Stomatal closure during water stress in the conifer Metasequoia glyptostroboides transitions from being entirely passive under moderate water stress to predominantly active, mediated by the level of foliar ABA, under more severe water stress.
Abstract
Motivated by studies suggesting that the stomata of ferns and lycophytes do not conform to the standard active abscisic acid (ABA) -mediated stomatal control model, we examined stomatal behavior in a conifer species (Metasequoia glyptostroboides) that is phylogenetically midway between the fern and angiosperm clades. Similar to ferns, daytime stomatal closure in response to moderate water stress seemed to be a passive hydraulic process in M. glyptostroboides immediately alleviated by rehydrating excised shoots. Only after prolonged exposure to more extreme water stress did active ABA-mediated stomatal closure become important, because foliar ABA production was triggered after leaf turgor loss. The influence of foliar ABA on stomatal conductance and stomatal aperture was highly predictable and additive with the passive hydraulic influence. M. glyptostroboides thus occupies a stomatal behavior type intermediate between the passively controlled ferns and the characteristic ABA-dependent stomatal closure described in angiosperm herbs. These results highlight the importance of considering phylogeny as a major determinant of stomatal behavior.
Stomata regulate parallel diffusive paths of water and carbon dioxide between leaves and the atmosphere, thus assuming a governing role over the processes of transpiration and photosynthesis. Guard cell movements that open and close stomata are increasingly characterized (similarly to animal cells) as being mediated by rapid changes in the polarization state of membranes (Schroeder et al., 2001; Hedrich, 2012; Hills et al., 2012). Despite this membrane-dominated view of stomatal function, the critical goal of modeling stomatal behavior to render predictions of transpiration and photosynthesis typically relies on a hydraulic framework built around the direct impact of leaf hydration on epidermal and guard cell turgor (Buckley, 2005; Damour et al., 2010). Although recent advances in modeling the ionic balance of guard cells (Chen et al., 2012; Hills et al., 2012) yield predictions of stomatal aperture, no macro-scale stomatal model has been able to predict stomatal conductance from the perspective of ion movements into and out of guard cells; however, the effects of key components, such as light, carbon dioxide, and abscisic acid (ABA), on membrane polarization have been studied in detail. Reconciling the dynamics of leaf-scale and canopy-scale transpiration with physical and chemical processes at the guard cell remains a major challenge.
Among the obstacles preventing the formulation of a large-scale transpiration model based on membrane ion transport is the fact that much of the characterization of guard cell membrane processes has been confined to a handful of small, ruderal, herbaceous angiosperms. Although species like Arabidopsis (Arabidopsis thaliana) provide the ideal molecular system for identifying guard cell signal transduction pathways, most of these model species are of little agricultural relevance and being herbaceous, poor physical analogs for the tree species that dominate terrestrial gas exchange. Hence, there is a need to understand whether the same principles governing stomatal control in angiosperm herbs, like Arabidopsis, equally apply to plants that dominate forests and agricultural production. Recent studies suggest that there are important differences in the ion-transport machinery among vascular plants, and despite the presence of potential guard cell signaling pathways throughout the plant kingdom (Dreyer et al., 2012; Brodribb and McAdam, 2013b; Chater et al., 2013), there is evidence of a systematic shift in the behavior of stomata among vascular plants (Doi et al., 2006; Brodribb and McAdam, 2011; McAdam and Brodribb, 2012a). In particular, the critical closing tendency of stomata during leaf water deficit seems to have evolved from a passive process mediated directly by water potential (passive hydraulic) to an active process controlled by the extrusion of anions from guard cells (active closure; Brodribb and McAdam, 2011). The stomata of ferns and lycophytes predictably respond to plant water deficit as passive hydraulic valves, closing rapidly on dehydration and opening on rehydration (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012a). Despite the stomata in these lineages only ever showing functionally passive responses to changes in leaf water status (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012a, 2013), some have challenged the concept of a passive origin of stomatal control in vascular plants by showing a conserved activity of key genes involved in active stomatal responses (Ruszala et al., 2011). In seed plants, the closure of stomata in response to water deficit is mediated by augmented levels of ABA, which leads to a depolarization of guard cell membranes triggering osmotic ion efflux and a loss of guard cell turgor (Mittelheuser and Van Steveninck, 1969; Thiel et al., 1992; Geiger et al., 2009, 2011; Bauer et al., 2013). In light of this variation in stomatal control, it seems that a key step to finding a general model for stomatal behavior would be to understand the interactions between active and passive processes in the stomatal movements of major lineages of plants.
Conifers contribute significantly to global transpiration and productivity and also seem to have a stomatal control system that is somewhat different from model angiosperm herbs. These distinctions include insensitivity to elevated carbon dioxide (Beadle et al., 1979; Morison and Jarvis, 1983; Brodribb et al., 2009); a lack of epidermal mechanical advantage, resulting in no Ivanov effect (the increase in transpiration from a leaf after excision or exposure to low humidity; Huber, 1923; Stålfelt, 1944; McAdam and Brodribb, 2012a), likely because of heavily lignified dorsal walls (the walls closest to the epidermal cells; Sack, 1987), and a very high length-to-width ratio of open stomatal pores (Copeland, 1902). Furthermore, recent research suggests that different conifer species depend more or less on ABA as an agent of stomatal closure during extended periods of water stress (Brodribb and McAdam, 2013a). The apparent lack of epidermal mechanical advantage in conifer stomata provides an unusual opportunity to examine the impacts of changing leaf water content and evaporation on stomatal conductance and guard cell turgor without the confusing Ivanov effect produced by changes in the ratio of epidermal and guard cell turgor pressure (Raschke, 1970). Manipulating the hydration status of the leaf, thus, allows quantification of the interacting influences of leaf water potential (Ψl) and ion transport on stomatal aperture (Brodribb and McAdam, 2013a).
Our aim in this study was to determine under what conditions passive (hydraulic) and active (ABA mediated) closures of stomata were important in a representative conifer species. We assumed that, in the absence of a mechanical interaction from the epidermis, it would be possible to characterize both dynamic and steady-state stomatal behavior based on intrinsic leaf properties of ABA sensitivity, hydraulic conductance, capacitance, and hydraulic vulnerability. We chose the conifer Metasequoia glyptostroboides (Cupressaceae) as our subject, because it has leaf characteristics within the range of deciduous angiosperm trees; also, it is one of the few conifer species where stomata are sufficiently visible (unoccluded by waxes) to observe stomatal responses in the isolated epidermis.
RESULTS AND DISCUSSION
Passive Hydraulic Regulation under Mild Stress
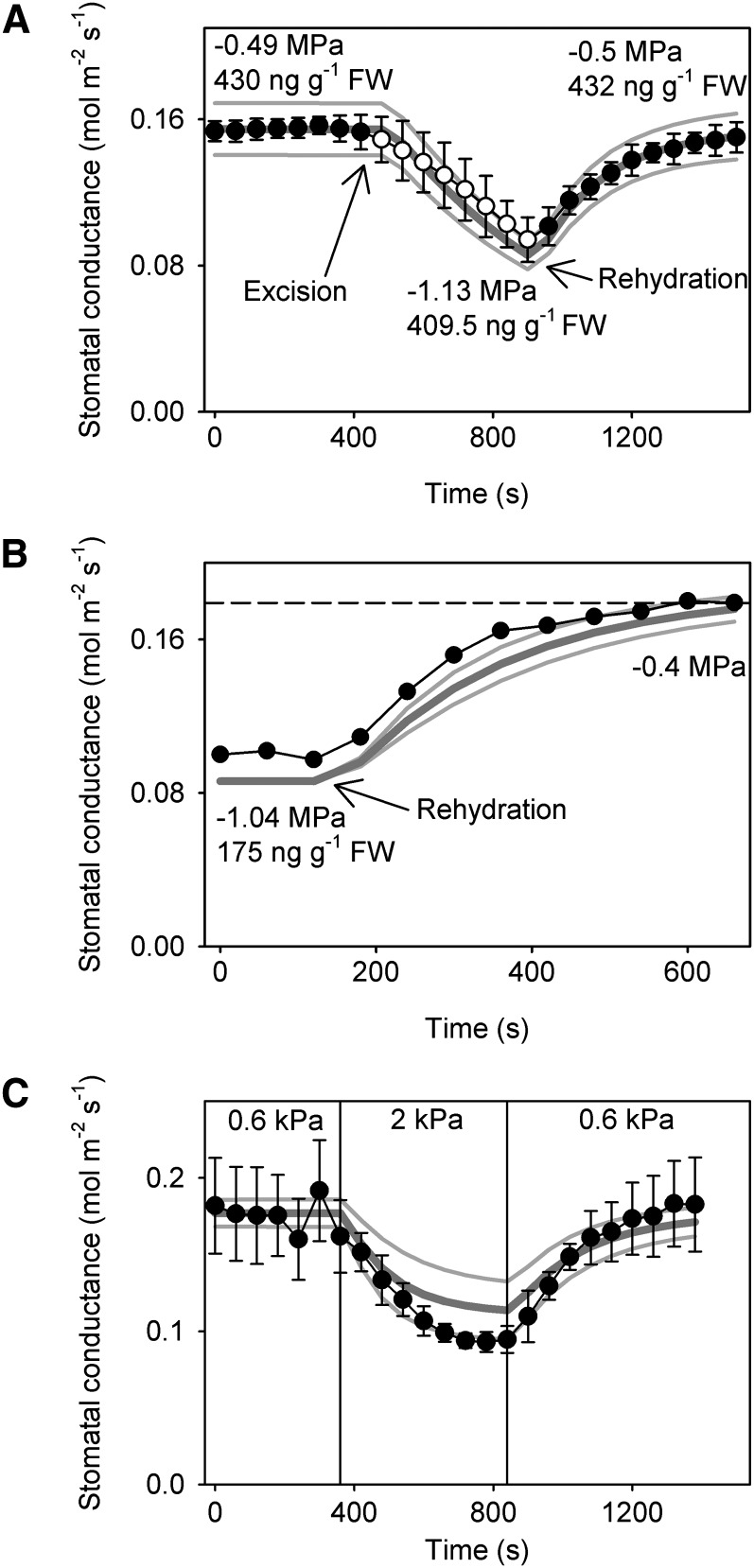
M. glyptostroboides leaves exposed to mild stresses (water stress that does not result in a decrease in leaf hydraulic conductivity [Kleaf]) caused by either increased vapor–pressure difference (VPD) of the leaf or short-term branch excision all closed their stomata over 5–8 min to about 60% of prestressed stomatal conductances (gs; Fig. 1). The dynamics of the closing response in each case followed very closely the expected trajectory of a passive hydraulic regulation of stomatal aperture, whereby gs was uniquely regulated by Ψl as determined by the leaf hydraulic capacitance (Cleaf) and Kleaf. No evidence of the Ivanov effect caused by epidermal mechanical advantage was observed, and there was no deviation in the dynamics or steady-state values that might indicate a metabolic component to the response (Fig. 1). Reversal of the imposed stress by rehydrating stems or returning to low VPD produced a nonhysteretic reopening of stomata to prestressed gs. The dynamics of stomatal reopening were modeled very faithfully by the same passive hydraulic model for gs used in dehydration. Longer-term exposure to mild stress after 6 d of soil drought produced similar results, with gs rapidly recovering from an approximately 60% reduction to prestress levels, again following a dynamic opening very close to the expected trajectory dictated by the passive hydraulic model (Fig. 1B).
Figure 1.
The stomata of M. glyptostroboides operate as passive hydraulic valves in response to moderate perturbations in water supply or evaporation. Changes in gs are shown in response to mild stresses imposed by (A) branch excision and dehydration in air for 8 min and then rehydration of the xylem by excision under water (arrows), (B) exposure to soil water deficit followed by branch rehydration (initial and final Ψl, foliar ABA level, and the point of rehydration are noted), and (C) step changes in VPD. Error bars show sds in mean gs (n = 3, ± sd). In each case, dynamic behavior of gs mirrored the behavior of a passive hydraulic model (dark gray lines). Variation in measured hydraulic parameter values from three branches are depicted as light gray lines.
These observations strongly suggest that, in M. glyptostroboides, the only processes regulating the dynamic responses of stomatal aperture to changes in leaf hydration were passive changes in guard cell turgor. A lack of hysteresis or any systematic deviation from the predictions of the hydraulic model indicates that metabolic modification of guard cell osmotic pressure was likely not a significant influence on the opening and closing of stomata in response to mild stress. This conclusion was supported by the observation that foliar ABA levels remained close to the range of control leaves (pretreatment) in all cases (Fig. 1).
Active Stomatal Regulation Triggered by Exposure to Moderate to Extreme Water Stress
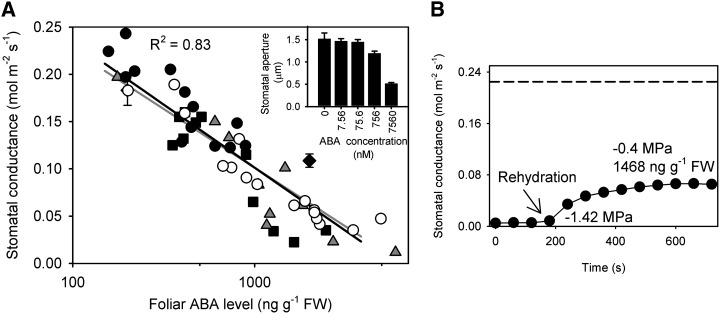
Although the passive control of stomatal aperture in M. glyptostroboides in response to mild stress was reminiscent of that control seen in early vascular plant groups, such as ferns and lycophytes (Brodribb and McAdam, 2011), there was an important difference. Stomata in ferns and lycophytes remain passively controlled, regardless of increasing foliar ABA levels during water stress (McAdam and Brodribb, 2012a), but the passive behavior of the conifer M. glyptostroboides here could be explained by the possibility that the water stress treatment was insufficiently severe to induce ABA production. To test this hypothesis, we sought to understand the relationship between ABA levels and stomatal opening by using four independent techniques to induce elevated levels of ABA: exogenous ABA feeding, water stress exposure in excised branches, water stress exposure in a drought-stressed plant, and incubation of stomata from isolated epidermes at various ABA concentrations. We found, in these four treatments, that increased ABA levels in the leaf or epidermis produced a very predictable closing response in stomata (Fig. 2).
Figure 2.
A, Sensitivity of gs to ABA in the absence of significant apoplastic water deficit was similar in leaves exposed to different pretreatments, including well-watered plants (black circles), bench-dried branches (black squares), declining soil water deficit (gray triangles), branches fed exogenous ABA (white circles), and mean gs calculated from the aperture dimensions of isolated epidermis incubated at various ABA concentrations (black diamonds; Inset, n = 50 viable stomata, ± sd). A highly significant logarithmic regression [gs = −0.058 ln (foliar ABA level) + 0.5019, P < 0.001] is shown for all data, whereas the gray line represents the significant logarithmic regression [gs = −0.054 ln (foliar ABA level) + 0.4742, P < 0.001] for the exogenous ABA feeding data used to quantify the effect of foliar ABA level on stomatal closure shown in Figure 3. B, The response of gs after instantaneous rehydration of a branch taken from a drought-stressed plant with high levels of foliar ABA; Ψl is shown before and after rehydration, with the dotted horizontal line representing unstressed, fully hydrated gs in the same leaf measured before drought stress.
First, feeding exogenous ABA through the transpiration stream in excised branches produced stomatal closure with a logarithmic dependence on foliar ABA at concentrations above the unstressed background level of ∼200 ng g−1 fresh weight (FW; Fig. 2, white circles). Second, we found that foliar ABA levels rose in leaves subjected to water stress by drying excised branches for more than 6 h or withholding water from whole plants. In each case, a rise in foliar ABA levels was associated with an incomplete recovery of gs when branches were excised underwater and rapidly rehydrated over 10 min (Fig. 2B). Water potentials in these shoots recovered quickly to prestressed levels on rehydration, but gs remained partially depressed by the presence of increased levels of foliar ABA (Fig. 2B). The relationship between gs and foliar ABA in these rehydrated branches was the same as the depression of gs caused by feeding exogenous ABA (Fig. 2). Third, we found that the initiation of stomatal closure in isolated epidermis was similar to stomata in planta when bathed in various ABA solutions (Fig. 2A, inset). A slightly lower sensitivity to ABA level in stomata from isolated epidermis was expected because of the incubation of stomata under conditions favoring stomatal opening (membrane polarizing solution and nominally carbon dioxide-free air; Dubbe et al., 1978). Regressions between foliar ABA level and gs on stomatal opening were highly significant in each case and not significantly different from one another. Foliar ABA levels were noted to influence gs in unstressed plants (Fig. 2), but these relatively low levels of foliar ABA did not influence the proportionality between active and passive responses of stomata to imposed water stress (Fig. 3B).
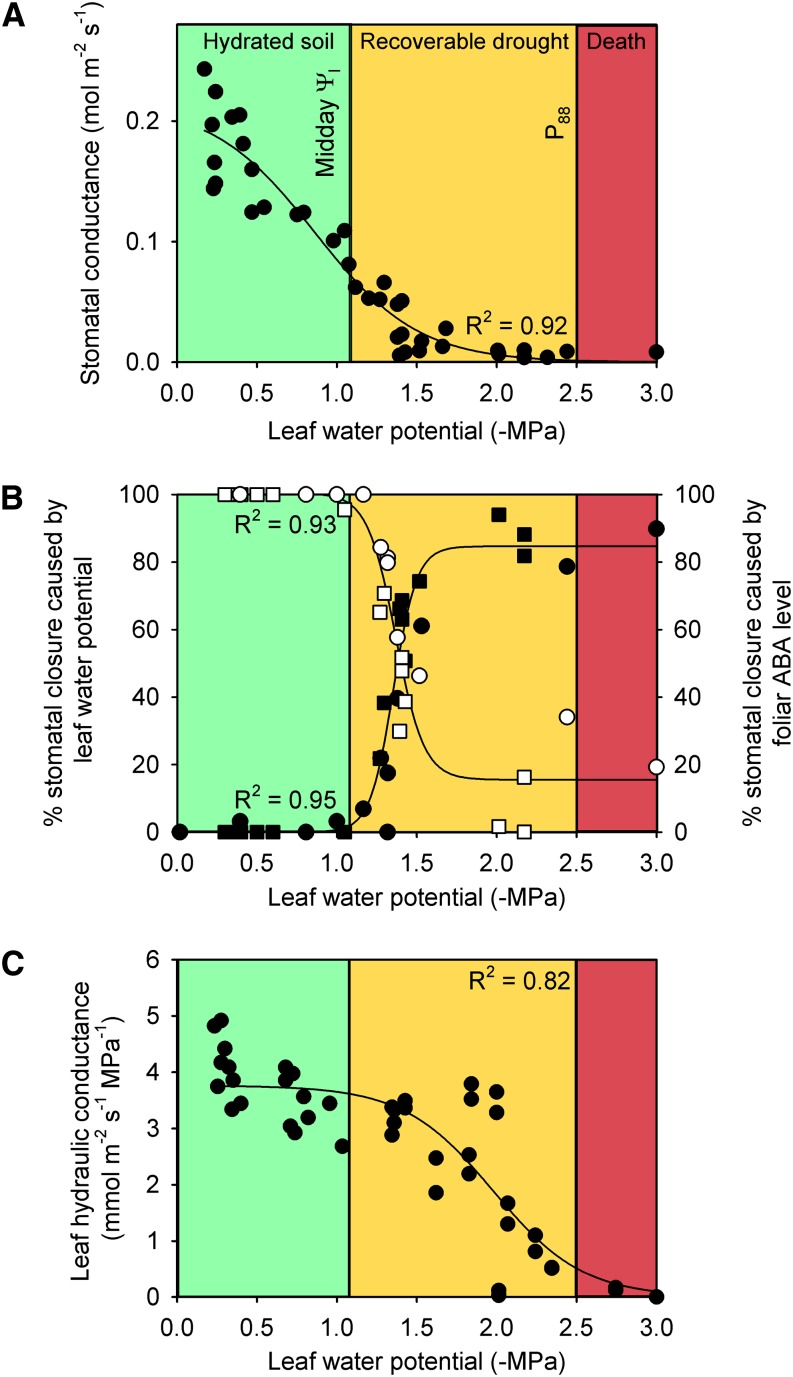
Figure 3.
Prolonged water stress exposure leads to a transition from passive stomatal closure to active ABA-mediated closure in M. glyptostroboides. A, gs predictably declines as plants experience increasingly negative Ψl. B, A significant change in stomatal control occurs as plants transition from unstressed (green box; Ψl > midday Ψl) to nonlethal water stress (yellow box; midday Ψl > Ψl > P88). Leaf death occurs as gs approaches zero and Kleaf declines by more than 88% (P88). (red box; P88 > Ψl). The percentage of stomatal closure that is caused by Ψl (white symbols; circles were determined from foliar ABA levels in bench-dried branches, and squares were determined from foliar ABA levels in droughted plants) rapidly declines beyond −1.1 MPa. At the same Ψl, the percentage of stomatal closure due to foliar ABA level rapidly increases (black symbols; circles were determined from rehydrated gs in bench-dried branches, and squares were determined from gs in rehydrated branches taken from a drought-stressed plant). C, Declining Kleaf in response to increasingly negative Ψl and the significant sigmoidal function used to determine P88.
Interactions between Passive and Active Control during Stomatal Closure
We found that both the passive and active components of stomatal closure were acting at different stages of water stress in M. glyptostroboides. Using two independent methods, we were able to quantify the relative contributions of passive (Ψl) and active (foliar ABA level) influences on stomatal closure during imposed water stress. Comparing the expected stomatal closure (using data from exogenous ABA feeding; Fig. 2) with observed stomatal closure in water-stressed leaves, we were able to calculate the percentage of stomatal closure that was attributable to ABA, with the remainder considered to be because of Ψl. On the basis of these calculations, passive hydraulic control was responsible for stomatal closure until leaves reached a water deficit of approximately −1.1 MPa (Fig. 3B). Only as water potentials fell below −1.1 MPa did the direct influence of Ψl on stomatal closure begin to rapidly diminish, such that by −2 MPa, Ψl was found to contribute <20% to stomatal closure (Fig. 3). An identical but opposite pattern was observed when the proportional contribution to stomatal closure caused by foliar ABA level was quantified by the percentage of depression in gs after water-stressed leaves were instantaneously rehydrated (Fig. 3). Strong agreement between these two independent methods illustrates that the influences of ABA and Ψl on stomatal conductance are independent. This result contrasts with the widely accepted model for angiosperms, where a compounding interaction between ABA level and Ψl has been suggested (Tardieu and Davies, 1992). Tardieu and Davies (1992) showed, in angiosperm species, that leaf water status modulated the sensitivity of stomata to ABA, with increasingly negative Ψl enhancing the closing response of stomata to increased ABA levels around the guard cells and in the xylem.
The key implication of these data is that the active stomatal closure produced by elevated levels of foliar ABA only seemed to be a significant agent for stomatal closure when plants were exposed to major water shortage (after gs had fallen to less than 20% of its prestress maximum; Fig. 3). Although the switch from passive to active stomatal closure occurred under significant plant stress (at a Ψl close to the turgor loss point of the leaf), it was still before the incipient point of leaf death, corresponding to an 88% loss of Kleaf (Fig. 3C). These data clearly illustrate that, in M. glyptostroboides, passive dehydration of guard cells remained the dominant force closing stomata as soil water was depleted and that foliar ABA only became the dominant contributor to stomatal closure as the plant approached severe levels of water stress, sufficient to cause damage to the hydraulic system and incipient leaf damage.
Conditions Triggering ABA Production in M. glyptostroboides
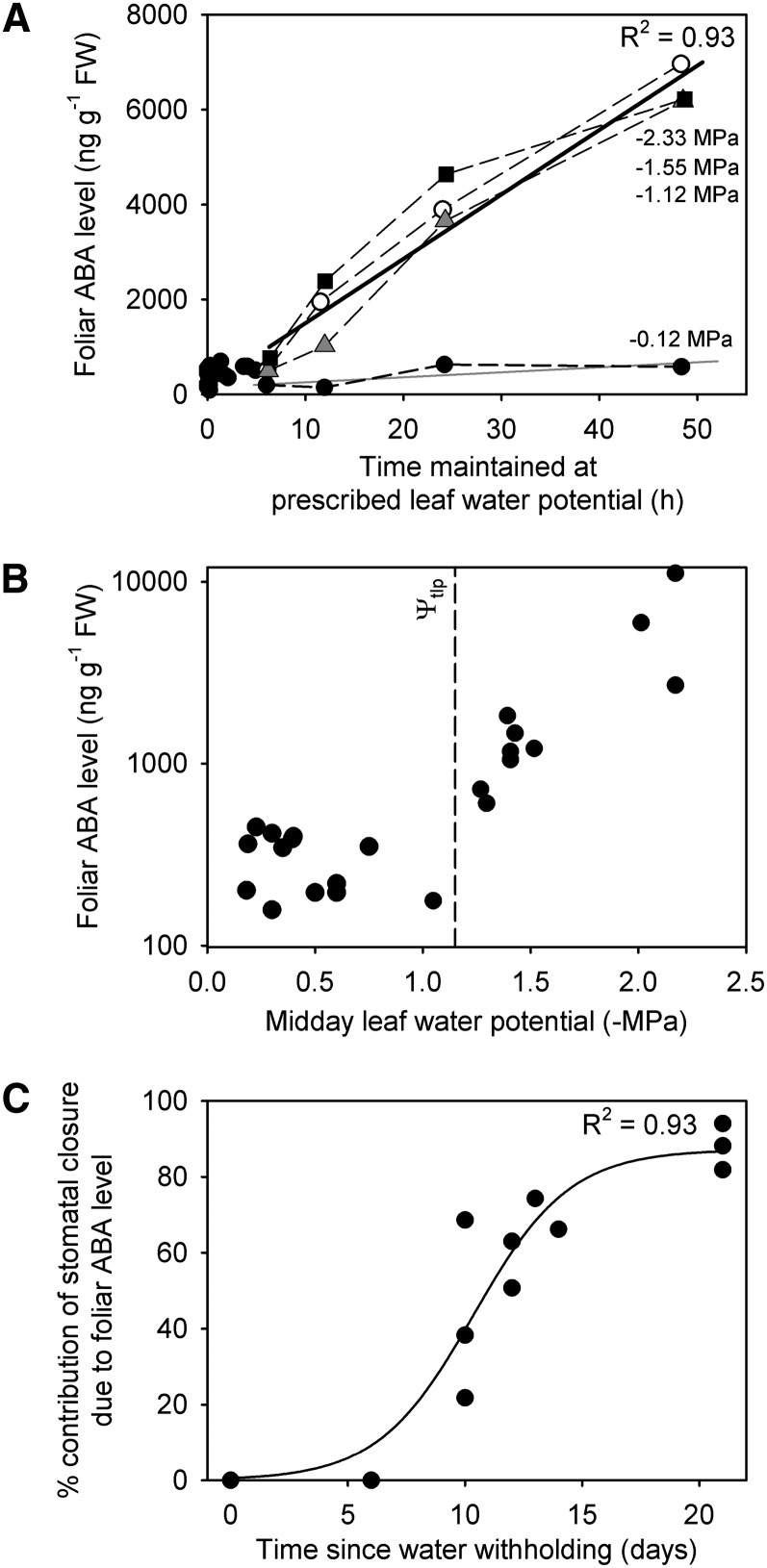
To understand the physical conditions triggering foliar ABA accumulation during soil drought, we separated the two most likely factors contributing to rising foliar ABA: the severity of stress and the time of stress exposure. By examining changes in foliar ABA levels in excised leaves maintained at different Ψl levels, we were able to separate the influences of the magnitude and duration of stress. Leaves maintained close to full hydration (−0.15 MPa) did not accumulate ABA above the control level found in normal unstressed leaves (150–400 ng g−1 FW), regardless of the time after excision (Fig. 4A). By contrast, leaves maintained at Ψl of −1.1 MPa or below synthesized ABA at a constant rate independent of the magnitude of the Ψl imposed (Fig. 4A). Foliar ABA level did not rise immediately; however, in each branch in these excised samples, we found that foliar ABA remained at control levels for a period of 6 h after the imposition of water stress (Fig. 4A). Only after this hiatus of 6 h did foliar ABA levels begin to rise, with a mean synthesis rate of 135 ng g−1 FW per hour−1 (Fig. 4A). From these data, we conclude that, for foliar ABA levels to rise above the levels found in unstressed leaves, Ψl must remain at or below −1.1 MPa for a period of more than 6 h (Fig. 4A). This finding contrasts with foliar ABA synthesis after leaf excision in angiosperm herbs, which generally increases after only a brief lag (30 min) after leaf excision for a period of 6 h, beyond which time, ABA levels cease to increase further because of enhanced catabolism (Zeevaart, 1980; Guerrero and Mullet, 1986). The 6-h delay before the commencement of foliar ABA augmentation in M. glyptostroboides also explained the observations that leaves rapidly bench dried to −1.5 MPa did not accumulate foliar ABA and were able to recover gs on rehydration.
Figure 4.
In M. glyptostroboides, foliar ABA level is augmented by the combination of both time and Ψl. A, Foliar ABA levels increased linearly with time (black line) after 6 h in branches that were maintained at prescribed Ψl below −1.1 MPa (gray triangles, −1.12 MPa; white circles, −1.55 MPa; black squares, −2.33 MPa). Foliar ABA levels did not increase in branches that were excised and dehydrated on the bench for times less than 6 h (black circles) or maintained at −0.12 MPa for 48 h (black circles; gray line). B, Foliar ABA levels in plants experiencing drought stress increase when the plant reaches Ψl more negative than turgor loss point (Ψtlp). C, The percentage contribution to stomatal closure that was caused by foliar ABA level increases in a drought-stressed plant beyond 6 d of water withholding and remains high until death beyond 21 d.
Droughted plants provided an opportunity to more closely examine the threshold Ψl required to induce a rise in foliar ABA, because the slow imposition of water stress ensured that there was more than the minimum of 6 h required for the initiation of foliar ABA synthesis. In this case, we found that foliar ABA levels rose rapidly after Ψl fell below −1.1 MPa, which corresponded with the turgor loss point, suggesting that this Ψl or the loss of leaf turgor was the trigger required for ABA synthesis (Fig. 4B). Our data agree with earlier suggestions that foliar ABA synthesis (Pierce and Raschke, 1981) or release (Georgopoulou and Milborrow, 2012) is triggered by changes in leaf turgor, but the 6-h delay before foliar ABA augmentation suggests an unknown step in the triggering process.
The rapid production of foliar ABA in excised water-stressed leaves after 6 h combined with the strongly predictive influence of foliar ABA level and Ψl on gs in water-stressed plants provide a compelling case for the autonomy of leaves in terms of regulating active ABA levels for stomatal control. These data contrast with the accepted view for angiosperms, whereby ABA involved in stomatal control is thought to be produced in the roots and delivered through the transpiration flux in the xylem (Zhang et al., 1987). In angiosperms, the change in foliar ABA level during soil drying is often less than the change in xylem ABA concentration, and in some angiosperm species, xylem ABA concentration seems to drive stomatal closure in the absence of either changes in Ψl or foliar ABA level (Zhang and Davies, 1989). Although we did not measure xylem ABA fluxes, the rate of ABA accumulation in excised shoots was much faster than in intact branches in the drought-stressed plant (135 compared with 20.5 ng g−1 FW per hour−1, respectively), suggesting, if anything, that the xylem connection to the roots dilutes rather than augments functional ABA levels in the leaf of M. glyptostroboides. This finding may be caused by diurnal metabolism of ABA by the mesophyll in drought-stressed plants (Trejo et al., 1993) or differences in leaf turgor between the two systems (Georgopoulou and Milborrow, 2012). It is uncertain whether this result represents a systematic difference between conifers and angiosperms, but recent evidence showing significant foliar ABA synthesis in leaves of angiosperms in both the stomata and xylem (Christmann et al., 2005) suggests that foliar ABA levels may, indeed, be functionally significant for angiosperm stomata as well.
Implications for Diversity in Stomatal Control of Conifers
These results from M. glyptostroboides greatly simplify our understanding of stomatal responses to water stress in conifers, illustrating a control process involving Ψl and foliar ABA level as independent drivers of stomatal closure (Figs. 1 and 2). The simplicity of this system of stomatal control contrasts with the models for angiosperm stomata, which invoke an osmotic dependence of guard cells on the epidermal turgor of the leaf to account for the apparent mechanical advantage of epidermal cells over guard cells (Buckley et al., 2003). In M. glyptostroboides, we found, under a moderate range of VPDs and soil moisture contents, that gs was a direct function of Ψl, with no apparent interacting role of foliar ABA level (Fig. 1). Thus, under most conditions of water availability, the stomata of M. glyptostroboides behave exactly like fern and lycophyte stomata, where dynamic and steady-state gs can be accurately predicted from a combination of plant hydraulics and leaf evaporation (Brodribb and McAdam, 2011). A transition to ABA-mediated stomatal closure under prolonged or severe stress in M. glyptostroboides provides evidence of a dual control mechanism that is widespread among conifers (Brodribb and McAdam, 2013a), with variation between species in foliar ABA sensitivity and the dynamics of foliar ABA synthesis. Water-stressed M. glyptostroboides plants exhibited similar behavior to the previously studied Pinus radiata in terms of a rapid triggering of ABA synthesis after Ψl fell below a threshold, but a higher foliar ABA sensitivity in P. radiata meant that stomata were influenced by foliar ABA level under mild water stress (Brodribb and McAdam, 2013a). A completely different pattern was observed in the fellow Cupressaceae species Callitris rhombiodea, in which stomatal control shifted from ABA-mediated closure to passive hydraulic control under severe water stress, thereby enabling an anisohydric stomatal closure response during soil drying (Brodribb and McAdam, 2013a). From these few examples, it is clear that the combination of active and passive regulation of stomatal aperture provides a large scope for adaptive variation in stomatal response and plant water management during drought (Franks, 2013). Combined with variation in the plant hydraulic system and plant allocation, a picture is emerging of how plants are able to adapt to changes in water availability, starting with the dynamic control of gs. Importantly, for conifers at least, the techniques required to assess the form of stomatal control as shown here are quite accessible and able to be undertaken in both field and laboratory conditions, which should facilitate additional investigations into the links between stomatal function and drought adaptation.
Implications for Stomatal Evolution
The fern-like and lycophyte-like stomatal behavior of M. glyptostroboides observed here has significant implications for our understanding of the evolution of stomatal function. Previous studies have suggested that stomatal function changed from a passive to active response to water stress at the transition from ferns to seed plants (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012b). Conifers represent the oldest living clade of seed plants, and the data presented here suggest that the stomata of conifers retain a strong direct influence of Ψl on stomatal turgor and aperture. The difference between ferns and M. glyptostroboides seems to be that, whereas augmented levels of foliar ABA in droughted ferns and lycophytes have no influence on stomatal aperture (McAdam and Brodribb, 2013), in M. glyptostroboides, foliar ABA level becomes important for stomatal closure in the later stages of drought stress as plants approach lethal levels of stress. This type of ABA interaction places M. glyptostroboides (and other conifers) in between the ABA-insensitive ferns and ABA-dependent angiosperms in terms of stomatal behavior. Although such a result seems surprising in the light of rather conservative ABA synthetic and sensing molecular toolboxes across all land plants (including some that lack stomata; McAdam and Brodribb, 2012a; Chater et al., 2013), recent work suggests that gymnosperms lack key parts of the ABA signal transduction pathway, namely calcium-dependent signaling and a related anion channel in guard cells (Brodribb and McAdam, 2013b). Hence, we suggest that ABA sensing and transduction in conifers are less sophisticated than in angiosperms, with the triggering of osmotic ion flux from guard cells by ABA regulated by fewer biochemical mechanisms as opposed to the multiple activation pathways evident in angiosperms (Geiger et al., 2011; Hills et al., 2012). This finding may explain the high levels of foliar ABA typically found in conifers relative to angiosperms (McAdam et al., 2011), indicating a much lower sensitivity of conifer stomata, compared with angiosperm stomata, to foliar concentrations ABA. Thus, gymnosperms seem to represent an important transition group in stomatal function from the ferns to the angiosperms.
CONCLUSION
Unless exposed to severe water stress, the stomata in M. glyptostroboides were found behave as passive hydraulic valves in response to changes in humidity and soil water content. An influence of ABA under prolonged soil water deficit contributed to stomatal closure only as plants approached damaging Ψl. This behavior suggests that, in M. glyptostroboides and perhaps, other gymnosperm species (Brodribb and McAdam, 2013a), stomata possess an unsophisticated control system that can be easily modeled using measurable characteristics of the hydraulic system and foliar ABA levels. The ability to separate active and passive responses to water stress in other gymnosperm species will provide opportunities to explain adaptive behavior in plant water management in terms of stomatal physiology.
MATERIALS AND METHODS
Plant Growth Conditions
Three 8-year-old individuals of Metasequoia glyptostroboides were used for all experiments, except for determining hydraulic vulnerability, and grown in 20-L pots containing an 8:2:1 mix of composted pine bark, coarse river sand, and peat moss, respectively. After a dormant overwintering period outside, approximately 10 weeks before experimentation, plants were grown under controlled environmental conditions in the glasshouses of the School of Plant Science, University of Tasmania. During this period in the glasshouses, plants were watered daily to full soil capacity and received weekly applications of liquid fertilizer (Aquasol; Hortico Ltd). Growth conditions were as described by Brodribb and McAdam (2013).
Stomatal Responses to Ψl and VPD
The stomatal response to instantaneous changes in Ψl was assessed in three excised branches. Leaves approximately halfway along the length of the branches were enclosed in the cuvette of an infrared gas analyzer (Li-6400; Li-Cor). The cuvette conditions of the infrared gas analyzer were controlled at a constant VPD of 1.2 kPa, a light intensity at 1,500 µmol quanta m−2 s−1, a carbon dioxide concentration at 390 µmol mol−1, and of leaf temperature of 22°C. Every 1 min, leaf gas exchange and cuvette conditions were automatically logged. The excised end of each branch, with periderm removed to prevent the blockage of xylem with resin, was cut under resin-filtered, deionized water, ensuring maximum hydration. The remainder of the branch outside the leaf cuvette was irradiated with a fiber-optic light source, providing a minimum light intensity of 300 µmol quanta m−2 s−1 at the leaf surface, which was near the light intensity required for saturating gs. After gas exchange reached stability (defined as less than a 3% change in gs over 8 min), Ψl was measured in an excised short shoot using a Scholander pressure chamber and microscope to precisely measure xylem balance pressure, after which time leaf tissue was immediately weighed for foliar ABA extraction, purification, and quantification (see below). The cut end of the branch was then removed from the water and allowed to dehydrate to a range of gs, after which time the stem was instantaneously rehydrated by excision under water, with paired sampling of gs, Ψl, and ABA occurring immediately before rehydration and on stability of gs after rehydration.
In three branches that were maintained in a hydrated state by excision under resin-filtered, deionized water, the stomatal response to step changes in VPD was also recorded. Leaf cuvette conditions were maintained as described above, with initial VPD controlled at 0.6 kPa. After gs reached stability, VPD was increased to 2 kPa for 10 min, after which time it was lowered back to 0.6 kPa and maintained until gs had again reached stability. VPD was regulated at the required level by adjusting the humidity in the inlet air of the gas analyzer by bubbling the incoming air through water and adjusting the amount passing through a desiccant column containing calcium sulfate.
Dynamic Passive Hydraulic Model for Stomatal Conductance
To test whether the stomata of M. glyptostroboides responded to changes in Ψl and VPD in a way that was consistent with passive hydraulic control of leaf hydration in the light, we used the dynamic, stepwise model described by Brodribb and McAdam (2011). Primarily, the model predicts gs in a stepwise nature based solely on the relationship between gs and Ψl. To avoid circularity, it was important to establish this passive gs (Ψl) function in the absence of changes in foliar ABA. Hence, we used only data from leaves that had not yet crossed the critical Ψl and 6-h time threshold for ABA production, such that stomatal closure was a product of changing Ψl and not ABA (Fig. 4B). Ψl was calculated from the balance between hydraulic supply and transpirational water loss using the leaf hydraulic parameters Kleaf and Cleaf. These leaf hydraulic parameters were quantified in three branches using a flow meter according to the bulk-flow method described by Blackman and Brodribb (2011). The three measurements of Kleaf and Cleaf were then each used to parameterize the model, allowing for the calculation of a mean predicted gs (± sd) for all modeled responses.
Assessments of Stomatal Sensitivity to ABA
Four independent techniques were used to assess the sensitivity of stomata to ABA, including (1) the tracking of gs and foliar ABA level after the feeding of exogenous ABA into the transpiration stream, the measurement of foliar ABA level and maximum gs after the instantaneous rehydration of the xylem of (2) excised bench-dried branches and (3) branches taken and rehydrated from a plant experiencing drought, and (4) the in vitro assessment of stomatal aperture in isolated epidermis incubated at increasing concentrations of exogenous ABA and subsequent estimation of gs.
ABA Feeding
Leaf gas exchange was measured (described above) in three branches that were excised under resin-filtered, deionized water (described above). Leaves were equilibrated in the leaf cuvette until maximum and stable gs was reached; then, an aliquot of a highly concentrated solution of exogenous ABA, prepared by dissolving 90% crystalline ABA of mixed isomers (Sigma Chemical Co.) in 1 mL methanol and then diluting up to 250 mL with resin-filtered, deionized water, was added to the leaf water supply, increasing the concentration of ABA in the water to 5,000 ng mL−1 (18.9 µm). Periodically, as stomata closed, short shoots were taken from the branch for paired sampling of Ψl and foliar ABA level (see below). Each branch contributed at least five paired gs, Ψl, and foliar ABA level data points.
Rehydration of Ex Situ Bench-Dried Branches
Branches (n = 15) were excised underwater from unstressed plants at midday; Ψl and foliar ABA level (see below) were determined in short shoots, whereas maximum gs was measured in a marked short shoot (described above). The excised branches were then allowed to desiccate under standard laboratory conditions for up to 24 h. After ex situ dehydration, gs was again measured in the same short shoot for which initial maximum gs had been assessed earlier, and at the same time, Ψl and foliar ABA level were again determined in neighboring short shoots. The branch was then instantaneously rehydrated by excision under resin-filtered, deionized water, whereas gs was continually recorded according to the methods by McAdam and Brodribb (2012). On completion of stomatal opening, Ψl was measured in a neighboring short shoot to verify effective leaf rehydration (Ψl > −0.4 MPa).
Rehydration of Branches over the Course of Drought Stress
In a single individual, maximum gs (as described above), Ψl, and foliar ABA level (see below) were determined in marked short shoots of 11 branches. Drought was then initiated by withholding water for a period of 21 d, by which time the plant had reached a water potential sufficient to cause 88% loss of leaf xylem hydraulic conductance (a damaging drought stress level; Brodribb and Cochard, 2009). Periodically during this time, Ψl and foliar ABA level were determined in neighboring short shoots on the marked branches, and gs was continually recorded as the selected branch was excised from the droughted plant and instantaneously rehydrated (described above); Ψl was measured on a short shoot after instantaneous rehydration to ensure that the stem was fully hydrated (>−0.1 MPa), ruling out a loss of hydraulic conductivity as the cause for any hysteresis in gs recovery and leaving foliar ABA level as the most parsimonious explanation for hysteresis in the recovery of gs.
In Vitro Response of Stomatal Aperture and Calculation of gs
The dose-dependent response of stomatal aperture in viable guard cells from isolated epidermis to exogenous ABA was also examined. Epidermes were prepared as described by Brodribb and McAdam (2013), and after incubation in the light (200 µmol quanta m−2 s−1) for 1 h in control (0 ng mL−1 ABA) buffer solution (50 mm KCl, 10 mm MES, 0.1 mm CaCl2, pH 6.15; rendered nominally CO2-free after 1 h of bubbling with N2 gas), at least two epidermes were then further incubated for 2 h in one of five concentrations (0, 2, 20, 200, and 2,000 ng mL−1) of exogenous ABA dissolved in the same buffer solution, after which time stomatal apertures (n = 50 minimum viable stomata) were observed. The work by Brodribb and McAdam (2013) has a description of the methods used to prepare epidermes, assess guard cell viability, and perform the double blind measurements of stomatal apertures. Stomatal conductance was calculated from the measurements of stomatal apertures at each concentration of ABA according to the formula given by Parlange and Waggoner (1970). The concentration of ABA in the buffer solution (nanograms per milliliter−1) was assumed to be the same as nanograms per gram−1 FW in the leaf. Stomatal length was determined from the same images used to quantify stomatal apertures, whereas stomatal density under ambient carbon dioxide concentrations and stomatal pore depth for M. glyptostroboides were taken from the works by Ogaya et al. (2011) and Li (2004), respectively.
Independent Quantifications of the Contribution of ABA and Ψl to Stomatal Closure
We calculated the percentage of stomatal closure that is caused by ABA using Equation 1 for instantaneously rehydrated ex situ bench-dried branches and branches from the droughted plant:
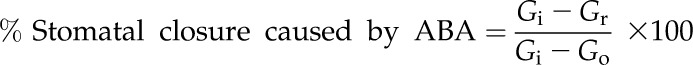 |
(1) |
where Gr is gs after instantaneous rehydration, Gi is initial maximum gs for that leaf and Go is the observed stomatal conductance immediately before instantaneous rehydration. This estimation of the percentage stomatal closure that is caused by ABA was calculated independently of the measurement of foliar ABA level and is based on the assumption that foliar ABA was the only driver for stomatal closure in fully rehydrated leaves that have experienced drought stress. We calculated the percentage stomatal closure that was caused by Ψl using Equation 2 by estimating the percentage reduction in gs based solely on foliar ABA levels in the instantaneously rehydrated ex situ bench-dried branches and branches from the droughted plant. Equation 2 assumes that ABA and Ψl are the only contributors to stomatal closure during drought and that other signals (Acharya and Assmann, 2009) play only a minor role in closing stomata during water stress:
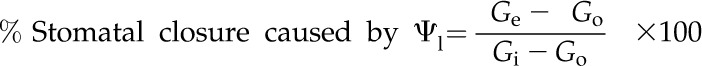 |
(2) |
where Ge is the expected gs in instantaneously rehydrated branches based on foliar ABA levels using the empirical relationship between foliar ABA level and gs determined in branches fed exogenous ABA (gray regression in Fig. 2A). Go is the observed stomatal conductance immediately before instantaneous rehydration.
Leaf Hydraulic Vulnerability and Turgor Loss Point
Leaf hydraulic vulnerability was measured using a rehydration technique (Brodribb and Cochard, 2009) to calculate Kleaf with a flow meter on branches excised and dehydrated to prescribed Ψl. Large amounts of branch material (>40 short shoots) were required to accurately determine the relationship between Ψl and hydraulic conductivity, and hence, measurements were made on a single large individual growing in the Royal Tasmanian Botanical Gardens in Hobart, Tasmania, Australia. Pressure–volume analysis was performed on five short shoots taken from an unstressed fully hydrated plant to determine turgor loss point according to the methods in the work by Tyree and Hammel (1972).
Effect of Leaf Water Status and Time on ABA Biosynthesis
To investigate the effect of Ψl and time on ABA biosynthesis in M. glyptostroboides, four branches were excised and allowed to dehydrate (over no more than 3 h) to a prescribed Ψl (−0.12, −1.12, −1.55, and −2.13 MPa). After the branch had reached the prescribed Ψl, a short shoot was excised for simultaneous Ψl and foliar ABA quantification (see below), after which time the branch was double bagged with damp paper towels to ensure maximum humidity, eliminating transpiration and maintenance of the prescribed Ψl. After this first sampling, a short shoot was excised for simultaneous Ψl and foliar ABA quantification at 6, 12, 24, and 48 h after the cessation of transpiration.
ABA Extraction, Purification, and Quantification
To extract foliar ABA, ∼0.07 g leaf tissue was weighed (±0.0001 g FW), roughly chopped into a 50-mL centrifuge tube (Cellstar; Greiner Bio-One), covered with 12 mL cold (−20°C) 80% (v/v) methanol in water, and stored at −20°C for 24 h. The tissue was then homogenized, and ABA was extracted by storing the sample at 4°C for another 24 h. After extraction, to each sample, 15 µL [2H6]ABA (National Research Council of Canada) was added as an internal standard. A 5-mL aliquot was then taken and dried to completeness under vacuum at 35°C; samples were then taken up in 500 µL 0.4% (v/v) acetic acid in water and partitioned two times against 300-µL aliquots of diethyl ether. The ether phases from each partition were pooled and dried under a stream of nitrogen gas. Samples were then taken up in 150 µL 5% (v/v) methanol, 94% (v/v) water, and 1% (v/v) acetic acid and centrifuged for 3 min; an aliquot of 50 µL was taken for quantification by ultraperformance liquid chromatography according to the methods in the work by McAdam and Brodribb (2012).
Acknowledgments
The authors thank John Ross and Noel Davies for assistance with ABA methodology.
Glossary
- ABA
abscisic acid
- Ψ
water potential
- VPD
vapor–pressure difference
Footnotes
Articles can be viewed online without a subscription.
References
- Acharya BR, Assmann SM. (2009) Hormone interactions in stomatal function. Plant Mol Biol 69: 451–462 [DOI] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Beadle CL, Jarvis PG, Neilson RE. (1979) Leaf conductance as related to xylem water potential and carbon dioxide concentration in Sitka spruce. Physiol Plant 45: 158–166 [Google Scholar]
- Blackman CJ, Brodribb TJ. (2011) Two measures of leaf capacitance: insights into the water transport pathway and hydraulic conductance in leaves. Funct Plant Biol 38: 118–126 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H. (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. (2013a) Abscisic acid mediates a divergence in the drought response of two conifers. Plant Physiol 162: 1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. (2013b) Unique responsiveness of angiosperm stomata to elevated CO2 explained by calcium signalling. PLoS One 8: e82057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS. (2009) Evolution of stomatal responsiveness to CO(2) and optimization of water-use efficiency among land plants. New Phytol 183: 839–847 [DOI] [PubMed] [Google Scholar]
- Buckley TN. (2005) The control of stomata by water balance. New Phytol 168: 275–292 [DOI] [PubMed] [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD. (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1785 [Google Scholar]
- Chater C, Gray JE, Beerling DJ. (2013) Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol 16: 638–646 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR. (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A, Hoffmann T, Teplova I, Grill E, Müller A. (2005) Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol 137: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland EB. (1902) The mechanism of stomata. Ann Bot 16: 327–364 [Google Scholar]
- Damour G, Simonneau T, Cochard H, Urban L. (2010) An overview of models of stomatal conductance at the leaf level. Plant Cell Environ 33: 1419–1438 [DOI] [PubMed] [Google Scholar]
- Doi M, Wada M, Shimazaki KI. (2006) The fern Adiantum capillus-veneris lacks stomatal responses to blue light. Plant Cell Physiol 47: 748–755 [DOI] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D. (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbe DR, Farquhar GD, Raschke K. (1978) Effect of abscisic acid on the gain of the feedback loop involving carbon dioxide and stomata. Plant Physiol 62: 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ. (2013) Passive and active stomatal control: either or both? New Phytol 198: 325–327 [DOI] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulou Z, Milborrow BV. (2012) Initiation of the synthesis of ‘stress’ ABA by (+)-[2 H6 ]ABA infiltrated into leaves of Commelina communis. Physiol Plant 146: 149–159 [DOI] [PubMed] [Google Scholar]
- Guerrero F, Mullet JE. (1986) Increased abscisic acid biosynthesis during plant dehydration requires transcription. Plant Physiol 80: 588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R. (2012) Ion channels in plants. Physiol Rev 92: 1777–1811 [DOI] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL. (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B. (1923) Transpiration in verschiedener Stammhöhe. I. Sequoia gigantea. Zeitschr f Bot 15: 465–501 [Google Scholar]
- Li X (2004) Development and light response of leaves of Metasequoia and close relatives. Master thesis. University of Maine, Orono, ME [Google Scholar]
- McAdam SAM, Brodribb TJ. (2012a) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. (2012b) Stomatal innovation and the rise of seed plants. Ecol Lett 15: 1–8 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. (2013) Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol 198: 429–441 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Ross JJ, Jordan GJ. (2011) Augmentation of abscisic acid (ABA) levels by drought does not induce short-term stomatal sensitivity to CO2 in two divergent conifer species. J Exp Bot 62: 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelheuser CJ, Van Steveninck RFM. (1969) Stomatal closure and inhibition of transpiration induced by (RS)-abscisic acid. Nature 221: 281–282 [Google Scholar]
- Morison JIL, Jarvis PG. (1983) Direct and indirect effects of light on stomata. I. In Scots pine and Sitka spruce. Plant Cell Environ 6: 95–101 [Google Scholar]
- Ogaya R, Llorens L, Peñuelas J. (2011) Density and length of stomatal and epidermal cells in “living fossil” trees grown under elevated CO2 and a polar light regime. Acta Oecol 37: 381–385 [Google Scholar]
- Parlange JY, Waggoner PE. (1970) Stomatal dimensions and resistance to diffusion. Plant Physiol 46: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Raschke K. (1981) Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 153: 156–165 [DOI] [PubMed] [Google Scholar]
- Raschke K. (1970) Leaf hydraulic system: rapid epidermal and stomatal responses to changes in water supply. Science 167: 189–191 [DOI] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM. (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Sack FD. (1987) The development and structure of stomata. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 59–89 [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Stålfelt MG. (1944) The water consumption of the spruce. Kungl. Lantbruksakademiens Tidskrift 88: 1–83 [Google Scholar]
- Tardieu F, Davies WJ. (1992) Stomatal response to abscisic acid is a function of current plant water status. Plant Physiol 98: 540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, MacRobbie EAC, Blatt MR. (1992) Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol 126: 1–18 [DOI] [PubMed] [Google Scholar]
- Trejo CL, Davies WJ, Ruiz L. (1993) Sensitivity of stomata to abscisic acid (an effect of the mesophyll). Plant Physiol 102: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Hammel HT. (1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot 23: 267–282 [Google Scholar]
- Zeevaart JAD. (1980) Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol 66: 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. (1989) Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ 12: 73–81 [Google Scholar]
- Zhang J, Schurr U, Davies WJ. (1987) Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J Exp Bot 38: 1174–1181 [Google Scholar]