Integrative soil-plant system approaches are needed to understand plant water uptake dynamics.
Abstract
Over the last decade, investigations on root water uptake have evolved toward a deeper integration of the soil and roots compartment properties, with the goal of improving our understanding of water acquisition from drying soils. This evolution parallels the increasing attention of agronomists to suboptimal crop production environments. Recent results have led to the description of root system architectures that might contribute to deep-water extraction or to water-saving strategies. In addition, the manipulation of root hydraulic properties would provide further opportunities to improve water uptake. However, modeling studies highlight the role of soil hydraulics in the control of water uptake in drying soil and call for integrative soil-plant system approaches.
The fundamental mechanism of water flow in plants has been described for many years (Steudle, 2001). Briefly, the diffusion of vapor through stomata leads to the evaporation of water from the surface of inner leaf tissues and an increase of tension in the xylem that propagates to each root segment following the cohesion-tension principle (in this context, a root segment can be seen as a portion of root with uniform hydraulic properties). Where this tension is higher than the surrounding soil, it induces an inflow of water from the rhizosphere, following paths of low soil hydraulic resistance. How far plants are able to sustain their leaf water demand is therefore largely dependent on the hydraulic properties of the soil-root system.
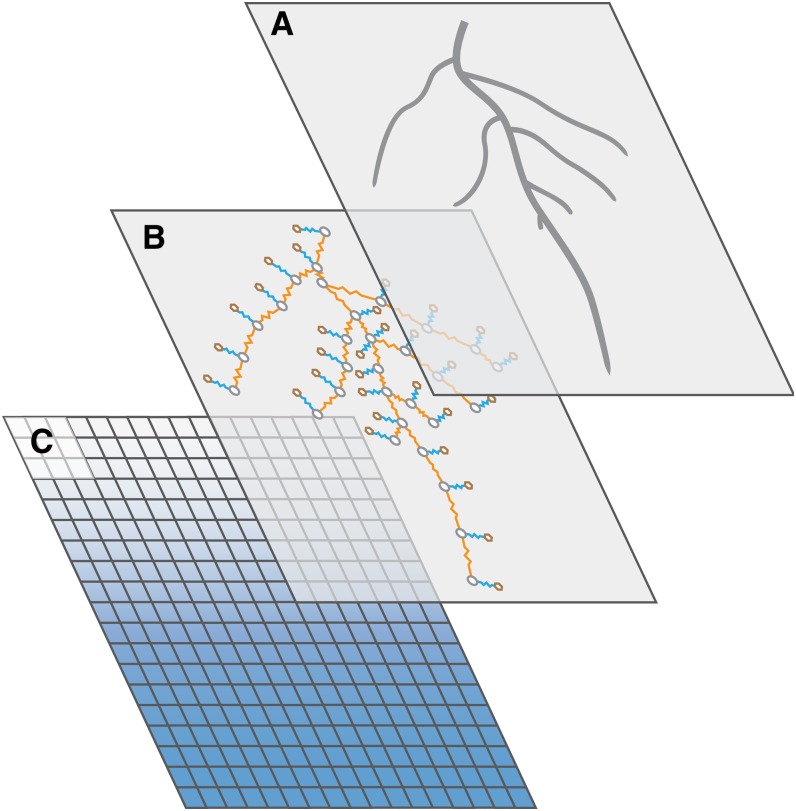
The spatial geometry of the root system is typically considered as a major determinant of water availability, essentially because the placement of roots in the soil domain delineates the extent of soil exploration and sets an upper limit to the volume of potentially accessible water (Fig. 1A). The level of details required to link the volume of accessible water to the spatial geometry of the root system depends on crop species, sowing patterns, and soil hydraulic properties. While a vertical profile of root density is generally sufficient for crops sown at very high densities in a highly conductive soil, two- or three-dimension descriptions are needed for crops with large interrows or in water-depleted soils (Couvreur, 2013).
Figure 1.
Properties of the soil-root system. A, Spatial geometry of the root system. B, Root hydraulic architecture is the integration of axial (orange lines) and radial (blue lines) hydraulic resistances of individual root segments (gray circles) and soil elements (brown circles). C, Soil water content distribution (white indicates dry and blue indicates wet).
Within the volume of soil explored by a root system, however, water uptake is unevenly shared among root segments. Individual segments differ by their axial and radial hydraulic conductivities and by the conductance of the shortest paths that links them to the shoot base. These properties, encapsulated in the concept of root hydraulic architecture (Fig. 1B), have a large impact on the hydraulic conductance of the root system and, together with the soil hydraulic status, on the distribution of water capture among individual root segments. Consequently, sites of higher uptake occur throughout the root zone and contribute to the heterogeneous spatial distribution of the plant-available soil water availability (Doussan et al., 2006). For a given root, these preferential sites are predicted a few centimeters from the root tip, where protoxylem and xylem elements are conductive and hydrophobic structures are lacking. This was recently confirmed experimentally by neutron radiography experiments (Zarebanadkouki et al., 2013).
The distribution and amount of water uptake in the root zone is also influenced by the distribution and amount of the available soil water (Fig. 1C). As the soil matric potential and hydraulic conductivity decrease with soil water content, dry soil portions contribute marginally to root water uptake but also limit the contribution of the surrounding (potentially wetter) bulk soil. As long as soil hydraulic conductivities do not limit the water flow to the rhizosphere, root placement and hydraulic properties (i.e. the root hydraulic architecture) have a limited impact on the uptake process, provided that the root system conductance is large enough (Passioura, 1984). The root hydraulic architecture essentially matters in water deficit conditions, when the soil hydraulic conductivity become limiting. Because the array of intermediate situations where the soil is neither completely dry nor wet is large, it has become obvious in the last decade that an appropriate framework to analyze water uptake should consider both root hydraulic architecture and soil hydraulic properties (Draye et al., 2010).
In this Update, we report on recent advances in the analysis of water flow and water uptake regulation within the soil-root domain. In the first three sections, we analyze root and soil features that influence water uptake, with a focus on conditions of limited water supply. In the last two sections, we highlight recent work in systems analysis of root water uptake and review methodological developments that will guide future research in this area.
COINCIDENCE BETWEEN ROOT FORAGING AND SOIL RESOURCES DISTRIBUTION
The importance of root placement for water extraction depends on the ability of the soil to redistribute its water to sustain the uptake of water that occurs in the rhizospheric compartment of the soil. In soils with high water conductivity throughout the season, fast soil water redistribution from the bulk soil to the rhizosphere limits the role of root foraging as long as the root system conductance is large enough. In drying soils, however, the smaller hydraulic conductivity of the soil reduces soil water redistribution and the soil volume from which individual root segments are able to obtain their water narrows down accordingly. In such conditions, even transient, the placement of roots and its correlation to the distribution of soil water sets an upper limit to the amount of water that can be extracted.
In transient or cyclic drought environments, the reserve of soil water can be temporarily restricted to deeper layers because water uptake (and evaporation) occurs preferentially in the topsoil, where the root length density (cumulated root length per unit soil volume) is the highest and the path to extract water the lowest. This situation is most pronounced under terminal drought, as the soil water reserve is not refilled over the growing season and is gradually restricted to deeper soil layers. Increasing the root system depth and tailoring deep water extraction was therefore proposed as a key element of a root system ideotype adapted to water-limited environments (Wasson et al., 2012; Comas et al., 2013; Lynch, 2013). Considering the construction and maintenance costs of root systems, the ideotype should preferably have few and long laterals, evenly distributed along the depth axis (Lynch, 2013). The rationale is that few long laterals have a small weight on the carbon budget and allow the exploration of a larger soil volume. Aerenchyma is also considered as a feature reducing the root construction cost, in favor of deep root extension. Wasson et al. (2012) also advocate for a greater root length density at depth and reduced density in the topsoil to favor deep soil water extraction.
Root system depth appears to be amenable to conventional breeding and has been shown to be under control of, at least, four different quantitative trait loci in rice (Oryza sativa; Courtois et al., 2013) and one major constitutive quantitative trait loci in maize (Zea mays; Landi et al., 2010). In addition, several traits that should contribute to a deep root phenotype have been proposed or identified. Increasing the diameter of the main roots is thought to be linked with a greater growth potential (Pagès et al., 2010) and a greater ability to explore hard soil (Bengough et al., 2011). In rice, the gene DEEPER ROOTING1 has been shown to steepen the root insertion angle and increase the rooting depth, conferring improved drought resistance (Uga et al., 2013). In groundnut (Arachis hypogaea), DEHYDRATION RESPONSE ELEMENT B1A has been shown to increase drought resistance by promoting root development in deep soil layers. Additionally, increasing the proportion of aerenchyma in main root axes reduces the metabolic cost of root exploration (Fan et al., 2007; Lenochova et al., 2009; Zhu et al., 2010). The manipulation of root branching in different layers, which is part of the deep root ideotype, is expected to be more difficult to achieve for practical observation constraints. While considering those traits, it should be reminded that deep rooting could be obtained differently in tap-rooted species compared with monocot root systems with continued production of gravitropic adventitious root axes.
The identification of root ideotypes is further complicated by the fact that root growth and development are strongly influenced by the soil environment. Root architecture remodeling in response to a wide range of nutrient deficiencies has been recently described and partly elucidated in Arabidopsis (Arabidopsis thaliana; Giehl et al., 2013; Gruber et al., 2013). Changes in root architecture in response to phosphate starvation occur under the control of Oryza sativa MYB2 phosphate-responsive gene1 in rice (Dai et al., 2012) and AtSIZ1 in Arabidopsis (Miura et al., 2005, 2011). Interestingly, alternative adaptations to the same adverse conditions exist among different genotypes, as illustrated by altered primary or lateral root growth conferring resistance to K starvation (Kellermeier et al., 2013). Local environmental conditions also contribute to root architecture remodeling. Individual roots are able to reorient toward water (hydrotropism) under the control of MIZU-KUSSEI1 (Iwata et al., 2013) and GNOM (Moriwaki et al., 2014) in Arabidopsis. Similarly, PIN-FORMED2 activity influences the capacity of individual roots to escape high-salinity patches (halotropism; Galvan-Ampudia et al., 2013). This plasticity of root development should not be overlooked in drought resistance studies given the role of water in nutrient uptake.
The benefit of deep root systems in drought-prone environments has been demonstrated experimentally in rice (Steele et al., 2013), wheat (Triticum aestivum; Manschadi et al., 2010), maize (Hammer et al., 2009, 2010), legumes (Vadez et al., 2013), grapes (Vitis vinifera; Alsina et al., 2011), or trees (Pinheiro et al., 2005). However, other results seem to indicate that deep root systems are not always linked to an increase in yield. Experiments with chickpea (Cicer arietinum; Zaman-Allah et al., 2011a, 2011b) and wheat (Schoppach et al., 2013) indicate that drought tolerance, especially in terminal drought conditions, can be linked to a conservative use of water throughout the season rather than deep rooting. In such cases, plants tailored for improved root length density at depth are likely to use too much water early in the season and reduce the reserve of water in the profile during grain filling. A similar behavior has been reproduced using modeling tools (Vadez et al., 2012). As suggested recently, benefits of any root-related trait could be highly dependent on the drought scenario (Genotype × Environment interactions; Tardieu, 2012).
ROOT SYSTEM HYDRAULIC ARCHITECTURE
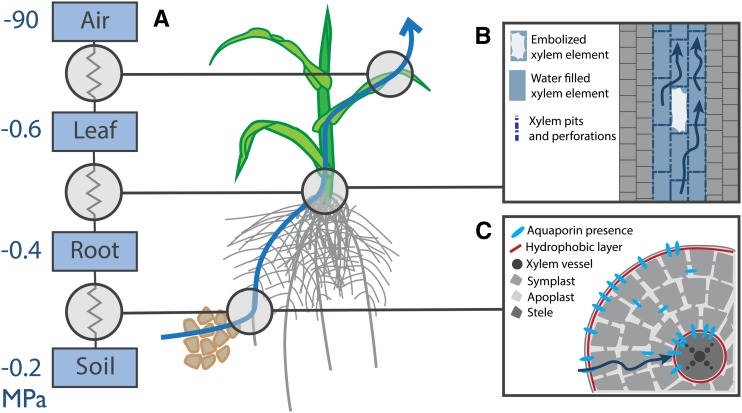
Although all root segments are somehow connected to the plant stem, the negative water potential that develops at their surface as a result of the xylem tension is not necessarily uniform. Individual root segments are not equally conductive to water, both radially and axially, and the paths that link them to the shoot base are unique (Fig. 1A). On the one side, from the root surface to the xylem vessels, water flows radially, following paths of lowest hydraulic resistance using apoplastic, symplastic, and cell-to-cell pathways. This radial water inflow into the root, described as a composite transport, can be characterized at the root segment level by a radial hydraulic conductance, which has been shown to be variable between species (Bramley et al., 2009; Knipfer et al., 2011) and even ecotypes (Sutka et al., 2011). On the other side, the axial flow along the xylem is characterized by the axial conductance of successive root segments. The complete hydraulic structure of the root system, comprising its topology and the size and hydraulic properties of its constituting segments, forms its root hydraulic architecture (Doussan et al., 1998). Under uniform soil water distribution, it has been shown that the hydraulic architecture allows for predicting the expected contribution of every root segment to the water uptake (Doussan et al., 2006), recently referred to as the standard uptake fractions distribution (Couvreur et al., 2012).
The tissular organization of root segments is a long-term determinant of their radial conductivity (Fig. 2C). This includes the number and anatomy of cell layers between the root surface and the xylem (Yang et al., 2012) and the presence of hydrophobic Casparian strips that occur typically at the endodermis and exodermis (Enstone et al., 2003). The formation of hydrophobic structures has been shown to be influenced by the growing medium (Hachez et al., 2012) and is triggered by drought conditions (Enstone and Peterson, 2005; Vandeleur et al., 2009). As the tissular organization is established permanently, this implies that the radial conductivity reflects the root segment history (its development, in relation with its environment). Beyond these structural features, the root radial conductivity is also controlled on a shorter term by the regulation of water channels, or aquaporins (Cochard et al., 2007b; Hachez et al., 2012) Presence of functional aquaporins in cell membranes highly facilitates the passive flow of water and has been shown to contribute to 20% to 80% of the radial water inflow into the root (Maurel and Chrispeels, 2001; Javot et al., 2003), although this contribution varies between species (Bramley et al., 2009, 2010). Aquaporin regulation is achieved through their expression intensity (Hachez et al., 2012) or subcellular localization (Li et al., 2011) or through the gating of the aquaporin pore (gating; Boursiac et al., 2008). In maize, aquaporins have been shown to be preferentially localized in the endodermis and exodermis (Hachez et al., 2006; Fig. 2C). For more details on aquaporins, see Chaumont and Tyerman (2014).
Figure 2.
Water flow in the plant. A, Water flow in the plant is a passive process driven by water potential differences and regulated by hydraulic conductivities between the compartments of the system (soil-root-shoot-atmosphere). B, Axial water flow is influenced by the anatomy of the xylem pipes (size, number, and presence of pits) and the occurrence of cavitation events (embolism of xylem elements). C, Radial water flow is influenced, in the long term, by the radial anatomy of the root, such as the number of cell layers and the presence of hydrophobic layers (endodermis and exodermis). In the short term, the radial flow is influenced by the expression and localization of aquaporins.
As for the radial conductance, both permanent and transient features affect the axial conductance of individual root segments. Structural features include the number, size, degree of interconnection, and decorations of xylem vessels (Vercambre et al., 2002; Domec et al., 2006; Tombesi et al., 2010; Fig. 2B). The number and size of xylem vessels increase during the maturation of root segments and decrease with branching order in cereals (Watt et al., 2008). The xylem diameter reflects the root segment history. For example, it tends to be lower in shallow roots than in deep roots for woody plants growing in environments subject to drought or freezing conditions (Gebauer and Volařík, 2012). The anatomy of xylem vessels also displays a large variability in Zea spp. (Burton et al., 2013), rice (Uga et al., 2008), legumes (Purushothaman et al., 2013), or coniferous (McCulloh et al., 2010). Transient modifications of the axial conductance occur as a result of xylem vessel embolism, or cavitation, following the nucleation and rapid expansion of gas bubbles under high tension. Because embolized vessels are not hydraulically conductive, the flow of water through the root segment is restricted to the remaining, noncavitated vessels. Different species are not equally susceptible to cavitation (Cochard et al., 2008) or even cultivars (Cochard et al., 2007a; Li et al., 2009; Rewald et al., 2011), but not always (Lamy et al., 2014). Susceptibility to cavitation has been linked to the large xylem vessels, anatomy of walls, and pits (Delzon et al., 2010; Herbette and Cochard, 2010; Christman et al., 2012). It has to be noted that xylem vessel cavitation is a reversible event, although the exact mechanisms underlying the refilling processes are not yet fully known (Zwieniecki and Holbrook, 2009). It is often considered that the axial conductance does not limit water flow in the root system by virtue of the large conductivity of xylem vessels (Steudle, 2000). However, recent experimental evidence has revealed the negative effect of cavitation on the plant water status (Zufferey et al., 2011; Johnson et al., 2012).
Novel root hydraulic architectures are being proposed to improve drought tolerance. Wasson et al. (2012) advocate for greater axial and radial conductivities in deep roots to increase the uptake and transport capacity of water from deep soil layers. In conditions of scarce deep water, Comas et al. (2013) recommend decreasing the axial conductance to save water for the end of the crop cycle. More generally, the importance of the ratio between axial and radial conductivities has also been stressed from modeling studies (Doussan et al., 2006; Draye et al., 2010). Large values of this ratio should lead toward a uniform distribution of the uptake throughout the entire root system, while low values would favor preferential uptake in the topsoil. Experimental evidence that the manipulation of root hydraulic architecture can improve the water status of plants under water deficit remains scanty (Passioura, 2012). Designing a root hydraulic architecture to improve drought tolerance is thus likely to be specific to the species and genotype, climatic scenario, soil hydraulic properties, and management practices (Draye et al., 2010).
INFLUENCE OF THE SOIL WATER DISTRIBUTION
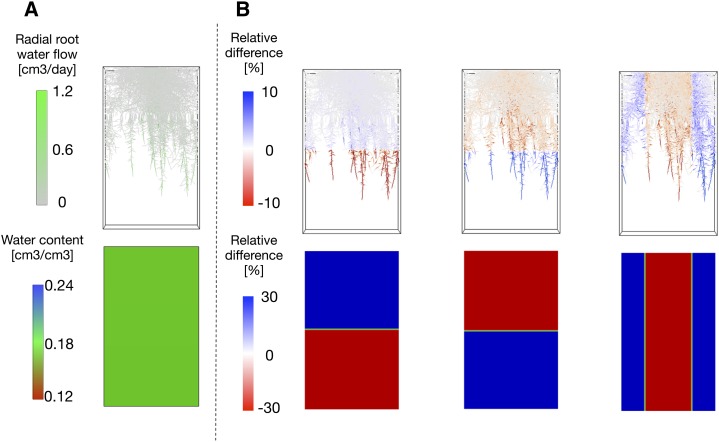
The above statement that the distribution of water uptake among root segments should be predictable from the sole root hydraulic architecture is only valid under conditions of uniform soil water potential that are generally encountered in well-watered soils (Doussan et al., 1998). Under heterogeneous conditions, at places where the soil water potential is low, soil capillary forces retain water more strongly in the remaining fraction of the soil porosity, comprised of small micropores. As this reduces the soil hydraulic conductivity, the flow of water toward the root surface is locally restricted, and water uptake by other root segments, located in portions of the soil where water is more readily available, should increase to maintain the global transpiration stream. This passive adjustment of the distribution of water uptake among root segments occurring as a consequence of the heterogeneity of soil water potential (Fig. 3) and conductivity was called compensatory root water uptake (Jarvis, 1976; Šimůnek and Hopmans, 2009). When compensation occurs, the root distribution becomes a very poor indicator of the distribution of the uptake sites, as root length density and uptake profiles become dissimilar (Javaux et al., 2013). Couvreur et al. (2012) recently highlighted that the compensatory uptake can be formulated as the product of three terms, the standard uptake fraction (see above), the difference between the local and spatially averaged soil water potential, and the root system conductance, which suggests that, in addition to defining the standard sites of water uptake, the root hydraulic architecture simultaneously contributes to the adjustment of the uptake to the soil water potential distribution and influences soil water potential heterogeneity. Interestingly, simulation studies indicate that compensatory root water uptake precedes the moment where transpiration is affected (Couvreur et al., 2012). All these results converge to a contribution of compensatory root water uptake to the maintenance of transpiration and assimilation.
Figure 3.
Influence of the soil water potential distribution on the water uptake process. The model (Javaux et al., 2008) was used to simulate the root radial water flow under different soil water potential distribution. A, Radial water flow (top) under hydrostatic equilibrium (bottom). B, Compensatory root water uptake (top) for different soil water potential distributions (bottom). Relative units compared with A.
A particular scenario of soil water redistribution involving the root hydraulic architecture can occur under low or negligible transpiration flow. In such conditions, the xylem water potential is a weighted value of the soil water potentials sensed by root segments, intermediate between the soil water potential of the driest and wettest soil parts in contact with roots. As long as root segments are radially conductive to water, the root system offers a long-distance path of low hydraulic resistance that allows the hydraulic lift phenomenon, whereby soil water is redistributed through the root system from the wetter soil regions toward the drier ones. This phenomenon, which has long been a matter of debate, would contribute to the night restoration of the soil hydraulic conductivity that decreased around part of the root system as a result of root water uptake during the day (McMichael and Lascano, 2010).
Other factors that reduce the soil hydraulic conductivity have been recently demonstrated. Following the mass conservation principle, the flux density of water (motion speed) increases as it gets closer to the root surface, and, in parallel, its water potential decreases as well as the soil conductivity. The rhizosphere is thus susceptible to a local drop of hydraulic conductivity that is favored by high rates of root water uptake and by soil properties, such as coarse textures, that steepen the relationship between soil conductivity and water potential (Shroeder et al., 2008). Soil hydraulic properties and water potential around each root segment therefore set a maximum uptake rate above which a soil restriction to water flow is likely to occur. Interestingly, this phenomenon would be difficult to distinguish from the limitation imposed by root hydraulic properties that is observed under drought (Schoppach et al., 2013).
The specific hydraulic properties of the rhizosphere have been reviewed recently (Carminati and Vetterlein, 2013). Strikingly, its complex constitution seems to generate hydrophilic or hydrophobic behaviors depending on the environmental conditions (Carminati et al., 2011; Moradi et al., 2012). The role of this plasticity is not yet fully understood but is proposed to participate in the control of the soil conductivity by the roots themselves, which would add a level of complexity in our model of the regulation of water uptake.
MODELING CAN HELP EXPLAIN THE DYNAMICS OF ROOT WATER UPTAKE
Despite the fact that water uptake follows simple rules of passive flow driven by water potential gradients and following paths of lowest resistance, and despite our knowledge of the main paths and factors affecting their conductivities, our understanding of water uptake at the plant and seasonal scale remains limited by the difficulties in integrating those interacting paths and factors, at the appropriate scales and in a spatial and temporal framework. Many of those factors have been evoked in the above sections, but many others have been deliberately set aside, such as the feedback effect of water uptake on root growth via its effects on, for example, assimilation and soil mechanical impedance. Because direct experimental observations are necessarily capturing limited aspects of water uptake, systems approaches gained much interest in the last decade (Dunbabin et al., 2013; Hill et al., 2013).
Doussan et al. (2006) presented the first model that simulates water flows explicitly in the soil-root continuum. Using the concept of hydraulic architecture to solve plant water flow (Doussan et al., 1998) and Richards equations to solve water flow in unsaturated soils, this model was able to simulate compensatory uptake and hydraulic lift in heterogeneous soil conditions. A very similar approach was taken by Javaux et al. (2008) to implement the soil-root hydraulic model R-SWMS. Using the model R-SWMS, Schroeder et al. (2009) illustrated the negative impact of local conductivity drops around roots in drying soils on the water uptake process. The importance of the ratio between axial and radial root conductivities and of the soil type was also highlighted (Draye et al., 2010). On the soil side, the model can be instrumental to investigate the influence of the root water uptake on water flow and nutrient transport in the surrounding soil (Schroeder et al., 2012). Recently, it was used to assess the impact of salinity on the plant transpiration reduction (Schroeder et al., 2013). To streamline the adoption of these tools by the plant science community, Couvreur et al. (2012) proposed a simplified version of R-SWMS that can be used at the crop level but still relies on a precise parameterization of root hydraulic architecture. This simplified model has also been shown to simulate behaviors such as compensatory uptake and hydraulic lift from hydraulic principles (Javaux et al., 2013).
METHODS TO INVESTIGATE ROOT WATER UPTAKE DYNAMICS
The development of measurement techniques and observation methods has been instrumental in many recent advance of our understanding of root water uptake dynamics. While traditional methods to investigate either plant or soil properties are mainly used at the plant scale, new techniques have empowered a more detailed approach of the system, down to the centimeter scale.
Several two- and three-dimensional observation methods have been developed that enable better or faster characterization of root system architecture. Pouches dipping in nutrient solution are becoming increasingly popular to screen early stages of root systems development in two dimensions (Hund et al., 2009). Recently, a scanning technique has been proposed for digitizing entire root systems of plants grown in rhizoboxes (Lobet and Draye, 2013). The two-dimensional restriction of pouches and rhizotrons was recently released by stereo imaging of root systems grown in tubes filled with gellan gum (Iyer-Pascuzzi et al., 2010; Clark et al., 2011). Lastly, x-ray computed tomography (Mooney et al., 2012) or magnetic resonance imaging (Jahnke et al., 2009), widely used in medical sciences, is now entering the plant research domain. These allow the three-dimensional noninvasive monitoring of root growth in realistic soil cores and, in the future, should provide many details on the precise soil conditions around individual root segments, including soil water content.
Following the development of these observation techniques, specific free software solutions were developed for the analysis of root system architecture and root anatomy. For example, RootNav (Pound et al., 2013), SmartRoot (Lobet et al., 2011), RootReader2D (Clark et al., 2013), EZ-Rhizo (Armengaud et al., 2009), and Root System Analyzer (Leitner et al., 2014) were developed for the analysis of two-dimensional root images, while RooTrak (Mairhofer et al., 2013) and RootReader3D (Clark et al., 2011) were designed for the analysis of stereo images. These tools ease the digitizing and analysis of complex root system architecture. At the organ scale, RootScan (Burton et al., 2012) was developed for the high-throughput analysis of the anatomy of root sections. The software automatically computes the area of multiple root tissues including the aerenchyma or the xylem vessels. These tools have been recently included on the Plant Image Analysis database (http://www.plant-image-analysis.org; Lobet et al., 2013).
The quantification of root hydraulic properties remains certainly one of the biggest challenges. Techniques suitable for global measurements have been established for many years. The pressure chamber is widely used and estimates the conductance from the measurement of the water flow induced by a known pressure differential. Other techniques estimate the conductance of individual root segments, yet remain extremely time consuming (e.g. pressure clamp [Bramley et al., 2007] and pressure probe [Steudle and Peterson, 1998]). Part of the challenge lies in the plasticity of root hydraulic properties as a function of segment type and age and environmental conditions and in the variability between measurement methods (Bramley et al., 2007).
On the opposite, an array of techniques is available to monitor soil water content in one, two, and even three dimensions. This includes time domain reflectometry (Robinson et al., 2003; Walker et al., 2004), electrical resistance tomography (Vanderborght et al., 2005; Cassiani et al., 2006; Beff et al., 2013), or, more recently, ground-penetrating radar (Lambot et al., 2008). The spatial resolution of these techniques ranges in the decimeter scale and is appropriate to study the distribution of water in rows or interrows. Recently, two techniques have been successfully tested for the observation of water flow down to the centimeter level. Light transmission imaging can be used to finely map changes in soil water content in transparent rhizotrons (Garrigues et al., 2006). Unfortunately, the technique is restricted to a specific type of substrate (white sand) and does not estimate water uptake by individual roots due to the unknown redistribution of the water in the substrate (Javaux et al., 2008). More recently, the use of neutron radiography (Esser et al., 2010) that is not bound to any specific type of substrate has been used to investigate water movement and determine water uptake sites in lupin (Lupinus albus) root systems. Using D2O injection in combination with a convection-diffusion model, water uptake by individual segments could be quantified in a complete root system (Zarebanadkouki et al., 2013). This technical evolution is therefore promising new insights on the water dynamics at smaller scales, while systems analysis frameworks will help to integrate this information.
CONCLUSION
The determinants of water flow through the soil-root system are well known and have been largely studied individually. However, their integration at the plant and canopy scales and over a whole crop cycle remains very limited. The spatial and temporal heterogeneity of the soil, the interactions between the soil and the root at multiple scales, and the need to combine very different disciplines makes this integration particularly difficult. With the development of functional-structural soil-plant models, root systems biology is bringing novel analytical tools to turn a vast amount of data into biological questions crossing scales and disciplines. We believe that new root system ideotypes could emerge from a more comprehensive and quantitative consideration of the many determinants of water flow during a whole crop cycle and in the framework of a cost-benefit analysis at the system level.
Footnotes
This work was supported by the Communauté Française de Belgique (Actions de Recherche Concertées, grant no. 11/16–036 to X.D.), the Belgian Science Policy Interuniversity Attraction Poles Program (grant no. P7/29 to G.L. and X.D.), the Fonds National Belge de la Recherche Scientifique (to F.M. and V.C.), the Belgian American Educational Foundation and the Wallonie-Bruxelles International (to V.C.), and the European Community’s Seventh Framework Programme (under the grand agreement no. FP7–244374; Drought Tolerant Yielding Plants [DROPs]).
References
- Alsina MM, Smart DR, Bauerle T, de Herralde F, Biel C, Stockert C, Negron C, Save R. (2011) Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J Exp Bot 62: 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A. (2009) EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture. Plant J 57: 945–956 [DOI] [PubMed] [Google Scholar]
- Beff L, Günther T, Vandoorne B, Couvreur V, Javaux M. (2013) Three-dimensional monitoring of soil water content in a maize field using electrical resistivity tomography. Hydrol Earth Syst Sci 17: 595–609 [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62: 59–68 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C. (2008) Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56: 207–218 [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner DW, Tyerman SD, Turner NC. (2007) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Adv Agron 96: 133–196 [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150: 348–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. (2010) The contrasting influence of short-term hypoxia on the hydraulic properties of cells and roots of wheat and lupin. Funct Plant Biol 37: 183–193 [Google Scholar]
- Burton AL, Brown KM, Lynch JP. (2013) Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Sci 53: 1042–1055 [Google Scholar]
- Burton AL, Williams M, Lynch JP, Brown KM. (2012) RootScan: software for high-throughput analysis of root anatomical traits. Plant Soil 357: 189–203 [Google Scholar]
- Carminati A, Schneider CL, Moradi AB, Zarebanadkouki M, Vetterlein D, Vogel J, Hildebrandt A, Weller U, Schueler L, Oswald SE. (2011) How the rhizosphere may favor water availability to roots. Vadose Zone J 10: 988–998 [Google Scholar]
- Carminati A, Vetterlein D. (2013) Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Ann Bot (Lond) 112: 277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiani G, Bruno V, Villa A, Fusi N. (2006) A saline trace test monitored via time-lapse surface electrical resistivity tomography. J Appl Geophys 59: 244–259 [Google Scholar]
- Chaumont F, Tyerman SD. (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164: 000–000 (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman MA, Sperry JS, Smith DD. (2012) Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. New Phytol 193: 713–720 [DOI] [PubMed] [Google Scholar]
- Clark RT, Famoso AN, Zhao K, Shaff JE, Craft EJ, Bustamante CD, McCouch SR, Aneshansley DJ, Kochian LV. (2013) High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development. Plant Cell Environ 36: 454–466 [DOI] [PubMed] [Google Scholar]
- Clark RT, MacCurdy RB, Jung JK, Shaff JE, McCouch SR, Aneshansley DJ, Kochian LV. (2011) Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiol 156: 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Barigah ST, Kleinhentz M, Eshel A. (2008) Is xylem cavitation resistance a relevant criterion for screening drought resistance among Prunus species? J Plant Physiol 165: 976–982 [DOI] [PubMed] [Google Scholar]
- Cochard H, Casella E, Mencuccini M. (2007a) Xylem vulnerability to cavitation varies among poplar and willow clones and correlates with yield. Tree Physiol 27: 1761–1767 [DOI] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. (2007b) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VM, Byrne PF, Dierig DA. (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois B, Audebert A, Dardou A, Roques S, Ghneim-Herrera T, Droc G, Frouin J, Rouan L, Gozé E, Kilian A, et al. (2013) Genome-wide association mapping of root traits in a japonica rice panel. PLoS ONE 8: e78037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur V (2013) Horizontal soil water potential heterogeneity: simplifying approaches for crop water dynamics models. In Emergent Properties of Plant Hydraulic Architecture a Modelling Study. Université Catholique de Louvain, Louvain-la-Neuve, Belgium, pp 57–102 [Google Scholar]
- Couvreur V, Vandenborg J, Javaux M. (2012) A simple three-dimensional macroscopic root water uptake. Hydrol Earth Syst Sci 16: 2957–2971 [Google Scholar]
- Dai X, Wang Y, Yang A, Zhang WH. (2012) OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiol 159: 169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S, Douthe C, Sala A, Cochard H. (2010) Mechanism of water-stress induced cavitation in conifers: bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant Cell Environ 33: 2101–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domec JC, Lachenbruch B, Meinzer FC. (2006) Bordered pit structure and function determine spatial patterns of air-seeding thresholds in xylem of Douglas-fir (Pseudotsuga menziesii; Pinaceae) trees. Am J Bot 93: 1588–1600 [DOI] [PubMed] [Google Scholar]
- Doussan C, Pagès L, Vercambre G. (1998) Modelling of the hydraulic architecture of root systems: an integrated approach to water absorption—model description. Ann Bot (Lond) 81: 213–223 [Google Scholar]
- Doussan C, Pierret A, Garrigues E, Pagès L. (2006) Water uptake by plant roots: II—Modelling of water transfer in the soil root-system with explicit account of flow within the root system—Comparison with experiments. Plant Soil 283: 99–117 [Google Scholar]
- Draye X, Kim Y, Lobet G, Javaux M. (2010) Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J Exp Bot 61: 2145–2155 [DOI] [PubMed] [Google Scholar]
- Dunbabin VM, Postma JA, Schnepf A, Pagès L, Javaux M, Wu L, Leitner D, Chen YL, Rengel Z, Diggle AJ. (2013) Modelling root–soil interactions using three–dimensional models of root growth, architecture and function. Plant Soil 372: 93–124 [Google Scholar]
- Enstone DE, Peterson CA. (2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ 28: 444–455 [Google Scholar]
- Enstone DE, Peterson CA, Ma F. (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21: 335–351 [Google Scholar]
- Esser HG, Carminati A, Vontobel P, Lehmann EH, Oswald SE. (2010) Neutron radiography and tomography of water distribution in the root zone. J Plant Nutr Soil Sci 173: 757–764 [Google Scholar]
- Fan M, Bai R, Zhao X, Zhang J. (2007) Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. J Integr Plant Biol 49: 598–604 [Google Scholar]
- Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C. (2013) Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23: 2044–2050 [DOI] [PubMed] [Google Scholar]
- Garrigues E, Doussan C, Pierret A. (2006) Water uptake by plant roots: I— Formation and propagation of a water extraction front in mature root systems as evidenced by 2D light transmission imaging. Plant Soil 283: 83–98 [Google Scholar]
- Gebauer R, Volařík D. (2012) Root hydraulic conductivity and vessel structure modification with increasing soil depth of two oak species: Quercus pubescens and Quercus robur. Trees (Berl) 27: 523–531 [Google Scholar]
- Giehl RFH, Gruber BD, von Wirén N. (2013) It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot 65: 769–778 [DOI] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von Wirén N. (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163: 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol Biol 62: 305–323 [DOI] [PubMed] [Google Scholar]
- Hachez C, Veselov D, Ye Q, Reinhardt H, Knipfer T, Fricke W, Chaumont F. (2012) Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ 35: 185–198 [DOI] [PubMed] [Google Scholar]
- Hammer G, Dong Z, Mclean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M. (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Sci 49: 299–312 [Google Scholar]
- Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC. (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. J Exp Bot 61: 2185–2202 [DOI] [PubMed] [Google Scholar]
- Herbette S, Cochard H. (2010) Calcium is a major determinant of xylem vulnerability to cavitation. Plant Physiol 153: 1932–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Porco S, Lobet G, Zappala S, Mooney S, Draye X, Bennett MJ. (2013) Root systems biology: bridging regulatory networks to rhizosphere-scale processes. Plant Physiol 163: 1487–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund A, Trachsel S, Stamp P. (2009) Growth of axile and lateral roots of maize: I. Development of a phenotying platform. Plant Soil 325: 335–349 [Google Scholar]
- Iwata S, Miyazawa Y, Fujii N, Takahashi H. (2013) MIZ1-regulated hydrotropism functions in the growth and survival of Arabidopsis thaliana under natural conditions. Ann Bot (Lond) 112: 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer-Pascuzzi AS, Symonova O, Mileyko Y, Hao Y, Belcher H, Harer J, Weitz JS, Benfey PN. (2010) Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol 152: 1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke S, Menzel MI, van Dusschoten D, Roeb GW, Bühler J, Minwuyelet S, Blümler P, Temperton VM, Hombach T, Streun M, et al. (2009) Combined MRI-PET dissects dynamic changes in plant structures and functions. Plant J 59: 634–644 [DOI] [PubMed] [Google Scholar]
- Jarvis PG. (1976) The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos Trans R Soc Lond B Biol Sci 273: 593–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaux M, Couvreur V, Vanderborght J. (2013) Root water uptake: from three-dimensional biophysical processes to macroscopic modeling approaches. Vadose Zone J 12: 1–16 [Google Scholar]
- Javaux M, Schroeder T, Vanderborght J, Vereecken H. (2008) Use of a three-dimensional detailed modeling approach for predicting root water uptake. Vadose Zone J 7: 1079–1088 [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D, et al. (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. (2012) Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant Cell Environ 35: 760–769 [DOI] [PubMed] [Google Scholar]
- Kellermeier F, Chardon F, Amtmann A. (2013) Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol 161: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Besse M, Verdeil JL, Fricke W. (2011) Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J Exp Bot 62: 4115–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambot S, Binley A, Slob E, Hubbard S. (2008) Ground penetrating radar in hydrogeophysics. Vadose Zone J 7: 137–139 [Google Scholar]
- Lamy JB, Delzon S, Bouche PS, Alia R, Vendramin GG, Cochard H, Plomion C. (2014) Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytol 201: 874–88 [DOI] [PubMed] [Google Scholar]
- Landi P, Giuliani S, Salvi S, Ferri M, Tuberosa R, Sanguineti MC. (2010) Characterization of root-yield-1.06, a major constitutive QTL for root and agronomic traits in maize across water regimes. J Exp Bot 61: 3553–3562 [DOI] [PubMed] [Google Scholar]
- Leitner D, Felderer B, Vontobel P, Schnepf A. (2014) Recovering root system traits using image analysis exemplified by two-dimensional neutron radiography images of lupine. Plant Physiol 164: 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenochova Z, Soukup A, Votrubova O. (2009) Aerenchyma formation in maize roots. Biol Plant 53: 263–270 [Google Scholar]
- Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu DT, Maurel C, Lin J. (2011) Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 23: 3780–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sperry JS, Shao M. (2009) Hydraulic conductance and vulnerability to cavitation in corn (Zea mays L.) hybrids of differing drought resistance. Environ Exp Bot 66: 341–346 [Google Scholar]
- Lobet G, Draye X. (2013) Novel scanning procedure enabling the vectorization of entire rhizotron-grown root systems. Plant Methods 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G, Draye X, Périlleux C. (2013) An online database for plant image analysis software tools. Plant Methods 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X. (2011) A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol 157: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot (Lond) 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairhofer S, Zappala S, Tracy S, Sturrock C, Bennett MJ, Mooney SJ, Pridmore TP. (2013) Recovering complete plant root system architectures from soil via X-ray μ-computed tomography. Plant Methods 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschadi AM, Christopher JT, Hammer GL, deVoil P. (2010) Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosys 144: 458–462 [Google Scholar]
- Maurel C, Chrispeels MJ. (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloh K, Sperry JS, Lachenbruch B, Meinzer FC, Reich PB, Voelker S. (2010) Moving water well: comparing hydraulic efficiency in twigs and trunks of coniferous, ring-porous, and diffuse-porous saplings from temperate and tropical forests. New Phytol 186: 439–450 [DOI] [PubMed] [Google Scholar]
- McMichael BL, Lascano RJ. (2010) Evaluation of hydraulic lift in cotton (Gossypium hirsutum L.) germplasm. Environ Exp Bot 68: 26–30 [Google Scholar]
- Miura K, Lee J, Gong Q, Ma S, Jin JB, Yoo CY, Miura T, Sato A, Bohnert HJ, Hasegawa PM. (2011) SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiol 155: 1000–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, Raghothama KG, Baek D, Koo YD, Jin JB, Bressan RA, et al. (2005) The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA 102: 7760–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ. (2012) Developing X-ray computed tomography to non-invasively image 3-D root systems architecture in soil. Plant Soil 352: 1–22 [Google Scholar]
- Moradi AB, Carminati A, Lamparter A, Woche SK, Bachmann J, Vetterlein D, Vogel HJ, Oswald SE. (2012) Is the rhizosphere temporarily water repellent? Vadose Zone J https://www.soils.org/publications/vzj/abstracts/11/3/vzj2011.0120 [Google Scholar]
- Moriwaki T, Moriwaki T, Miyazawa Y, Miyazawa Y, Fujii N, Fujii N, Takahashi H, Takahashi H. (2014) Plant science. Plant Sci 215–216: 141–149 [Google Scholar]
- Pagès L, Serra V, Draye X, Doussan C, Pierret A. (2010) Estimating root elongation rates from morphological measurements of the root tip. Plant Soil 328: 35–44 [Google Scholar]
- Passioura JB. (1984) Roots and water economy of wheat. In W. Day, R.K. Atkin, eds, Wheat Growth and Modelling, NATO ASI Series A Life Science, Plenum, New York 86: 185–198 [Google Scholar]
- Passioura JB. (2012) Phenotyping for drought tolerance in grain crops: when is it useful to breeders? Funct Plant Biol 39: 851. [DOI] [PubMed] [Google Scholar]
- Pinheiro HA, Damatta FM, Chaves ARM, Loureiro ME, Ducatti C. (2005) Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Ann Bot (Lond) 96: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T. (2013) RootNav: navigating images of complex root architectures. Plant Physiol 162: 1802–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman R, Zaman-Allah M, Mallikarjuna N, Pannirselvam R, Krishnamurthy L, Gowda CLL. (2013) Root anatomical traits and their possible contribution to drought tolerance in grain legumes. Plant Prod Sci 16: 1–8 [Google Scholar]
- Rewald B, Ephrath JE, Rachmilevitch S. (2011) A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell Environ 34: 33–42 [DOI] [PubMed] [Google Scholar]
- Robinson DA, Jones SB, Wraith JM, Or D, Friedman SP. (2003) A review of advances in dielectric and electrical conductivity measurement in soils using time domain reflectometry. Vadose Zone J 2: 444–475 [Google Scholar]
- Schoppach RM, Wauthelet D, Jeanguenin L, Sadok W. (2013) Conservative water use under high evaporative demand associated with smaller root metaxylem and limited trans-membrane water transport in wheat. Funct Plant Biol 41: 257–269 [DOI] [PubMed] [Google Scholar]
- Schroeder N, Javaux M, Vanderborght J, Steffen B, Vereecken H (2012) Effect of root water and solute uptake on apparent soil dispersivity: a simulation study. Vadoze Zone J 11: 1–14 [Google Scholar]
- Schroeder N, Lazarovitch N, Vanderborght J, Vereecken H, Javaux M. (2013) Linking transpiration reduction to rhizosphere salinity using a 3D coupled soil-plant model. Plant Soil http://dx.doi.org/10.1007/s11104-013-1990-8 [Google Scholar]
- Schroeder T, Tang L, Javaux M, Vanderborght J, Koerfgen B, Vereecken H. (2009) A grid refinement approach for a three-dimensional soil-root water transfer model. Water Resour Res 45: W10412 [Google Scholar]
- Shroeder T, Javaux M, Vanderborght J, Koerfgen B, Vereecken H. (2008) Effect of local soil hydraulic conductivity drop using a three-dimensional root water uptake model. Vadose Zone J 7: 1089–1098 [Google Scholar]
- Šimůnek J, Hopmans JW. (2009) Modeling compensated root water and nutrient uptake. Ecol Modell 220: 505–521 [Google Scholar]
- Steele KA, Price AH, Witcombe JR, Shrestha R, Singh BN, Gibbons JM, Virk DS. (2013) QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theor Appl Genet 126: 101–108 [DOI] [PubMed] [Google Scholar]
- Steudle E. (2000) Water uptake by plant roots: an integration of views. Plant Soil 226: 45–56 [Google Scholar]
- Steudle E. (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 847–875 [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. (1998) How does water get through roots? J Exp Bot 49: 775–788 [Google Scholar]
- Sutka M, Li G, Boudet J, Boursiac Y, Doumas P, Maurel C. (2011) Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol 155: 1264–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F. (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63: 25–31 [DOI] [PubMed] [Google Scholar]
- Tombesi S, Johnson RS, Day KR, DeJong TM. (2010) Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann Bot (Lond) 105: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M. (2008) QTLs underlying natural variation in stele and xylem structures of rice root. Breed Sci 58: 7–14 [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Vadez V, Rao JS, Bhatnagar-Mathur P, Sharma KK. (2013) DREB1A promotes root development in deep soil layers and increases water extraction under water stress in groundnut. Plant Biol (Stuttg) 15: 45–52 [DOI] [PubMed] [Google Scholar]
- Vadez V, Soltani A, Sinclair TR. (2012) Modelling possible benefits of root related traits to enhance terminal drought adaptation of chickpea. Field Crops Res 137: 108–115 [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149: 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderborght J, Kemna A, Hardelauf H. (2005) Potential of electrical resistivity tomography to infer aquifer transport characteristics from tracer studies: a synthetic case study. Water Resour Res 41: 1–23 [Google Scholar]
- Vercambre G, Doussan C, Pagès L, Habib R, Pierret A. (2002) Influence of xylem development on axial hydraulic conductance within Prunus root systems. Trees (Berl) 16: 479–487 [Google Scholar]
- Walker JP, Willgoose GR, Kalma JD. (2004) In situ measurement of soil moisture: a comparison of techniques. J Hydrol (Amst) 293: 85–99 [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SVS, Rebetzke GJ, Kirkegaard JA, Christopher JT, Watt M. (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63: 3485–3498 [DOI] [PubMed] [Google Scholar]
- Watt M, Magee LJ, McCully ME. (2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytol 178: 135–146 [DOI] [PubMed] [Google Scholar]
- Yang X, Li Y, Ren B, Ding L, Gao C, Shen Q, Guo S. (2012) Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol 53: 495–504 [DOI] [PubMed] [Google Scholar]
- Zaman-Allah M, Jenkinson DM, Vadez V. (2011a) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J Exp Bot 62: 4239–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman-Allah M, Jenkinson DM, Vadez V. (2011b) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Funct Plant Biol 38: 270–281 [DOI] [PubMed] [Google Scholar]
- Zarebanadkouki M, Kim YX, Carminati A. (2013) Where do roots take up water? Neutron radiography of water flow into the roots of transpiring plants growing in soil. New Phytol 199: 1034–1044 [DOI] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP. (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zufferey V, Cochard H, Améglio T, Spring JL, Viret O. (2011) Diurnal cycles of embolism formation and repair in petioles of grapevine (Vitis vinifera cv. Chasselas). J Exp Bot 62: 3885–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki MA, Holbrook NM. (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534 [DOI] [PubMed] [Google Scholar]