-1,3-glucan (Ragni et al. 2007). However, a pool of Gas1 also localizes to the nuclear periphery (Huh et al. 2003) and genome-wide studies have identified genetic and physical interactions between Gas1 and diverse components of the chromatin modifying machinery (www.thebiogrid.org). Reflecting these findings, deletion of GAS1 was recently discovered to lead to a unique constellation of silencing defects in yeast. Specifically, loss of Gas1 catalytic activity increases rDNA silencing and decreases telomeric silencing, yet has no observable change at the HM cryptic mating-type loci. These alterations in silencing are not remediated by the osmoregulator sorbitol (Koch and Pillus 2009), which rescues the cell wall-associated defects of gas1 and other cell wall mutants (Turchini et al. 2000; Levin 2005). Combined, these data support a function for Gas1 in chromatin-mediated processes that is separable from its role at the cell wall.
A genome-wide screen reported that GAS1 has a negative genetic interaction with GCN5 (Costanzo et al. 2010), which encodes a prominent lysine acetyltransferase (KAT). Gcn5-catalyzed acetylation of histone and nonhistone substrates affects numerous chromatin-dependent processes (reviewed in Lee and Workman 2007; Koutelou et al. 2010). Gcn5 functions in several important complexes including SAGA, ADA, and SLIK/SALSA (Grant et al. 1997; Pray-Grant et al. 2002) to acetylate nucleosomal substrates on histone H3, with lysine 14 (K14) as a predominant target (Kuo and Andrews 2013). Gcn5 acts as a coactivator, with H3K14 acetylation correlating with active transcription (Pokholok et al. 2005) and Gcn5 is enriched at the promoters of active genes (Robert et al. 2004).
Gcn5 functionally overlaps with another KAT, Sas3. Gcn5 and Sas3 share nucleosomal H3 targets (reviewed in Lafon et al. 2007) and deletion of both GCN5 and SAS3 is synthetically lethal (Howe et al. 2001). Further, both Gcn5 and Sas3 are recruited to similar genomic regions (Rosaleny et al. 2007). Whereas Gcn5 has been studied extensively, less is known about Sas3, due in part to the functional overlaps with Gcn5 as well as the limited independent phenotypes defined for SAS3 mutants. Deletion of SAS3 leads to a modest increase in silencing at the HM loci (Reifsnyder et al. 1996) and Sas3 localizes at the boundary of the HM loci, blocking the spread of silent chromatin (Tackett et al. 2005). Sas3 physically associates with the N terminus of Spt16, a subunit of the FACT elongation complex (John et al. 2000), which is essential for recovery from replication stress (O’Donnell et al. 2004) and boundary formation (Tackett et al. 2005).
In addition to functions in transcriptional regulation and silencing, Gcn5 and other histone modifying enzymes also have crucial roles in the DDR. One of the earliest marks associated with DDR activation in yeast is the phosphorylation of H2A at serine 129 (S129), which serves as a scaffold that amplifies the DNA damage signal in part by recruiting the repair machinery (reviewed in Rossetto et al. 2010). Subsequently, phosphorylation of other mediators, prominently including the Rad53 kinase, triggers a cascade that leads to changes in transcription and activation of cell cycle checkpoints, which foster the repair of damaged DNA (reviewed in Branzei and Foiani 2006; Sirbu and Cortez 2013).
Deletion of GCN5 renders cells sensitive to DNA damaging agents such as the topoisomerase I inhibitor camptothecin (CPT), the radiomimietic drug methyl methanesulfonate (MMS) and the replication inhibitor hydroxyurea (HU) (Choy and Kron 2002; Burgess et al. 2010). Indeed, Gcn5-catalzyed acetylation of both histone and nonhistone substrates features prominently at numerous stages of the DNA damage response (Burgess et al. 2010; Lee et al. 2010; Charles et al. 2011).
There is also some evidence that Sas3 may play a role in the DDR. For example, Sas3 has a reported physical interaction with the DNA damage checkpoint effector kinase Chk1 (Liu et al. 2000), although the functional significance of this interaction has not been established. Further, mutants of H3K14 and H3K23, nucleosomal substrates of Gcn5 and Sas3, are sensitive to DNA-damaging agents (Qin and Parthun 2002; Tamburini and Tyler 2005). However, what role, if any, Sas3 may play in DNA damage has not been defined.
Here we report that GAS1 has strong genetic interactions with the histone H3 lysine acetyltransferases encoded by both GCN5 and SAS3. The gas1gcn5 combination was synthetically lethal. In contrast, the gas1sas3 double mutant was viable and, moreover, displayed selective mutual suppression of each individual mutant’s phenotypes. We also discovered that gas1 has broad DNA damage sensitivity following exposure to the genotoxins MMS, HU, and CPT. Sensing and initial activation of the DNA damage response was intact in gas1 strains, as evidenced by phosphorylation of histone H2A. However, the MMS and HU sensitivity of gas1 reflects failure to trigger the DNA damage cell cycle checkpoint as demonstrated by loss of both the cell cycle delay and Rad53 phosphorylation. The deletion of SAS3 in the gas1 background specifically suppressed both MMS and HU sensitivity, leading to restoration of cell cycle delay and Rad53 phosphorylation. These findings define a role for Gas1 in the DNA damage response that is separable from its cell wall function. We have also identified a specific role for Sas3 in antagonizing the replication checkpoint, which is unique and opposite to the role previously identified for Gcn5.
Materials and Methods
Yeast strains and plasmids
Strains are listed in Supporting Information, Table S1, plasmids in Table S2, and oligonucleotides in Table S3. All mutations are deletions, unless otherwise noted, and were constructed using standard techniques (Amberg et al. 2005).
Growth, silencing, and DNA damage assays
Plate assays are fivefold serial dilutions adjusted to an A600 of 1.0 after growth to saturation in synthetic complete (SC) medium. Dilution assays were incubated at 30°, except where noted. Telomeric silencing assays were performed with the TELVR::URA3 reporter strain grown in SC medium and plated on SC as growth control or SC supplemented with 0.1% 5-fluoroorotic acid (5-FOA) to assay silencing (Renauld et al. 1993; Van Leeuwen and Gottschling 2002). Silent mating-type analysis was performed with the hml::TRP1 reporter (Le et al. 1997). Silencing of the rDNA was assayed using the RDN::Ty-1-mURA3 construct (Smith and Boeke 1997). Strains were plated on SC as a growth control and SC −Ura for rDNA silencing. HU sensitivity was analyzed with 0.2 M HU. MMS sensitivity was analyzed with 0.015% MMS. CPT sensitivity was analyzed using 20 μg/ml CPT dissolved in DMSO added to plates buffered with 100 mM potassium phosphate (pH 7.5) to maintain CPT activity (Nitiss and Wang 1988) with growth control plates at the same concentration of DMSO. DMSO is shown as a control with all CPT images as gas1 is mildly sensitive to DMSO. For ultraviolet light (UV) sensitivity, strains were plated at A600 of 1.0 and exposed to 60 J/m2. Where indicated, plates were supplemented with 1 M sorbitol.
Protein immunoblots
Strains for analysis of H2AS129 and Rad53 phosphorylation following genotoxin exposure were incubated at 30° to an A600 of 0.4. Cultures were then treated with either indicated genotoxin or untreated as a control. The concentrations of HU, MMS, and CPT were the same as in dilution assays. Cells were incubated with genotoxin for 2 hr at 30° with shaking. Cell extracts were prepared by bead beating (Clarke et al. 1999). Proteins were separated on 18% (H2A) or 8% (Rad53) SDS-polyacrylamide gels and transferred to nitrocellulose. H2AS129 phosphorylation levels were analyzed with the primary antiserum anti-H2A phospho S129 (1:5000, Abcam) and blots were imaged using ECL Plus (GE Healthcare Amersham) with anti-H2A (1:5000, Abcam) used as a probe for protein loading. For analysis of Rad53 phosphorylation, the primary antiserum was anti-Rad53 (1:5000 dilution; Pike et al. 2003, a gift from J. Heierhorst). Antitubulin (1:10000; Bond et al. 1986) used as a probe for protein loading.
Flow cytometry
Cells were grown in SC with genotoxin conditions as used for immunoblots, fixed with ethanol overnight, and then treated with RNase A (Clarke et al. 1999). Cells were stained with propidium iodide for 2 days at 4°, sonicated, and analyzed with Accuri (BD).
Results
The synthetic lethality of GAS1 with GCN5 is separable from cell wall functions
The function of Gas1 at the cell wall has been studied extensively (reviewed in Popolo and Vai 1999; Orlean 2012), but less is known about the pool of Gas1 that is contiguous with the nuclear periphery (Huh et al. 2003). Genome-wide studies report >50 interactions of GAS1 with genes encoding nuclear proteins, many of which are active in chromatin dynamics and/or the DDR (www.thebiogrid.org). However, few of these interactions have been independently validated. Based on the silencing defects of gas1 and its reported interactions, we chose to further define the chromatin-based functions of Gas1 by analyzing interactions with genes encoding nuclear factors. We selected these based on previous genome-wide analysis of synthetic interactions, such as the synthetic lethality for gas1 and orc2-1 (Suter et al. 2004) or based on independent observations from our laboratory. The initial analysis included genes encoding the Orc2 subunit of the DNA replication origin recognition complex, the histone lysine deacetylase Rpd3, and the ATPase Swr1. The double mutants gas1rpd3 and gas1orc2-1 were synthetically lethal; however, these interactions were at least partially rescued by the osmoregulator sorbitol (Figure S1, A and B), which rescues phenotypes of cell wall-defective mutants, including gas1 (Turchini et al. 2000; Levin 2005). Conversely, deletion of SWR1 rescued both gas1 temperature and calcofluor white (CFW) sensitivity (Figure S1C), which disrupts the cell wall by inhibiting chitin synthesis (Roncero and Duran 1985). Although these results do not eliminate the possibility that the proteins encoded by these genes may also be significant for Gas1-related chromatin functions, we directed our focus to other chromatin modifying enzymes as a means to define the roles of Gas1 in chromatin dynamics that are separable from its cell wall function.
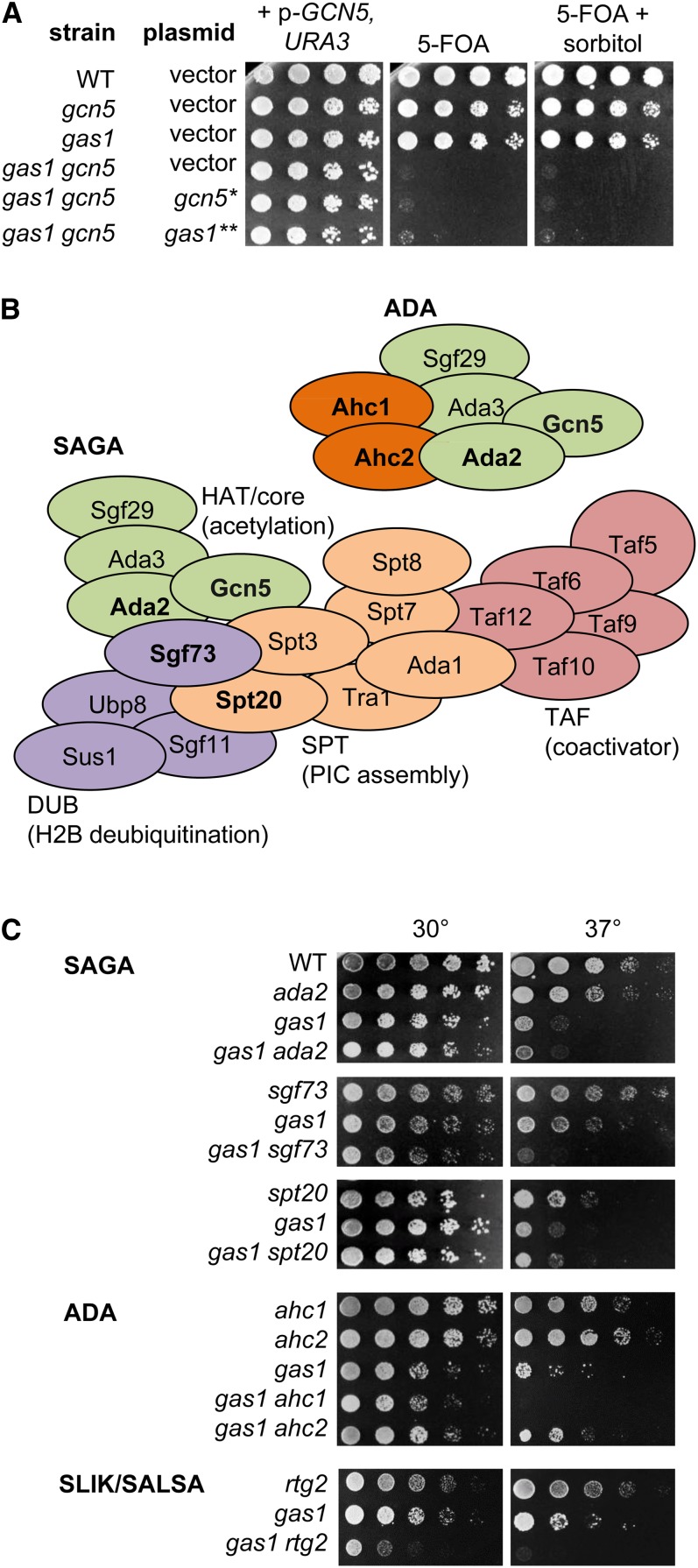
A recent genome-wide study indicated that GAS1 and GCN5 have a negative genetic interaction (Costanzo et al. 2010). We found that the gas1gcn5 heterozygous double mutant failed to sporulate unless covered by a plasmid encoding either GAS1 or GCN5. When dissected, the resulting haploid double mutants were not viable without the covering plasmid as demonstrated in two ways: first by the inferred genotype of dead spores and second by inability to grow on 5-FOA, which selects against the URA3-marked covering plasmids. The catalytic activity of both Gas1 and Gcn5 is required for viability, as neither of the previously defined catalytically inactive mutants, gcn5-KQL (gcn5*; Wang et al. 1998) or gas1-E161Q, E262Q (gas1**; Carotti et al. 2004), rescued the lethality of the double mutant in plasmid-shuffle tests. Additionally, the osomoregulator sorbitol did not rescue the synthetic lethality of gas1gcn5 (Figure 1A). Thus, the synthetic lethality of gas1gcn5 is due to loss of the catalytic activities of Gas1 and Gcn5 and is separable from cell wall-associated functions.
Figure 1.
The gas1 gcn5 double mutant is synthetically lethal. (A) Synthetic lethality of gas1 gcn5 is due to loss of catalytic activity of both Gas1 and Gcn5 and is not rescued by the osomoregulator sorbitol. Serial dilutions of wild type (LPY18050), gcn5 (LPY12264), gas1 (LPY18081), gas1 gcn5 (LPY16798), and gas1 gcn5 covered by plasmid-born p-gcn5-KQL (gcn5*; LPY16800) or p-gas1-E161Q, E262Q (gas1**; LPY16801) were plated on selective media with 5-FOA, to counterselect the p-GCN5, URA3 plasmid, with or without 1 M sorbitol at 30°. (B) Primary Gcn5-containing complexes are shown with color coding to highlight defined subunits in each functional module. Boldface type indicates subunits analyzed in this study (adapted from Lee et al. 2011). (C) gas1 has modest synthetic interactions with components of all three complexes tested, including increased temperature sensitivity with gas1 sgf73 and gas1 ahc1 at 37°. A more severe effect is observed in which gas1 rtg2 is synthetic sick at 30° and dead at 37°. Serial dilutions of wild type (LPY5), ada2 (LPY6439), gas1 (LPY10129), gas1 ada2 (LPY19197), sgf73 (LPY19816), gas1 sgf73 (LPY19771), spt20 (LPY16914), gas1 spt20 (LPY19630), ahc1 (LPY17370), ahc2 (LPY18518), gas1 ahc1 (LPY19467), gas1 ahc2 (LPY19414), rtg2 (LPY18206), and gas1 rtg2 (LPY18372) were plated on SC at either 30° or 37°. Here, and in other figures, gas1 and gcn5 refer to the null alleles, whereas the gas1 catalytic mutant (Carotti et al. 2004) is denoted as gas1** and the gcn5 catalytic mutant (Wang et al. 1998) as gcn5*.
The substrate specificity of Gcn5 is largely defined by the macromolecular complexes in which it is found, including SAGA, ADA, and SLIK/SALSA (Grant et al. 1999; Lee et al. 2011; Figure 1B). To determine whether the synthetic lethality observed for gas1gcn5 was specifically mediated through one complex or functional module, double mutants were generated with gas1 to include genes encoding components of the SAGA modules and unique subunits for both SLIK/SALSA and ADA. These included genes encoding a central component of the HAT module (ADA2), key structural or functional components of other SAGA modules including DUB (SGF73) and SPT (SPT20), in addition to genes encoding unique components of SLIK/SALSA (RTG2) and ADA (AHC1 and AHC2). The TAF module subunits are essential and shared with TFIID (Grant et al. 1998) and thus were not analyzed.
Deletion of ADA2, which is required for Gcn5 association with all complexes and nucleosome acetylation (Candau et al. 1997; Balasubramanian et al. 2002), did not have a synthetic interaction with gas1; however, modest interactions were observed with distinct subunits of each Gcn5 complex (Figure 1C). As deletion of no single subunit defining modules or complexes recapitulated the synthetic lethality of gas1gcn5 at 30°, it is likely that Gcn5 catalytic activity itself is the critical factor in the interaction with Gas1, as is observed for the gcn5sas3 synthetic lethality (Howe et al. 2001).
The gas1 sas3 double mutant mutually suppresses select phenotypes
In addition to the synthetic lethality, Gcn5 and Sas3 have overlapping sites of genomic localization (Rosaleny et al. 2007) and share nucleosomal substrates (Howe et al. 2001). Based on the similarities between Gcn5 and Sas3, we chose to analyze the gas1sas3 double mutant to determine if the synthetic lethality observed with gas1gcn5 was gene specific.
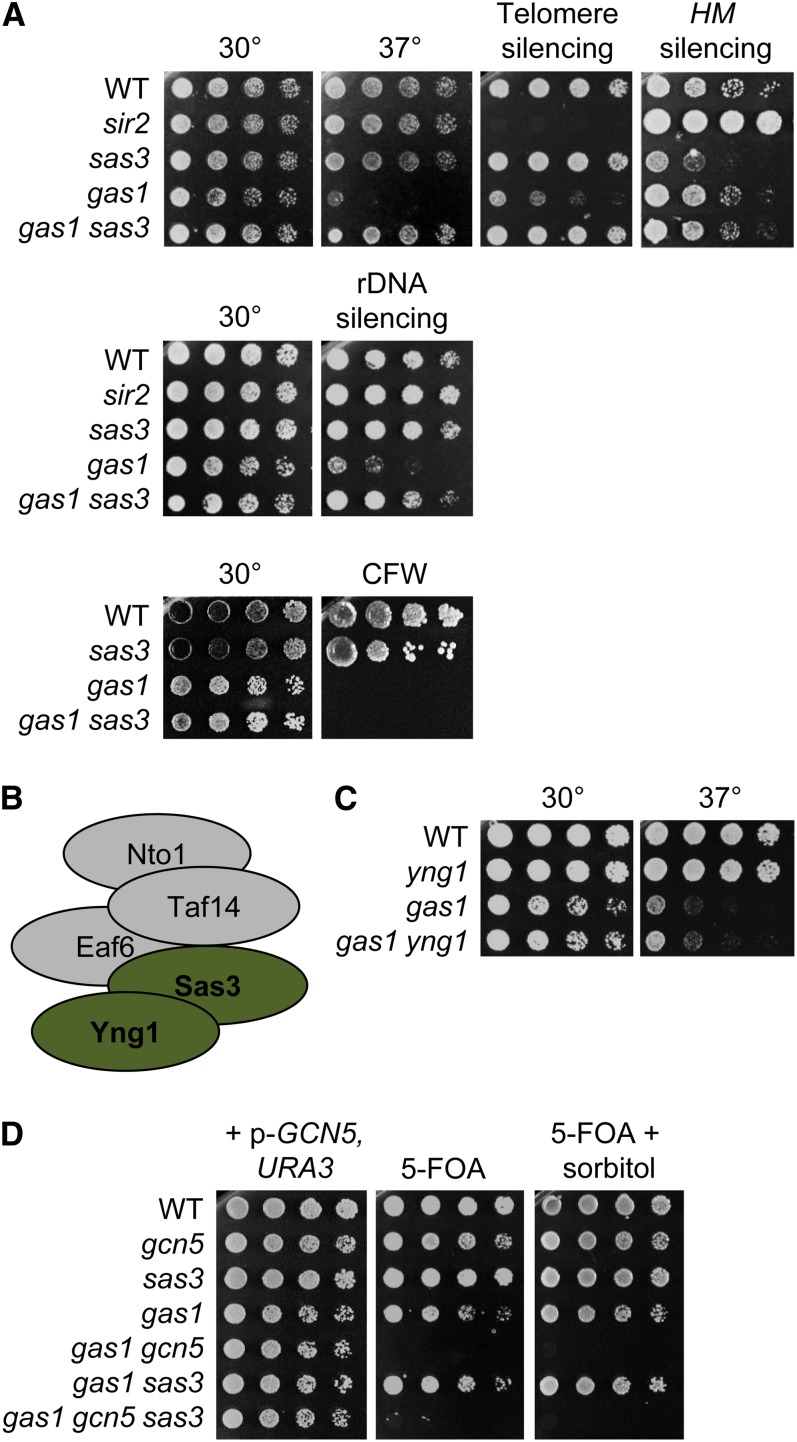
In sharp contrast to gas1gcn5, not only was gas1sas3 viable but the double mutant also displayed mutual suppression of select phenotypes (Figure 2A). Deletion of SAS3 suppressed phenotypes of gas1, including temperature sensitivity and telomeric and rDNA silencing defects. In turn, deletion of GAS1 restored normal levels of cryptic mating-type silencing in sas3. Deletion of SAS3 did not suppress the sensitivity of gas1 to CFW. This suggests that, like the gas1gcn5 mutant, the interaction between GAS1 and SAS3 is separable from cell wall functions of Gas1.
Figure 2.
Mutual suppression of phenotypes in the gas1 sas3 double mutant. (A) Deletion of SAS3 rescues gas1 temperature sensitivity and silencing defects at the telomere and rDNA array but not CFW sensitivity. In turn, deletion of GAS1 restores HM silencing in sas3 to wild-type levels. The sir2 mutant is included as a positive control for disruption of silencing. Top panel: Serial dilutions of wild type (LPY4924), sir2 (LPY5035), sas3 (LPY19731), gas1 (LPY19773), and gas1 sas3 (LPY16444) were plated on SC at 30° and 37°, SC with 5-FOA (TELVR::URA3) or SC −Trp (hml::TRP1). Middle panel: Serial dilutions of wild type (LPY2444), sir2 (LPY2447), sas3 (LPY17686), gas1 (LPY10074), and gas1 sas3 (LPY17685) were plated on SC or SC −Ura (RDN::Ty-1-mURA3) at 30°. Bottom panel: wild type (LPY5), sas3 (LPY8256), gas1 (LPY10129), and gas1 sas3 (LPY17520) were plated on either SC or SC with 10 μg/ml CFW. (B) NuA3 complex with subunits analyzed herein shaded green (adapted from Lafon et al. 2007). (C) Deletion of YNG1 does not have synthetic interactions with gas1. Serial dilutions of wild type (LPY6285), yng1 (LPY5526), gas1 (LPY9820), and gas1 yng1 (LPY16997) were plated on SC at either 30° or 37°. (D) Analysis of GAS1, GCN5, and SAS3 reveals distinct and opposing outcomes for synthetic interactions. Serial dilutions of wild type (LPY5), gcn5 (LPY8242), sas3 (LPY16039), gas1 (LPY10129), gas1 gcn5 + p-GCN5, URA3 (LPY16736), gas1 sas3 (LPY19823), and gas1 gcn5 sas3 + p-GCN5, URA3 (LPY19101) were plated on SC or SC with 5-FOA, to select against p-GCN5, URA3, at 30° with and without 1 M sorbitol.
Sas3 is targeted to specific chromatin regions by the NuA3 complex (Howe et al. 2002; Figure 2B), which includes the subunit Yng1, a PHD-finger protein that recognizes methylated H3K4 (Martin et al. 2006). To determine whether the NuA3 complex plays a role in suppression of gas1 phenotypes, we generated the double mutant gas1yng1. This mutant did not display synthetic interactions and phenocopied gas1 (Figure 2C). Thus the interaction observed between GAS1 and SAS3 depends on Sas3 activity but is independent of specific substrate targeting properties of NuA3.
Based on the mutual suppression observed in the gas1sas3 double mutant, we next tested whether deletion of SAS3 suppressed the gas1gcn5 synthetic lethality. The triple mutant gas1gcn5sas3 was not viable (Figure 2D). These results suggest that the interactions between GAS1 and GCN5 or SAS3 are of distinct and opposite outcomes.
Due to the strength of the genetic interactions with H3 KATs, we analyzed H3 acetylation (H3Ac) levels under suppressing conditions. As previously reported, deletion of GAS1 did not alter levels of H3K9Ac, H3K14Ac at 30° (Koch and Pillus 2009), which are targets of both Gcn5 and Sas3 (reviewed in Lafon et al. 2007). At 37°, a condition under which deletion of SAS3 suppresses gas1 temperature sensitivity, neither the gas1 strain nor gas1sas3 had altered global levels of H3K9Ac, H3K14Ac (Figure S2). This suggests that the suppression phenotypes of gas1sas3 are not mediated through changes in global H3K9Ac, H3K14Ac levels, which are largely intact in sas3 strains due to Gcn5.
Deletion of GAS1 leads to broad DNA damage sensitivity with specific suppression in the absence of SAS3
Several studies have demonstrated a role for Gcn5-based acetylation of histone and nonhistone substrates in the DDR (Choy and Kron 2002; Qin and Parthun 2002; Tamburini and Tyler 2005; Liang et al. 2007; Burgess et al. 2010; Wang et al. 2012). GAS1, SAS3, and GCN5 also all have numerous genetic and physical interactions with key components of the DDR, as defined from previous genome-wide screens (www.thebiogrid.org). Based on these connections, we asked whether the chromatin functions of GAS1 may also influence DDR.
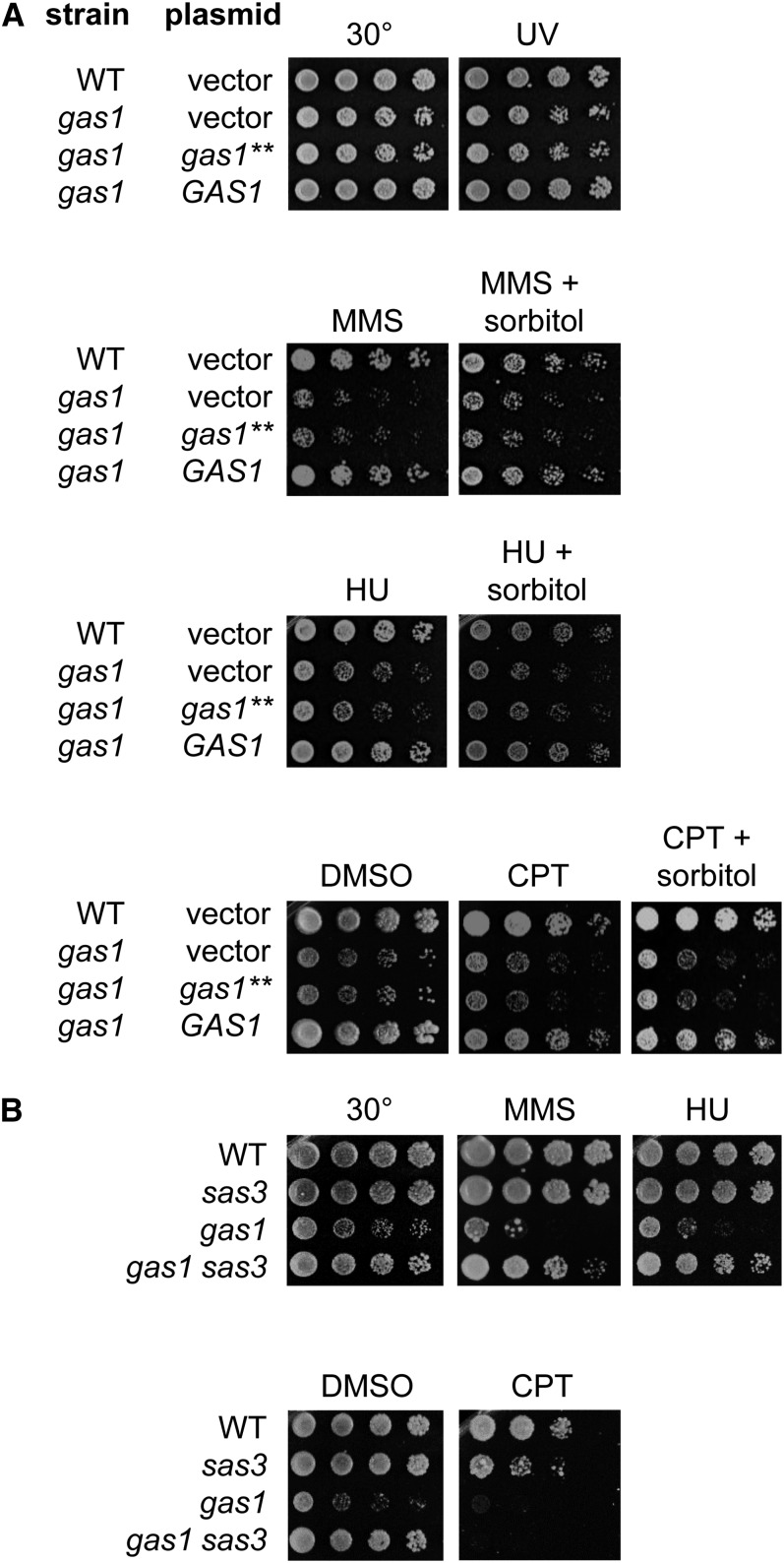
We evaluated the sensitivity of gas1 to a spectrum of DNA damaging agents including MMS, HU, CPT, and UV irradiation, which generates bulky DNA adducts (Sertic et al. 2012). Deletion of GAS1 led to sensitivity to all chemical agents tested, but not to UV. The genotoxin sensitivity was due to loss of the β-1,3-glucanosyltransferase activity of Gas1 and was not rescued by sorbitol (Figure 3A). DNA damage sensitivity was not shared with other members of the GAS family, nor other components of the cell wall machinery tested (Figure S3), demonstrating that the sensitivity was not a general phenotype of mutants with cell wall defects.
Figure 3.
Loss of GAS1 leads to broad DNA damage sensitivity with phenotype-specific suppression by deletion of SAS3. (A) gas1 mutants are sensitive to MMS, HU, and CPT but not exposure to UV. Sensitivity is due to loss of Gas1 catalytic activity and separable from cell wall function as demonstrated by failure of sorbitol to rescue these phenotypes. Serial dilutions of wild type (LPY18050), gas1 (LPY12247), gas1 + p-gas1-E161Q, E262Q (gas1**; LPY12251), and gas1 + p-GAS1 (LPY12326) were plated on selective media with 0.015% MMS, 0.2 M HU, or 20 μg/ml CPT in DMSO with or without 1 M sorbitol or on SC buffered with phosphate and supplemented with DMSO as a control. UV exposure was 60 J/m2. (B) Deletion of SAS3 specifically suppressed the MMS and HU sensitivity of gas1, but not CPT sensitivity. Serial dilutions of wild type (LPY5), sas3 (LPY8256), gas1 (LPY10129), and gas1 sas3 (LPY17520) were plated on SC plates using the same concentration of genotoxins and plate conditions as in A.
As deletion of SAS3 suppressed specific phenotypes of gas1, we analyzed the gas1sas3 double mutant upon DNA damage. Deletion of SAS3 suppressed both the MMS and HU sensitivity of gas1 but did not rescue the CPT sensitivity (Figure 3B). These results indicated that, whereas Gas1 has a broad role in the DDR, Sas3 has a more specific, and antagonistic, function.
Based on the DNA damage phenotypes, we performed genetic analysis of nucleosomal targets of Sas3 that have been implicated in DDR. The residues, H3K14 and H3K23, have increased sensitivity to DNA damaging agents when mutated to arginine (Qin and Parthun 2002; Tamburini and Tyler 2005). Lysine-to-arginine mutations both block acetylation and maintain a positive charge, thus mimicking the nonacetylated form. Conversely, mutation of lysine to alanine, which neutralizes lysine’s positive charge, leads to disruption of DNA-nucleosome contacts, as occurs with acetylation.
The H3K23A mutant suppressed all gas1 phenotypes, including temperature- and genotoxin sensitivity, whereas the H3K14A, H3K23A double mutant suppressed temperature sensitivity alone (Figure S4A). Conversely, the H3K23R mutant had no obvious phenotype compared to the single gas1 mutant and the double H3K14R, H3K23R was synthetically sick at elevated temperature and with DNA damage (Figure S4B). The H3K14A mutant was also synthetically sick with gas1 (Figure S4A) as were wild type and sas3 (Figure S4C). The increased sensitivity of the H3K14R, H3K23R mutant is consistent with reports that this double mutant has increased sensitivity to genotoxic stress (Tamburini and Tyler 2005) and was also observed in both the wild-type and sas3 histone mutant background (Figure S4D).
These findings indicate that changes in the acetylation status of Sas3 histone substrates can influence gas1 phenotypes. However, analysis of whether this is mediated by Sas3 is complicated in the histone mutant background. Here, gas1 growth is improved and suppression by deletion of SAS3 is less apparent (compare growth in Figure 2A and Figure 3B to Figure S4, A and B). It is possible that HHT1-HHF1 may modulate suppression of gas1 phenotypes, as this histone locus is deleted in the histone mutant background. Indeed, we found that adding a centromeric plasmid containing wild-type HHT1-HHF1 restored suppression of gas1 phenotypes by deletion of SAS3 (Figure S5A), although global histone levels remained comparable to the histone mutant strain (Figure S5B).
Deletion of SAS3 selectively restores DNA damage cell cycle arrest control in gas1
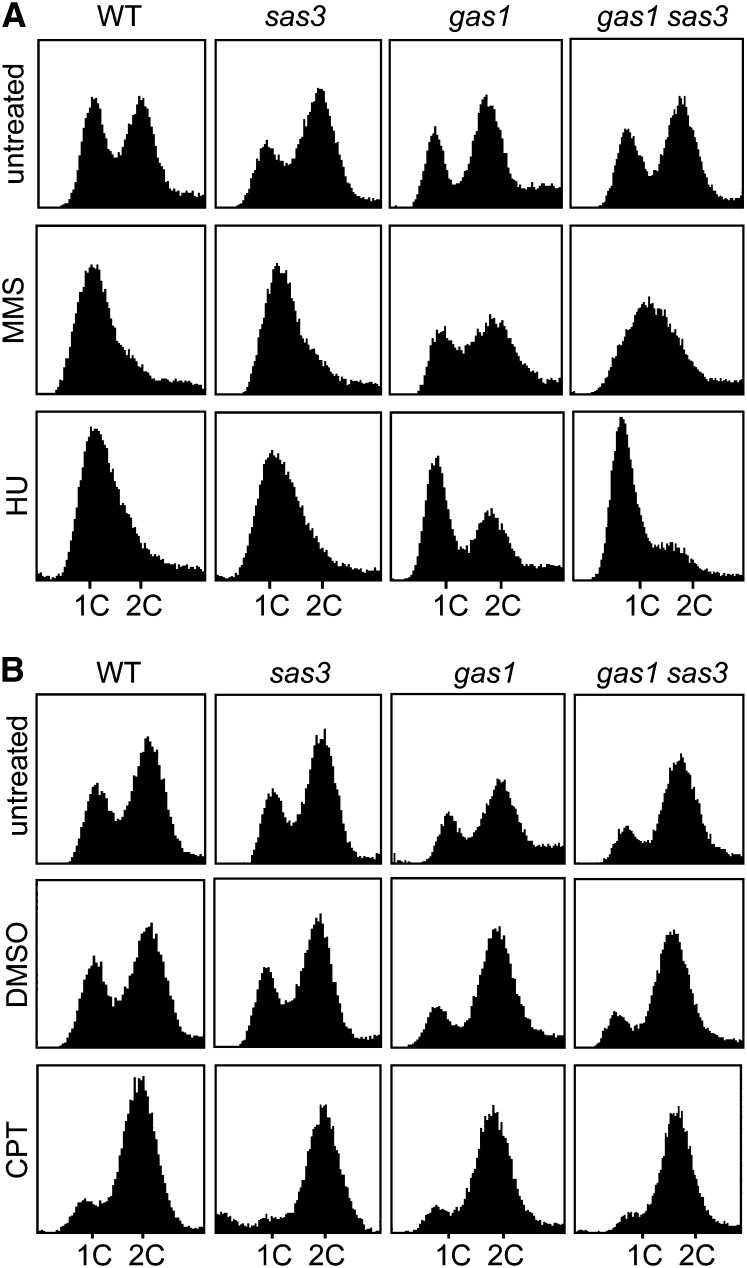
Genotoxin exposure triggers activation of cell cycle checkpoints via a kinase cascade that allow cells time to repair damaged DNA (reviewed in Sirbu and Cortez 2013). To determine how the DNA damage sensitivity of gas1 and its suppression by deletion of SAS3 may be linked to events in the DDR pathway, we analyzed gas1, sas3, and gas1sas3 cell cycle profiles by flow cytometry. Whereas the wild-type and sas3 strains had the expected cell cycle delay blocking replication following MMS and HU treatment, the gas1 strain did not have a delayed cycle, with cells remaining distributed throughout the cell cycle. The genotoxin-associated delay was restored with deletion of SAS3, although to a lesser extent with MMS treatment (Figure 4A). Conversely, upon treatment with CPT, the gas1 strain displayed a clear cell cycle arrest at G2/M similar to wild type, and this response was not altered in the gas1sas3 double mutant (Figure 4B). Thus, whereas there are distinct functions for Gas1 under a spectrum of DNA damage conditions, Sas3 may act specifically to antagonize the DNA replication checkpoint (DRC), as both MMS and HU trigger the DRC, but CPT does not (Redon et al. 2003).
Figure 4.
Deletion of SAS3 rescues gas1 defects in cell cycle arrest. (A) Treatment of gas1 with HU fails to trigger the cell cycle delay observed in wild type, whereas the cell cycle delay following treatment with MMS is severely impaired in gas1. Cycle delay is significantly restored in the double mutant gas1 sas3. (B) CPT treatment triggers cell cycle arrest in all strains tested. Strains and genotoxin concentrations are as in Figure 3A.
Cell cycle defects in gas1 correspond to loss of Rad53 phosphorylation and are restored by deletion of SAS3
Analysis of cell cycle profiles following DNA damage suggested that the rescue of gas1 by SAS3 deletion may specifically occur by restoring activation of the cell cycle checkpoint. One of the initial events following DNA damage in yeast is the phosphorylation of histone H2A at serine 129, which is indicative of sensing of DNA damage (reviewed in Rossetto et al. 2010). Downstream of H2AS129 phosphorylation, the effector kinase Rad53 is hyperphosphorylated, which is largely responsible for triggering cell cycle delay or arrest (Branzei and Foiani 2006; Sirbu and Cortez 2013).
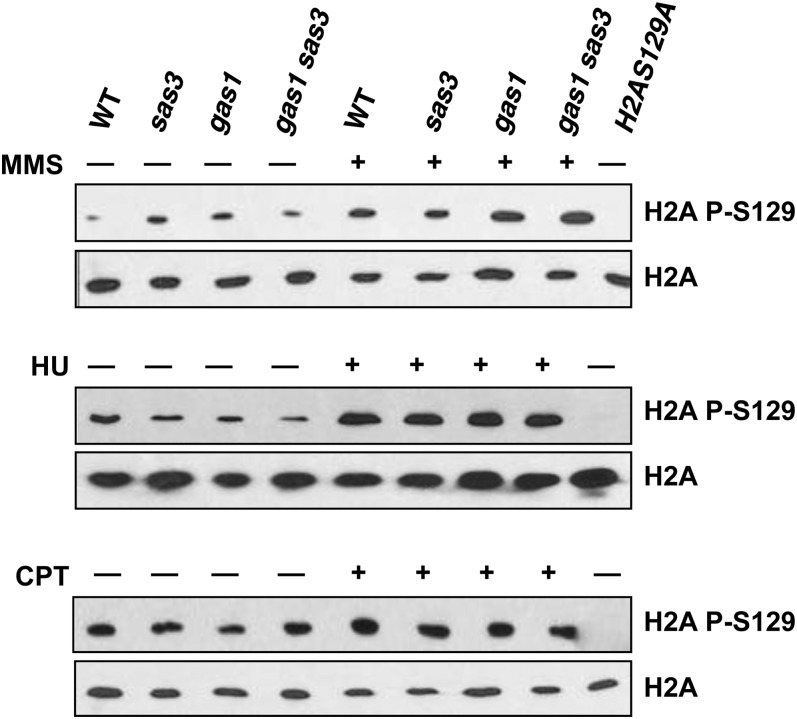
To determine whether gas1 is defective in sensing DNA damage we analyzed H2A phosphorylation in gas1 and gas1sas3 by immunoblotting. In all mutants, H2AS129 phosphorylation levels are comparable to WT levels following exposure to MMS, HU, and CPT (Figure 5), consistent with accurate sensing of damage.
Figure 5.
H2AS129 is phosphorylated following genotoxin exposure in all strains. Levels of H2AS129 phosphorylation following exposure to MMS (top), HU (middle), and CPT (bottom) are comparable to wild type in all strains analyzed. Strains and genotoxin concentrations are as in Figure 3A.
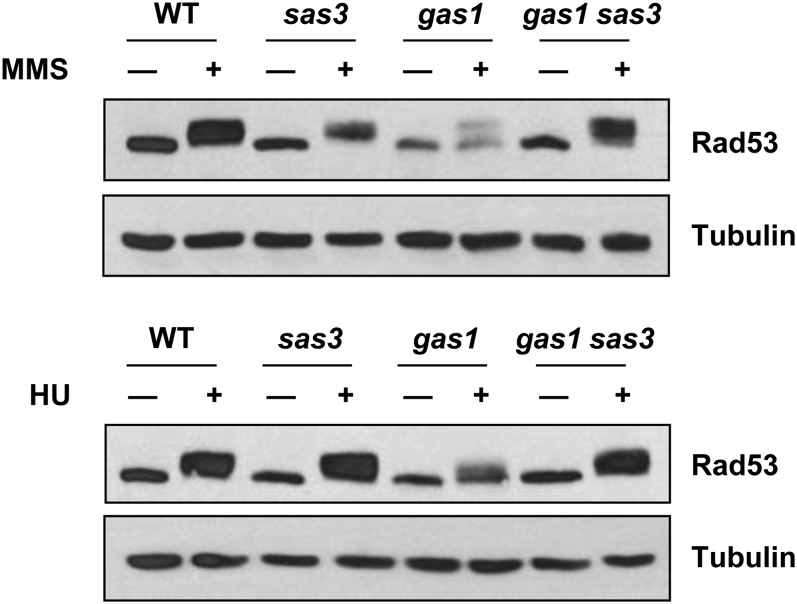
To monitor subsequent activation of the downstream effectors, we evaluated Rad53 phosphorylation. In the gas1 strain, Rad53 phosphorylation was severely impaired upon treatment with MMS and HU. However, moderate phosphorylation of Rad53 was evident following MMS exposure (Figure 6), consistent with the partial activation of the cell cycle checkpoint observed in Figure 4A. The reduced level of Rad53 phosphorylation was due to loss of Gas1 activity, as the catalytically inactive gas1-E161Q, E262Q mutant was also defective for Rad53 phosphorylation (Figure S6). As with the rescue of gas1 MMS and HU sensitivity by deletion of SAS3, in the double mutant, Rad53 phosphorylation was restored to near wild-type levels (Figure 6). Rad53 phosphorylation following CPT treatment is negligible (Figure S7), consistent with previous reports that CPT only minimally triggers Rad53 phosphorylation (Redon et al. 2003). Together, these data demonstrate that sas3 suppression of gas1 MMS and HU sensitivity is linked to reactivation of the cell cycle delay via restoration of Rad53 phosphorylation.
Figure 6.
Rad53 phosphorylation is significantly reduced in gas1 and restored in gas1 sas3 following exposure to MMS (top) and HU (bottom). Note that overall levels of Rad53 are diminished in gas1. Strains and genotoxin concentrations are as in Figure 3A.
Discussion
Our findings demonstrate that GAS1 has striking yet distinct genetic interactions with genes encoding the lysine acetyltransferases Gcn5 and Sas3, which themselves are synthetically lethal and have overlapping nucleosomal substrates (Howe et al. 2001) and genome-wide localization patterns (Rosaleny et al. 2007). Whereas the gas1gcn5 double mutant is dead, there is mutual suppression of specific phenotypes in the gas1sas3 strain. The suppression phenotypes include both silencing defects and specific relief of the newly identified gas1 sensitivity to genotoxins. The strong genetic interactions with the acetyltransferases and the DNA damage sensitivity of the gas1 mutant demonstrate that Gas1 plays an important role in chromatin dynamics, which is separable from its cell wall function. Further, whereas Gcn5 and Sas3 have often been considered to be largely functionally overlapping, our results distinguish the biological roles of Sas3 and Gcn5 in the important process of DNA repair.
Gas1 and Sas3 counterbalance silencing at all three silenced regions
Previous research indicates that Gas1 and Sas3 contribute to transcriptional silencing at distinct loci. Whereas loss of SAS3 leads to an increase in silencing at the HM loci (Reifsnyder et al. 1996), gas1 mutants have impaired silencing at telomeric loci and improved silencing within rDNA (Koch and Pillus 2009). We demonstrate that deletion of both enzymes leads to restoration of silencing to wild-type levels at all loci analyzed (Figure 2A). Locus-specific silencing relies on a balance of silencing proteins and other chromatin factors, some of which are limiting (Smith et al. 1998; Benbow and Dubois 2008). Altering the distribution of these factors can lead to changes in the strength of silencing between loci (Lustig et al. 1996). As silencing is both strengthened and/or disrupted at specific loci in the mutants under study, one potential explanation for the mutual suppression observed in the gas1sas3 strain is that localization of limiting silencing factors is normalized. In this case, Sas3 and Gas1 counteract the influence of each other, such that in the absence of both enzymes balance is restored. This idea is in agreement with our previous observation of a physical interaction between Gas1 and the deacetyltransferase Sir2 (Koch and Pillus 2009), a limiting factor essential for establishment and maintenance of silencing (Rusche et al. 2003).
Analysis of DNA damage sensitivity in gas1 cells reveals that Sas3 antagonizes the DNA replication checkpoint
In addition to previously defined silencing defects (Koch and Pillus 2009) we found that deletion of GAS1 led to DNA damage sensitivity. Strains lacking Gas1, or with defective catalytic activity, were sensitive to the genotoxins MMS, HU, and CPT but not UV (Figure 3A). Thus, although Gas1 plays a broad role in DNA damage, there are distinctions for particular types of damage or repair pathways.
Whereas H2AS129 phosphorylation, indicating sensing and initial DDR activation, was intact in all strains analyzed, the levels of Rad53 phosphorylation were significantly reduced in gas1 and restored by deletion of SAS3. Impairment of the MMS or HU DNA damage-associated cell cycle delay and Rad53 phosphorylation levels in gas1 strains (Figure 4 and Figure 5) indicates that Gas1 may function in triggering hyperphosphorylation of Rad53 and the subsequent cell cycle checkpoint. Although GAS1 mutants failed to arrest in response to MMS and HU they did undergo CPT-induced G2/M arrest. These observations strengthen the idea that Gas1 is broadly relevant to DDR, yet its contributions appear to depend on the type of lesion.
Distinct mechanistic roles for Gas1 in DNA damage are further supported by the suppression seen with deletion of SAS3, which rescued MMS and HU sensitivity but not CPT sensitivity (Figure 3B). MMS and HU elicit a largely overlapping transcriptional response, which is primarily dependent on Rad53 phosphorylation of substrates. By contrast, CPT leads to induction of a markedly different set of genes (Travesa et al. 2012; Travesa and Wittenberg 2012). Both MMS and HU trigger the replication checkpoint via fork arrest or by slowing fork progression by reducing dNTP pools, respectively (reviewed in Branzei and Foiani 2007). Conversely, CPT is considered to be “checkpoint blind” as exposure leads to only modest induction of Rad53 phosphorylation and does not trigger the replication checkpoint (Redon et al. 2003; Tourriere and Pasero 2007).
The primary checkpoints activated by DNA damage include delay of the G1/S transition and block of the G2/M transition and the S-phase checkpoints. Although there are overlaps in the proteins mediating these checkpoints there are also distinctions that depend on the phase of the cell cycle, type of DNA damage, and repair pathway choice (reviewed in Warmerdam and Kanaar 2010; Symington and Gautier 2011; Gobbini et al. 2013). Cell cycle checkpoints and DNA damage repair require both positive and negative regulation to ensure proper spatiotemporal dynamics and maintenance of genomic integrity (reviewed in Panier and Durocher 2013). Thus, Sas3 may be particularly relevant in antagonizing activation of the replication checkpoint pathway, specific to the repression of the cell cycle delay prior to DNA replication mediated by Rad53 phosphorylation.
DNA damage occurs within the context of chromatin, yet the functions of the chromatin-modifying enzymes and histone post-translational modifications in the DNA damage response remain incompletely defined (reviewed in Papamichos-Chronakis and Peterson 2013). Multiple chromatin factors, including key silencing enzymes, are known to dynamically redistribute from telomeres to sites of double-strand breaks (Martin et al. 1999; Mills et al. 1999). Further, silencing at the HM loci was recently found to involve key factors of the homologous recombination pathway (Kirkland and Kamakaka 2013). If Sas3 and Gas1 act to balance chromatin-modifying enzymes, as proposed above, the suppression of gas1 genotoxin sensitivity could relate to redistribution of the same or similar factors that alter silencing phenotypes in the double mutant. Indeed, localization of chromatin to the nuclear periphery is linked to both maintenance of silencing (reviewed in Zimmer and Fabre 2011; Taddei and Gasser 2012) and regulation of the DNA damage response (reviewed in Bermejo et al. 2012). Thus the pool of Gas1 at the nuclear periphery may be optimally localized at the interface of both silencing and DDR.
We found that the HHT1-HHF1 locus may, at least in part, mediate the suppression observed by deletion of SAS3 in the gas1 background (Figure S5A). The role of histones in the DNA damage response is complex, such that even modest imbalances in histone levels can alter DNA damage sensitivity (see for example Gunjan and Verreault 2003; Sanders et al. 2004; Du et al. 2006). Whereas the duplicate histone loci are believed to be largely redundant, there are distinctions both at the level of dosage (Cross and Smith 1988; Libuda and Winston 2010) and in regulation of their expression (Zunder and Rine 2012). Our findings here and previous work of others (Sanders et al. 2004; Du et al. 2006) suggest that the HHT1-HHF1 locus may indeed have a unique function in DNA damage. Histones are highly regulated at multiple levels including expression, localization, PTM, and degradation (reviewed in Kurat et al. 2013). Whether the restoration of suppression by HHT1-HHF1 is relevant to precise histone levels or some other aspect of this locus’s biology has yet to be determined.
Distinct functions for Gcn5 and Sas3
Although the function of Gcn5 in both transcription and DNA damage has been analyzed extensively (Robert et al. 2004; Burgess et al. 2010; Lee et al. 2010), less is known about functions of Sas3. Several lines of research indicate that Sas3 may have a role in cell cycle regulation and DDR. Using a sas3 allele with diminished function, it was found that both Gcn5 and Sas3 play a role in cell cycle regulation, with decreased Sas3 activity coupled with deletion of GCN5 leading to G2/M arrest (Howe et al. 2001). Loss of SAS3 leads to a decrease of H3K14Ac, primarily at genes involved in cell cycle regulation and cell division (Rosaleny et al. 2007). Sas3 physically interacts with Chk1 (Liu et al. 2000), a Mec1 DNA damage pathway effector kinase and Dpb4, which regulates DNA replication and telomere silencing (Tackett et al. 2005). As noted above, Sas3 physically associates with the FACT remodeling complex via interaction with the N terminus of Spt16 (John et al. 2000), which is necessary for the DNA replication stress response (O’Donnell et al. 2004). Several chromatin-remodeling complexes have been linked to the synthetic lethality observed between SAS3 and GCN5, including RSC (Choi et al. 2008) and ISWI (Lafon et al. 2012). Chromatin remodeling complexes have well-established roles in the DDR, with Gcn5-based acetylation of Rsc4 identified as a key factor in replication stress resistance (Charles et al. 2011).
Although Sas3 has often been considered to be largely functionally redundant with Gcn5, previous research indicated that Sas3 can disrupt Gcn5-based acetylation of H3K14 at distinct genomic loci (Rosaleny et al. 2007). They may also compete during other dynamic processes. Whereas Gcn5 has primarily been implicated as a broad positive regulator of the DNA damage response, our finding that Sas3 may function antagonistically in DDR further demonstrates a unique, and opposing, function for Sas3. This possibility is consistent with the strong yet opposing genetic interactions observed between GAS1 and GCN5 and SAS3. Future studies should reveal how the protein modifications controlled by these three enzymes are balanced to respond to distinct forms of cellular and genotoxic stresses.
Supplementary Material
Acknowledgments
We thank members of the Pillus lab, Douglass Forbes, Melissa Koch, and Christie Chang for helpful discussion and critical reading of the manuscript. We thank J. Heierhorst and S. Elledge for anti-Rad53 reagents. M.E. is supported by the Graduate Assistance in Areas of National Need Award P200A100159 and the Eugene-Cota Robles fellowship. This project was initiated with support from National Institutes of Health GM054778, GM09177, and GM033279.
Footnotes
Communicating editor: M. Hampsey
Literature Cited
- Amberg D., Burke D., Strathern J., 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Balasubramanian R., Pray-Grant M. G., Selleck W., Grant P. A., Tan S., 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277: 7989–7995. [DOI] [PubMed] [Google Scholar]
- Benbow S. Z., Dubois M. L., 2008. The dosage of chromatin proteins affects transcriptional silencing and DNA repair in Saccharomyces cerevisiae. FEBS Lett. 582: 497–502. [DOI] [PubMed] [Google Scholar]
- Bermejo R., Kumar A., Foiani M., 2012. Preserving the genome by regulating chromatin association with the nuclear envelope. Trends Cell Biol. 22: 465–473. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Fridovich-Keil J. L., Pillus L., Mulligan R. C., Solomon F., 1986. A chicken-yeast chimeric β-tubulin protein incorporated into mouse microtubules in vivo. Cell 44: 461–468. [DOI] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2006. The Rad53 signal transduction pathway: replication fork stabilization, DNA repair, and adaptation. Exp. Cell Res. 312: 2654–2659. [DOI] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2007. Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amst.) 6: 994–1003. [DOI] [PubMed] [Google Scholar]
- Burgess R. J., Zhou H., Han J., Zhang Z., 2010. Gcn5 in replication-coupled nucleosome assembly. Mol. Cell 37: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos E. I., Reinberg D., 2009. Histones: annotating chromatin. Annu. Rev. Genet. 43: 559–599. [DOI] [PubMed] [Google Scholar]
- Candau R., Zhou J. X., Allis C. D., Berger S. L., 1997. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 16: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotti C., Ragni E., Palomares O., Fontaine T., Tedeschi G., et al. , 2004. Characterization of recombinant forms of the yeast Gas1 protein and identification of residues essential for glucanosyltransferase activity and folding. Eur. J. Biochem. 271: 3635–3645. [DOI] [PubMed] [Google Scholar]
- Charles G. M., Chen C., Shih S. C., Collins S. R., Beltrao P., et al. , 2011. Site-specific acetylation mark on an essential chromatin-remodeling complex promotes resistance to replication stress. Proc. Natl. Acad. Sci. USA 108: 10620–10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. K., Grimes D. E., Rowe K. M., Howe L., 2008. Acetylation of Rsc4p by Gcn5p is essential in the absence of histone H3 acetylation. Mol. Cell. Biol. 28: 6967–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy J. S., Kron S. J., 2002. NuA4 subunit Yng2 function in intra-S-phase DNA damage response. Mol. Cell. Biol. 22: 8215–8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. S., Lowell J. E., Jacobson S. J., Pillus L., 1999. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol. Cell. Biol. 19: 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E. D., et al. , 2010. The genetic landscape of the cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Smith M. M., 1988. Comparison of the structure and cell cycle expression of mRNAs encoding two histone H3–H4 loci in Saccharomyces cerevisiae. Mol. Cell. Biol. 8: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L.-L., Nakamura T. M., Russell P., 2006. Histone modification-dependent and independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev. 20: 1583–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini E., Cesena D., Galbiati A., Lockhart A., Longhese M. P., 2013. Interplays between ATM/Tel1 and ATR/Mec1 in sensing and signaling DNA double-strand breaks. DNA Repair (Amst.) 12: 791–799. [DOI] [PubMed] [Google Scholar]
- Grant P. A., Duggan L., Côté J., Roberts S. M., Brownell J. E., et al. , 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- Grant P. A., Schieltz D., Pray-Grant M. G., Steger D. J., Reese J. C., et al. , 1998. A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94: 45–53. [DOI] [PubMed] [Google Scholar]
- Grant P. A., Eberharter A., John S., Cook R. G., Turner B. M., et al. , 1999. Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 274: 5895–5900. [DOI] [PubMed] [Google Scholar]
- Gunjan A., Verreault A., 2003. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 115: 537–549. [DOI] [PubMed] [Google Scholar]
- Howe L., Auston D., Grant P., John S., Cook R. G., et al. , 2001. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 15: 3144–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L., Kusch T., Muster N., Chaterji R., Yates J. R., 3rd, et al. , 2002. Yng1p modulates the activity of Sas3p as a component of the yeast NuA3 histone acetyltransferase complex. Mol. Cell. Biol. 22: 5047–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., et al. , 2003. Global analysis of protein localization in budding yeast. Nature 425: 686–691. [DOI] [PubMed] [Google Scholar]
- John S., Howe L., Tafrov S. T., Grant P. A., Sternglanz R., et al. , 2000. The something about silencing protein, Sas3, is the catalytic subunit of NuA3, a yTAF(II)30-containing HAT complex that interacts with the Spt16 subunit of the yeast CP (Cdc68/Pob3)-FACT complex. Genes Dev. 14: 1196–1208. [PMC free article] [PubMed] [Google Scholar]
- Kirkland J. G., Kamakaka R. T., 2013. Long-range heterochromatin association is mediated by silencing and double-strand DNA break repair proteins. J. Cell Biol. 201: 809–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. R., Pillus L., 2009. The glucanosyltransferase Gas1 functions in transcriptional silencing. Proc. Natl. Acad. Sci. USA 106: 11224–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y., 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98: 285–294. [DOI] [PubMed] [Google Scholar]
- Koutelou E., Hirsch C. L., Dent S. Y., 2010. Multiple faces of the SAGA complex. Curr. Opin. Cell Biol. 22: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., 2007. Chromatin modifications and their function. Cell 128: 693–705. [DOI] [PubMed] [Google Scholar]
- Kuo Y. M., Andrews A. J., 2013. Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS ONE 8: e54896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurat C. F., Recht J., Radovani E., Durbic T., Andrews B., et al. , 2013. Regulation of histone gene transcription in yeast. Cell. Mol. Life Sci. 71: 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon A., Chang C. S., Scott E. M., Jacobson S. J., Pillus L., 2007. MYST opportunities for growth control: yeast genes illuminate human cancer gene functions. Oncogene 26: 5373–5384. [DOI] [PubMed] [Google Scholar]
- Lafon A., Petty E., Pillus L., 2012. Functional antagonism between Sas3 and Gcn5 acetyltransferases and ISWI chromatin remodelers. PLoS Genet. 8: e1002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S., Davis C., Konopka J. B., Sternglanz R., 1997. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast 13: 1029–1042. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Park J. H., Kim S. J., Kwon S. J., Kwon J., 2010. A cooperative activation loop among SWI/SNF, gamma-H2AX and H3 acetylation for DNA double-strand break repair. EMBO J. 29: 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Sardiu M. E., Swanson S. K., Gilmore J. M., Torok M., et al. , 2011. Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes. Mol. Syst. Biol. 7: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Workman J., 2007. Histone acetyltransferase complexes: one size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 8: 284–295. [DOI] [PubMed] [Google Scholar]
- Levin D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Reinberg D., 2011. Chromatin higher-order structures and gene regulation. Curr. Opin. Genet. Dev. 21: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Qiu J., Ratnakumar K., Laurent B. C., 2007. RSC functions as an early double-strand-break sensor in the cell’s response to DNA damage. Curr. Biol. 17: 1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libuda D. E., Winston F., 2010. Alterations in DNA replication and histone levels promote histone gene amplificaition in Saccharomyces cerevisiae. Genetics 184: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Vidanes G., Lin Y. C., Mori S., Siede W., 2000. Characterization of a Saccharomyces cerevisiae homologue of Schizosaccharomyces pombe Chk1 involved in DNA-damage-induced M-phase arrest. Mol. Gen. Genet. 262: 1132–1146. [DOI] [PubMed] [Google Scholar]
- Lustig A. J., Liu C., Zhang C., Hanish J. P., 1996. Tethered Sir3p nucleates silencing at telomeres and internal loci in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2483–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. G., Baetz K., Shi X., Walter K. L., MacDonald V. E., et al. , 2006. The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol. Cell. Biol. 26: 7871–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G., Laroche T., Suka N., Grunstein M., Gasser S. M., 1999. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97: 621–633. [DOI] [PubMed] [Google Scholar]
- Mills K. D., Sinclair D. A., Guarente L., 1999. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97: 609–620. [DOI] [PubMed] [Google Scholar]
- Nitiss J., Wang J. C., 1988. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc. Natl. Acad. Sci. USA 85: 7501–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell A. F., Brewster N. K., Kurniawan J., Minard L. V., Johnston G. C., et al. , 2004. Domain organization of the yeast histone chaperone FACT: the conserved N-terminal domain of FACT subunit Spt16 mediates recovery from replication stress. Nucleic Acids Res. 32: 5894–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P., 2012. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192: 775–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panier S., Durocher D., 2013. Push back to respond better: regulatory inhibition of the DNA double-strand break response. Nat. Rev. Cancer 13: 661–672. [DOI] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Peterson C. L., 2013. Chromatin and the genome integrity network. Nat. Rev. Genet. 14: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Yongkiettrakul S., Tsai M. D., Heierhorst J., 2003. Diverse but overlapping functions of the two forkhead-associated (FHA) domains in Rad53 checkpoint kinase activation. J. Biol. Chem. 278: 30421–30424. [DOI] [PubMed] [Google Scholar]
- Pokholok D. K., Harbison C. T., Levine S., Cole M., Hannett N. M., et al. , 2005. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122: 517–527. [DOI] [PubMed] [Google Scholar]
- Popolo L., Vai M., 1999. The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426: 385–400. [DOI] [PubMed] [Google Scholar]
- Pray-Grant M. G., Schieltz D., McMahon S. J., Wood J. M., Kennedy E. L., et al. , 2002. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol. 22: 8774–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Parthun M. R., 2002. Histone H3 and the histone acetyltransferase Hat1p contribute to DNA double-strand break repair. Mol. Cell. Biol. 22: 8353–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni E., Fontaine T., Gissi C., Latge J. P., Popolo L., 2007. The Gas family of proteins of Saccharomyces cerevisiae: characterization and evolutionary analysis. Yeast 24: 297–308. [DOI] [PubMed] [Google Scholar]
- Redon C., Pilch D. R., Rogakou E. P., Orr A. H., Lowndes N. F., et al. , 2003. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep. 4: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder C., Lowell J., Clarke A., Pillus L., 1996. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nat. Genet. 14: 42–49. [DOI] [PubMed] [Google Scholar]
- Renauld H., Aparicio O. M., Zierath P. D., Billington B. L., Chhablani S. K., et al. , 1993. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 7: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Robert F., Pokholok D. K., Hannett N. M., Rinaldi N. J., Chandy M., et al. , 2004. Global position and recruitment of HATs and HDACs in the yeast genome. Mol. Cell 16: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C., Duran A., 1985. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J. Bacteriol. 163: 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosaleny L. E., Ruiz-Garcia A. B., Garcia-Martinez J., Perez-Ortin J. E., Tordera V., 2007. The Sas3p and Gcn5p histone acetyltransferases are recruited to similar genes. Genome Biol. 8: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto D., Truman A. W., Kron S. J., Côté J., 2010. Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin. Cancer Res. 16: 4543–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche L. N., Kirchmaier A. L., Rine J., 2003. The establishment, inheritance, and funciton of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481–516. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Portoso M., Mata J., Bahler J., Allshire R. C., et al. , 2004. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119: 603–614. [DOI] [PubMed] [Google Scholar]
- Sertic S., Pizzi S., Lazzaro F., Plevani P., Muzi-Falconi M., 2012. NER and DDR: classical music with new instruments. Cell Cycle 11: 668–674. [DOI] [PubMed] [Google Scholar]
- Sirbu B. M., Cortez D., 2013. DNA damage response: three levels of DNA repair regulation. Cold Spring Harb. Perspect. Biol. 5: a012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Boeke J. D., 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11: 241–254. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Brachmann C. B., Pillus L., Boeke J. D., 1998. Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics 149: 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter B., Tong A., Chang M., Yu L., Brown G. W., et al. , 2004. The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae. Genetics 167: 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Gautier J., 2011. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45: 247–271. [DOI] [PubMed] [Google Scholar]
- Tackett A. J., Dilworth D. J., Davey M. J., O’Donnell M., Aitchison J. D., et al. , 2005. Proteomic and genomic characterization of chromatin complexes at a boundary. J. Cell Biol. 169: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A., Gasser S. M., 2012. Structure and function in the budding yeast nucleus. Genetics 192: 107–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini B. A., Tyler J. K., 2005. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol. Cell. Biol. 25: 4903–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H., Pasero P., 2007. Maintenance of fork integrity at damaged DNA and natural pause sites. DNA Repair (Amst.) 6: 900–913. [DOI] [PubMed] [Google Scholar]
- Travesa A., Kuo D., de Bruin R. A., Kalashnikova T. I., Guaderrama M., et al. , 2012. DNA replication stress differentially regulates G1/S genes via Rad53-dependent inactivation of Nrm1. EMBO J. 31: 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travesa A., Wittenberg C., 2012. Turned on by genotoxic stress. Cell Cycle 11: 3145–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchini A., Ferrario L., Popolo L., 2000. Increase of external osmolarity reduces morphogenetic defects and accumulaiton of chitin in a gas1 mutant of Saccharomyces cerevisiae. J. Bacteriol. 182: 1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F., Gottschling D. E., 2002. Assays for gene silencing in yeast. Methods Enzymol. 350: 165–186. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu L., Berger S. L., 1998. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 12: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kallgren S. P., Reddy B. D., Kuntz K., Lopez-Maury L., et al. , 2012. Histone H3 lysine 14 acetylation is required for activation of a DNA damage checkpoint in fission yeast. J. Biol. Chem. 287: 4386–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmerdam D. O., Kanaar R., 2010. Dealing with DNA damage: relationships between checkpoint and repair pathways. Mutat. Res. 704: 2–11. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Fabre E., 2011. Principles of chromosomal organization: lessons from yeast. J. Cell Biol. 192: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunder R. M., Rine J., 2012. Direct interplay among histones, histone chaperones, and a chromatin boundary protein in the control of histone gene expression. Mol. Cell. Biol. 32: 4337–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.