Reactive oxygen metabolism affects physiology during drought, with implications for the potential roles of antioxidant systems in restricting oxidative stress and in transmitting oxidative stress signals in these conditions.
Abstract
Drought is considered to cause oxidative stress, but the roles of oxidant-induced modifications in plant responses to water deficit remain obscure. Key unknowns are the roles of reactive oxygen species (ROS) produced at specific intracellular or apoplastic sites and the interactions between the complex, networking antioxidative systems in restricting ROS accumulation or in redox signal transmission. This Update discusses the physiological aspects of ROS production during drought, and analyzes the relationship between oxidative stress and drought from different but complementary perspectives. We ask to what extent redox changes are involved in plant drought responses and discuss the roles that different ROS-generating processes may play. Our discussion emphasizes the complexity and the specificity of antioxidant systems, and the likely importance of thiol systems in drought-induced redox signaling. We identify candidate drought-responsive redox-associated genes and analyze the potential importance of different metabolic pathways in drought-associated oxidative stress signaling.
Suboptimal water availability is a major factor limiting plant growth and performance. The ability of plants to acclimate to such conditions through appropriate signaling is a key determinant of survival, and hence identification of the genes involved is a major interest of plant scientists (Claeys and Inzé, 2013). Research in recent years has clearly demonstrated that plant responses to stress rely on the functioning of complex gene networks. Oxidative signaling is now considered to be a key element of these networks, underpinning cross tolerance responses to stress and leading not only to defense but also to regulation of growth. Although the importance of redox regulation in linking the fundamental energetic processes of the cell to developmental regulation required for stress survival has become increasingly accepted, some stresses may depend on redox processes to a greater degree than others. Drought is now widely considered to induce oxidative stress. This implies that like other environmental stresses, limited water availability favors a shift in the balance between reactive oxygen species (ROS) production and their elimination. It is generally assumed that this means an increase in the levels of ROS such as hydrogen peroxide (H2O2) and singlet oxygen (de Carvalho, 2013), motivating many authors to attempt to measure these compounds. In addition, many embedded notions continue to underpin and drive research, for example, on the importance of H2O2 generated in the chloroplast during drought. Although rarely acknowledged, uncertainty remains over the accuracy of ROS measurements, the relative importance of each ROS form, and the subcellular localization of ROS production in relation to the redox-dependent signaling pathways that may contribute to acclimation and drought tolerance. Moreover, the effects of ROS are often viewed independently from their interactions with the antioxidative machinery, with the role of the latter being restricted to that of elimination (negative control) of ROS. Few authors acknowledge that effective ROS signaling may require increased flux through antioxidative components, notably those that are thiol dependent, as we discuss further below.
A substantial body of literature concerns the importance of oxidative stress in plant drought responses, ranging from oxidative damage to the role of ROS in local and systemic signaling (for review, see Smirnoff, 1993; Miller et al., 2010; de Carvalho, 2013). Despite this information leading to apparently robust concepts, no simple picture emerges from the data and there is wide variation in effects reported both for oxidant production and for antioxidant responses. It is therefore opportune to examine what appear to be increasingly complex roles of ROS and related redox processes in drought responses. Our aim in this Update is to critically examine the extent to which oxidative stress and related redox signaling are crucial factors in plant responses to this challenging condition. To this end, we provide an overview of the sources of ROS as well as the antioxidative systems that limit or process these signals, and we present a meta-analysis of transcriptomic data to scrutinize the importance and specificity of redox changes and components during drought.
HORMONES AND ROS
It is becoming increasingly clear that ROS and antioxidants exert many of their effects through the redox-dependent regulation of components of hormone signaling. For example, H2O2-mediated control of auxin, salicylic acid, and jasmonate responses is probably mediated at least in part by thiol regulation linked to the glutathione pool (Han et al., 2013a, 2013b; Gao et al., 2014). Although thiol-dependent processes are clearly important in the control of growth linked to auxins and strigonolactones (Marquez-Garcia et al., 2014), relatively little is known about interactions between redox processes and hormones in the control of growth during drought (Claeys and Inzé, 2013).
Salt stress may restrict plant growth through decreased GAs and increased accumulation of the DELLA proteins, which are repressors of GA signaling (Achard et al., 2008). Mutants multiply deficient in DELLA proteins showed compromised tolerance to salt stress (Achard et al., 2008). This effect was linked to redox processes, although the details remain to be elucidated. Enhanced drought tolerance in the spindly3 (spy3) mutant, which has compromised activity of an O-linked GlcNAc transferase activity that antagonizes GA signaling, was linked to up-regulation of GA-related gene expression as well as decreased cytokinin signaling (Qin et al., 2011). Whether the drought tolerance of spy mutants is exclusively related to GA signaling is unclear, because the transferase activity that is affected in this line may modify proteins involved in several pathways (Qin et al., 2011). In addition to abscisic acid (ABA) and GA, other hormones are integrated with ROS signaling in the drought response. Overexpression of an H2O2-induced UDP-glucosyl transferase with indole-3-butyric acid glycosylation activity modified the concentrations of different auxins in planta and led to altered root architecture and increased tolerance to drought and salt stress (Tognetti et al., 2010).
INCREASED ROS PRODUCTION IN PHOTOSYNTHESIS DURING DROUGHT
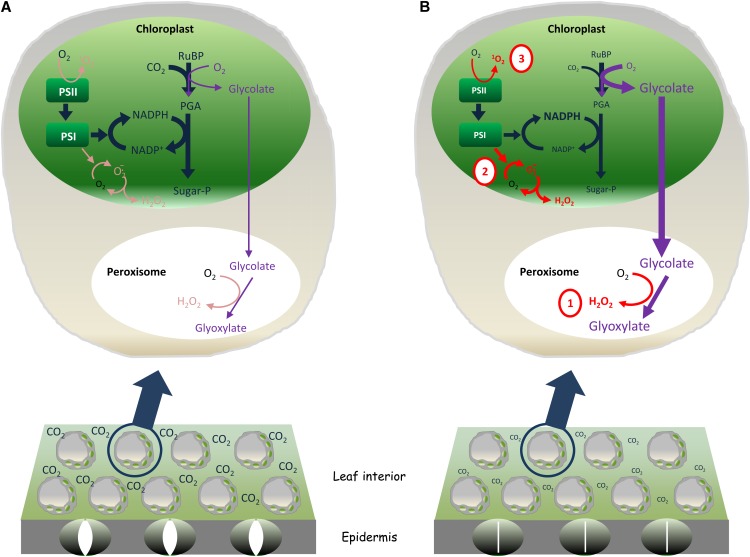
Much of the work on redox changes triggered by drought has focused on shoots. According to the dominant view of drought-induced oxidative stress in these organs, ROS production is increased by redox changes associated with photosynthesis. Even under optimal conditions, ROS can be produced at considerable rates inside the cell as part of metabolism (Foyer and Noctor, 2003). In terms of production of H2O2, the most stable of the major ROS species, the chloroplast continues to receive the most attention; however, in many conditions in C3 plants, the rate of H2O2 production may actually be higher in the peroxisomes (Noctor et al., 2002). Peroxisomal H2O2 production in the green tissues of C3 plants is largely a result of the activity of glycolate oxidase. This enzyme is an essential part of the photorespiratory recycling pathway that is initiated by oxygenation of ribulose-1,5-bisphosphate (RuBP) in the chloroplast (Foyer et al., 2009). Glycolate production is considered to be accelerated during drought as intercellular CO2 drops as a result of drought-induced stomatal closure. This favors RuBP oxygenation (Cornic and Briantais, 1991) and hence increased peroxisomal H2O2 production (Fig. 1B, site 1).
Figure 1.
Current concepts of how drought increases the generation of ROS in photosynthesis. A, Cartoon of leaf section in well-watered plants in which relatively high intercellular CO2 concentrations (Ci) allow efficient regeneration of terminal oxidants and limit RuBP oxygenation. B, Drought-induced stomatal closure restricts CO2 uptake, favoring photorespiratory production of H2O2 in the peroxisome (1) and possibly favoring production of superoxide and H2O2 (2) or singlet oxygen (3) by the photosynthetic electron transport chain. PGA, 3-Phosphoglyceric acid. [See online article for color version of this figure.]
In the chloroplast, restrictions over reductant and ATP consumption during drought may also favor ROS production at two distinct sites within the electron transport chain. First, decreased availability of other oxidants for the chain may promote electron flow to O2 in the Mehler reaction, thus stimulating superoxide and H2O2 production and accelerating the water-water cycle (Asada, 2006; Figure 1B, site 2). Second, any overreduction of the electron transport chain is expected to enhance the probability of singlet oxygen generation in PSII (Fischer et al., 2013; Figure 1B, site 3).
INTERACTIONS BETWEEN PHOTOSYNTHETIC ROS-PRODUCING PATHWAYS
The commonly considered sources of ROS shown in Figure 1 potentially allow specificity because of their differences in chemical nature or location. Although drought may stimulate all three sources simultaneously, and ROS are frequently associated with damage, at least some of these pathways may act rather as damage limitation (protective) processes. First, although increased photorespiration will promote H2O2 generation in the peroxisomes, it is also likely to limit chloroplast ROS generation and photoinhibition (Osmond and Grace, 1995). This is because RuBP oxygenation maintains production of 3-phosphoglycerate, thereby sustaining the reductive phase of the Benson-Calvin cycle. Together with the reassimilation of photorespiratory ammonia, this allows metabolism to continue to consume the products of the electron transport chain (ferredoxin/NADPH) and the proton gradient that is concomitantly generated (ATP; Foyer et al., 2012). Another accepted concept is that accelerated water-water cycle activity also has a dissipative function as it consumes electrons and thereby reoxidizes the electron transport chain (Osmond and Grace, 1995; Foyer et al., 2012). According to this view, production of superoxide and H2O2 at both sites 1 and 2 is associated with limitation of the accumulation of reduced intermediates and hence a decrease in the probability of singlet oxygen generation at site 3 (Fig. 1). These energy-dissipative functions may be important because singlet oxygen is a powerful oxidant and signaling molecule that probably accounts for the vast majority of damage measured as lipid peroxidation in chloroplasts (Triantaphylidès et al., 2008). However, although photorespiration undoubtedly has a dissipative role by consuming both reductant and ATP, any such role for the water-water cycle must take into account the often overlooked notion of photosynthetic control.
COUPLING IN CHLOROPLASTS: DO NOT FORGET THE PROTONS
A predominant concept is that drought, like some other stresses, favors electron flux to O2 based on the notion that the regeneration of NADP+ cannot keep pace with NADPH production (Fig. 1, site 2). However, any marked increase in the NADPH:NADP+ ratio will have profound effects on the regulation of photosynthesis because of the obligatory coupling of electron transport to ATP synthesis (Foyer et al., 2012). In terms of the sustainability of alternative electron flow, a key point is the average turnover times of ATP and NADPH in the chloroplast stroma, which do not exceed a few seconds at moderate to high rates of steady-state photosynthesis (Noctor and Foyer, 2000). In addition to any promotion of the Mehler reaction, overreduction of the stroma will favor engagement of other ATP-generating processes such as cyclic electron transport and chlororespiratory pathways. As a consequence, photosynthetic control over plastoquinol oxidation at the cytochrome b6f complex will restrict overreduction of PSI and tend to build electron pressure in PSII (Joliot and Johnson, 2011). Increased photosynthetic control will not only promote energy dissipation in PSII but will also tend to increase the likelihood of singlet oxygen production (Fig. 1B, site 3). Unless uncoupling mechanisms exist in the thylakoid electron transport chain, sustained high rates of the water-water cycle are not possible without an additional sink for ATP. Although leaf ATP contents have been reported to decrease during drought stress, this was ascribed to inhibition of the ATP synthase, rather than uncoupling (Tezara et al., 1999). Consistent with this interpretation, nonphotochemical quenching of chlorophyll fluorescence (NPQ), an indicator of transthylakoid pH difference, increased with progressive decreases in water potential (Tezara et al., 1999). Increases in NPQ during drought have commonly been observed in other studies of intact leaves, providing little evidence of significant uncoupling to allow electron transport-pumped protons to flow back more easily from the lumen to the stroma. Therefore, unlike photorespiration, which consumes both ATP and reductant, any acceleration of the water-water cycle activity may only be transient. Moreover, rather than a simple safety valve to relieve overreduction of PSII, the water-water cycle might be predicted to enhance photosynthetic control, favoring singlet oxygen production unless alternative sinks are present.
OTHER POTENTIAL SOURCES OF ROS DURING DROUGHT
The mitochondrial electron transport chain is another possible source of superoxide and H2O2 during drought. In addition to any drought-induced changes in dark respiration, increases in photorespiration could enhance mitochondrial electron pressure linked to accelerated production of Gly, hence favoring mitochondrial ROS production in the light. Interestingly, the Gly decarboxylase complex can undergo oxidative inactivation and be subject to posttranslational redox modifications including S-glutathionylation and S-nitrosylation of several Cys residues (Taylor et al., 2002; Palmieri et al., 2010), although the physiological impact of these modifications is not yet clear. The ROS-inducible alternative oxidase (AOX) is considered a crucial player in limiting ROS production by the mitochondrial electron transport chain and, possibly, in cellular redox homeostasis in general (Vanlerberghe, 2013). Although rates of photosynthesis are very sensitive to drought, overall respiration rates appear to be less affected (Ribas-Carbo et al., 2005). However, drought induced a shift from the cytochrome oxidase to the AOX pathway, although this was not associated with altered AOX abundance (Ribas-Carbo et al., 2005). Current evidence suggests that deficiency in the AOX pathway enhances drought sensitivity (Giraud et al., 2008; Wang and Vanlerberghe, 2013).
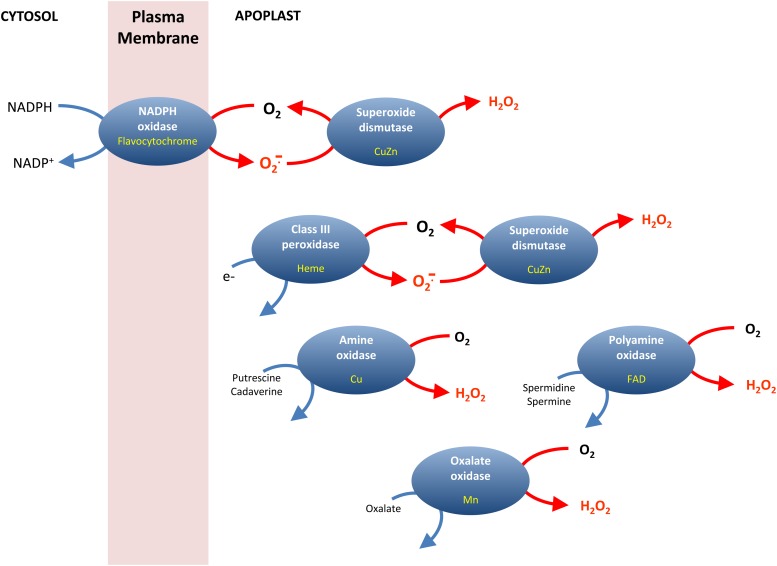
Other than the organelles discussed above, the plasma membrane together with the cell wall and apoplast could make an important contribution to drought-induced ROS production. Adjustments in the cell wall are part of drought responses in many species and involve processes such as both wall loosening and tightening, the latter associated with lignin formation (Moore et al., 2008). Several genes encoding expansins are among genes that are up-regulated at an early stage or in response to moderate drought (Harb et al., 2010). Some of these adjustments in cell wall structure may involve ROS and thus one or more of several types of enzymes that are localized at the cell surface or apoplast and that use different reductants and cofactors to produce either superoxide or H2O2 (Fig. 2). These include NADPH oxidases, amine oxidases, polyamine oxidases, oxalate oxidases, and a large family of class III heme peroxidases (Moschou et al., 2008; Angelini et al., 2010; Marino et al., 2012; O’Brien et al., 2012). Class III heme peroxidases may either use H2O2 to oxidize apoplastic substrates or reductants to produce superoxide from O2 (O’Brien et al., 2012). Although most of these ROS-producing enzymes have been implicated in pathogenesis responses (Zhou et al., 1998; Torres et al., 2006; Angelini et al., 2010; O’Brien et al., 2012), the roles of many of them in drought are less clear. However, enzymes such as oxalate oxidases may play important roles in the acclimation of root growth to drought (Voothuluru et al., 2011).
Figure 2.
Multiple ROS-producing enzymes at the cell surface/exterior. Enzymes are shown in blue and their redox cofactors are indicated in yellow. Class III peroxidases may accept electrons from several types of compounds to generate superoxide, but in many cases their physiological reductant is not established (O’Brien et al., 2012). [See online article for color version of this figure.]
Specific NADPH oxidases play indispensable roles in stomatal regulation in response to stress-induced hormones such as ABA (Kwak et al., 2003). Increasing attention has recently focused on the central role of NADPH oxidases in stress-induced systemic signaling involving fast waves of ROS production at the cell surface (Miller et al., 2009; Steinhorst and Kudla, 2013). It is not yet clear whether and how such systemic cell-to-cell signaling interacts with ROS-linked processes at the local intracellular level. A key difference could be the timescale, with intracellular events being the result of more prolonged, sustained ROS production. Another important point is that the apoplast lacks much of the antioxidative machinery of the cell interior. It is therefore a relatively oxidized compartment that tolerates, or indeed requires, strong oxidants such as the hydroxyl radical for important reactions in cell wall synthesis and expansion (Müller et al., 2009). In addition, the plasma membrane is rich in stress perception proteins such as receptor-like kinases that fulfill important roles in drought tolerance and cell wall function (Steinwand and Kieber, 2010; Marshall et al., 2012). Many of these are regulated at the level of expression by changes in the cell redox state (Munné-Bosch et al., 2013).
ANTIOXIDATIVE DEFENSES AND REDOX HOMEOSTATIC MECHANISMS IN DROUGHT
The predominant mechanisms for the removal of superoxide and H2O2 are enzyme-catalyzed reactions, which are in some cases linked to low Mr antioxidants such as ascorbate and reduced glutathione (GSH). Within the antioxidant network, catalases (CATs) and ascorbate peroxidases (APXs), are the main enzymes involved in H2O2 removal. In parallel, glutathione peroxidases (GPXs; Iqbal et al., 2006), glutathione S-transferases (GSTs; Dixon et al., 2009), and peroxiredoxins (PRXs; Dietz, 2011) reduce H2O2 and organic hydroperoxides by ascorbate-independent thiol-mediated pathways. The nucleophile in these reactions is usually GSH or thioredoxin (TRX).
Many studies have reported effects of drought on the expression and activities of the major antioxidant enzymes such as superoxide dismutase (SOD), CAT, APX, and so forth. This has involved two main types of approaches: Expression and/or activities are either measured after drought in comparison with well-watered controls, or they are compared between plants that have differing drought sensitivity. A summary of data on total extractable enzyme activities provides little evidence for a striking, consistent response between different plant species, but the reported effects generally involve unchanged or increased activities (de Carvalho, 2013). In terms of extractable activities, the information provided by such analyses is potentially useful to understand the physiology underlying the response and/or as a marker in breeding programs. However, the conclusions that can be drawn from such analyses are complicated by several factors, notably the existence of multiple isoforms for these enzymes as well as the now-evident complexity of plant antioxidative systems, meaning that only a partial picture of ROS metabolism is obtained. A recent proteomics study of wheat (Triticum aestivum) cultivars with different drought tolerance reported that several antioxidative enzymes showed altered abundance in response to drought (Ford et al., 2011). Enhanced CAT accumulation was the clearest response, although no marked difference was observed between the three cultivars (Ford et al., 2011). Because CAT is predominantly located in peroxisomes, sufficient activity of this enzyme may be particularly important to metabolize photorespiratory H2O2, which, as noted above, can be produced at higher rates when water becomes limiting.
A transcriptomics-based study of Arabidopsis (Arabidopsis thaliana) exposed to mild drought stress provided little evidence for an acute, generalized response of antioxidative systems (Harb et al., 2010). To investigate this question in detail, we defined 406 Arabidopsis genes encoding core antioxidative and redox homeostatic components (Supplemental Table S1A). Although even this set of genes does not represent a comprehensive survey of proteins potentially involved in ROS-linked metabolism or repair, our analysis included many of the recognized antioxidative and associated reductant-regenerating enzymes, as well as ROS sources such as those shown in Figure 2. Genes were assigned loosely into classes according to their likely or known substrates/products; in some cases, these categories are difficult to assign unambiguously (e.g. for some glutaredoxins [GRXs] or GRX-linked PRX, SOD, and dehydroascorbate reductase). Of these genes, 302 were represented by probes on the most commonly used microarray chip (ATH1) and therefore Genevestigator data were available on their expression in response to drought and associated stresses (Supplemental Table S1B). The available relevant data sets were as follows: two recent drought experiments, osmotic stress, salt, and ABA treatment. Details of the design of these transcriptomic experiments are given in Supplemental Table S2.
Identification of redox-linked genes that are responsive to drought reveals that many antioxidative and redox homeostatic systems respond; however, within each class, the response is both complex and specific. Most notably, there is no general up-regulation of either ROS-producing enzymes or antioxidative and redox homeostatic pathways (Table I). In all categories, as many genes are repressed as are induced.
Table I. Response of redox-associated genes to drought in Arabidopsis.
Genevestigator data were used from experiments described in Supplemental Table S2. A full list of data and gene identities are given in Supplemental Table S1. The percentage of hits is calculated as the number of responsive genes as a percentage of the maximum possible number of responsive genes in the two experiments (e.g. for ROS sources = 100 × 17/80).
| Category | Genes | Drought 1 |
Drought 2 |
Total Hits | Hits | ||
|---|---|---|---|---|---|---|---|
| Repressed >2-fold | Induced >2-fold | Repressed >2-fold | Induced >2-fold | ||||
| n | % | ||||||
| ROS sources | 40 | 7 | 4 | 5 | 1 | 17 | 21 |
| CAT | 3 | 0 | 0 | 0 | 1 | 1 | 17 |
| Ascorbate metabolism | 37 | 4 | 2 | 5 | 3 | 14 | 19 |
| Glutathione metabolism | 106 | 12 | 4 | 13 | 16 | 45 | 21 |
| Thioredoxin-linked | 96 | 4 | 5 | 8 | 4 | 21 | 11 |
| NADPH generation | 20 | 1 | 1 | 3 | 3 | 8 | 20 |
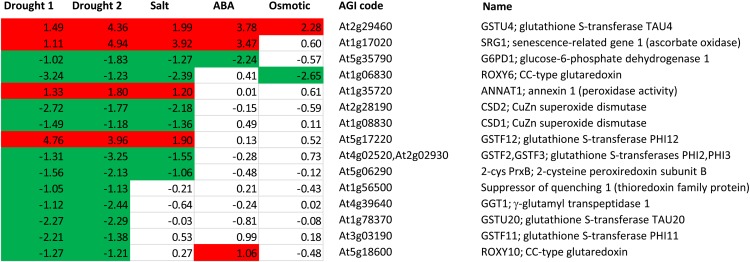
Fifteen genes were found to respond in the same direction at the >2-fold level in both drought experiments, and some of these genes also responded to ABA, salt, or osmotic stress (Fig. 3; Supplemental Table S1C). The only potential ROS-producing enzyme that was induced in both drought data sets was annexin1, which is known to have peroxidase activity. None of the 10 recognized Arabidopsis thaliana Respiratory burst oxidase homolog-encoded NADPH oxidases responded at the 2-fold level in any treatment. Similarly, the abundance of transcripts for the major Arabidopsis leaf CAT2 was not affected. Two genes encoding cytosolic and chloroplastic SODs were repressed in both drought experiments, as well as by salt treatment (Fig. 3). The clearest response of genes involved in ascorbate metabolism was Senescence-Related Gene1, encoding an ascorbate oxidase, which was induced in the drought experiments and by ABA and salt (Fig. 3). Repression of transcripts for the major leaf chloroplast Glc-6-P dehydrogenase1 is possibly consistent with an increased NADPH:NADP+ ratio after adjustments of photosynthetic metabolism during drought (Fig. 1), although the encoded protein is also addressed to the peroxisomes (Meyer et al., 2011). Two TRX-linked genes were also repressed, including a chloroplast 2-Cys-PRX subunit and suppressor of quenching1, a thylakoid-located TRX-like protein involved in regulating NPQ (Brooks et al., 2013). Responsive glutathione-associated genes included two CC-type GRXs, some of which are known to interact with transcription factors (Zander et al., 2012), a γ-glutamyl transpeptidase1 involved in degradation of glutathione or glutathione S-conjugates in the apoplast (Ohkama-Ohtsu et al., 2007), and several GSTs. Among the last type, the response of GSTU4 seems particularly noteworthy. This gene was the only redox-associated gene that was consistently induced in response to drought, salt, ABA, and osmotic stress (Fig. 3). The precise nature of the in vivo reactions catalyzed by many GSTs remains uncertain. In many cases, it is not clear whether their primary function is as a conjugase, a peroxidase, or both (Dixon et al., 2009). Purified recombinant GSTU4 shows both types of activity in vitro (Dixon et al., 2009).
Figure 3.
The 15 of the 302 redox-linked genes that respond >2-fold in the same direction in both drought 1 and drought 2 data sets and their response in related conditions. Data extracted from Genevestigator are shown as log2 values compared with controls. Red and green indicate induction and repression. Genes are ordered from the top according to the number of conditions in which they respond. The full list of genes and their expression values is given in Supplemental Table S1. For details of experiments, see Supplemental Table S2. [See online article for color version of this figure.]
In addition to enzymatic H2O2-metabolizing systems, many metabolites may act as antioxidants. Most of these are considered to be sacrificial. This means that they lack recognized, high-capacity systems to regenerate their reduced forms. Hence, their ongoing function requires resynthesis, which is likely to occur at significantly slower rates than regeneration of the classical antioxidants ascorbate or glutathione. Given this, they are not likely to play a major direct role in peroxide metabolism. Their most important function could be as scavengers of the highly reactive hydroxyl radical, which is notably formed by reductive cleavage of H2O2. Attention has been drawn to the roles of compatible solutes in this regard (Smirnoff and Cumbes, 1989). Other metabolites could be important in redox homeostatic pathways that act to enable ongoing NADPH consumption and/or production. For instance, although Pro has long been proposed to accumulate during drought and related stresses as a compatible solute, mutants in both Pro synthesis and degradation were found to show enhanced sensitivity to drought (Sharma et al., 2011). One possibility is that Pro-Glu cycling plays a role in NADP(H) homeostasis (Sharma et al., 2011 and refs. therein).
ANTIOXIDANT SYSTEMS AS REDOX SIGNAL TRANSMITTERS
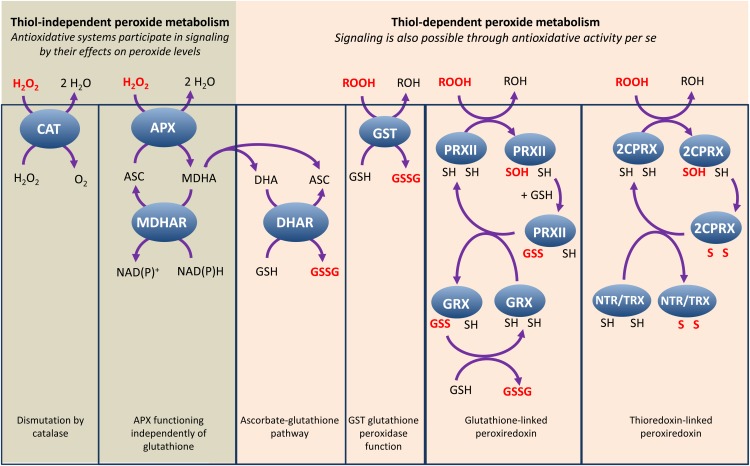
By definition, the primary function of antioxidant defenses is to restrict the accumulation of ROS. It is therefore often considered that ROS can only act in signaling by escaping this removal system. Although it is easy to understand how this can happen at the cell surface or in the case of singlet oxygen produced in the PSII reaction center, it is less clear how H2O2 generated in the aqueous phase of the highly reducing cell interior can work. We consider that one physiologically relevant way to classify the major antioxidative enzymes, which is sometimes overlooked, is according to their dependence or not on reducing cofactors. Such a classification divides the numerous H2O2-removing enzymes into CATs, which dismutate H2O2 to water and O2 through heme-dependent dismutation, and peroxidases, which require reducing cofactors.
Peroxidases are diverse enzymes, but include two major groups: heme-based and thiol-based peroxidases. In plants, the first group includes the best-characterized antioxidative heme-based peroxidase, APX (a class I heme peroxidase), and class III heme peroxidases, which, as noted above, may be as important in ROS generation (Fig. 2) as in ROS consumption. APX reduces H2O2 at the expense of ascorbate, which is then regenerated by monodehydroascorbate reductases and dehydroascorbate reductases using NAD(P)H and GSH, respectively. Thiol-based peroxidases include enzymes that notably use reducing equivalents from TRX- and/or GSH. They include glutathione peroxidases (GPXs, which probably function as TRX-dependent PRXs), PRX, and GSTs (Iqbal et al., 2006; Dixon et al., 2009; Tripathi et al., 2009).
Although many of the details of redox signaling pathways remain unclear, they are likely to involve changes in thiol status. Thus, thiol-based enzymes may have antioxidative and signaling functions through essentially similar chemistry so that their increased engagement in H2O2 reduction can be sensed by the cell (Fig. 4). This could be mediated either by changes in glutathione or TRX redox potentials with repercussions for sensitive target proteins, or by structural changes in the enzymes themselves and knock-on effects on their partners. For heme-based enzymes, even if their activities are greatly increased (for instance, in response to accelerated photorespiration), this increased activity will not necessarily in itself affect cell thiol status. We suggest that enhanced APX activity may only involve signaling if it is linked to thiol oxidation in the ascorbate-glutathione pathway, a question that is still not resolved (Fig. 4; Foyer and Noctor, 2011; Rahantaniaina et al., 2013). Thus, although the main role of heme-based antioxidative enzymes may be to antagonize ROS signaling by decreasing ROS concentrations, increased engagement of thiol enzymes may itself be an integral part of the signals that are generated (Tripathi et al., 2009).
Figure 4.
Peroxide-removing enzymes: roles as antioxidants, in signaling, or both? Cartoon of the best characterized peroxide-metabolizing enzymes in plants. Other mechanisms are possible and for ease of display reactions are not shown stoichiometrically. 2CPRX, 2-cys-PRX; ASC, ascorbate; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase; NTR, NADPH-thioredoxin C; PRXII, PRX type II; ROH, water or organic alcohol; ROOH, H2O2 or organic peroxide; S, sulfur atom in disulfide bond; SH, sulfhydryl (thiol) group; SOH, sulfenic acid group. [See online article for color version of this figure.]
If drought leads to even a small switch between these two types of systems, then ROS signaling could become intrinsically linked to the resulting changes in thiol oxidation (Fig. 4). The effects of such shifts are apparent in CAT-deficient mutants, in which oxidative signaling linked to photorespiration is forced through thiol-based pathways (Mhamdi et al., 2010), causing marked changes in cell thiol-disulfide status that have been shown to be required for increased oxidation to activate signaling through salicylic acid and jasmonic acid pathways (Han et al., 2013a, 2013b). Specific thiol-dependent peroxidases that couple H2O2 reduction to oxidation of a yeast activator protein1 transcription factor were described in yeast (Saccharomyces cerevisiae) several years ago (Delaunay et al., 2002), and a subsequent study reported that an Arabidopsis GPX can function in a similar manner to allow oxidation of components involved in ABA signaling during drought (Miao et al., 2006).
Although Figure 4 places emphasis on thiol-based pathways in redox signaling, we do not discount roles for CAT that are additional to its primary antioxidative function. For instance, evidence from forward genetics screens suggests that this enzyme acts as a ROS-dependent activator of signals involved in cell death or autophagy, possibly through a secondary CAT-peroxidase reaction (Juul et al., 2010; Hackenberg et al., 2013).
The Arabidopsis flu (fluorescent) mutant has been extensively used to study singlet oxygen signaling (Kim and Apel, 2013). The excess singlet oxygen production in this line does not occur within the PSII reaction center, which is probably the most relevant site for electron transport-linked singlet oxygen signaling. However, because of the spatial separation of scavengers from the site of generation, singlet oxygen produced in the reaction center can largely evade removal by cellular antioxidants. Hence, drought-induced increases in singlet oxygen generation in the chloroplasts will facilitate the operation of oxylipin and jasmonate-dependent pathways that underpin further local and systemic responses in gene expression (Kim and Apel, 2013). Recent evidence suggests that oxidation products produced by interactions with carotenoids are also important in singlet oxygen signaling (Ramel et al., 2012).
MEASURABLE CHANGES IN REDOX STATE DURING DROUGHT
Increased expression or activities of major antioxidative enzymes is often taken as an indicator of increases in ROS. One often overlooked problem is the difficulty of quantifying ROS in plants. For instance, nitroblue tetrazolium is frequently used as an in situ indicator of superoxide but such data should be treated with some skepticism, because of problems with specificity and the artifactual generation of superoxide triggered by the presence of nitroblue tetrazolium itself (Fridovich, 1997). Assays of the more stable nonradical ROS, H2O2, are also plagued by potential problems linked to extraction and artifactual effects in the assay (Queval et al., 2008). Although extracellular ROS signaling can occur over long distances (Miller et al., 2009), the extent to which ROS move between compartments within the highly reducing cell interior is unclear. One mechanism is through aquaporins (Henzler and Steudle, 2000; Bienert et al., 2007; Mubarakshina Borisova et al., 2012), so that any drought-triggered effects on aquaporin activity could conceivably affect movement of H2O2. Inversely, water transport through aquaporins may be affected by ROS-triggered gating, caused by direct hydroxyl radical-mediated closure or by more indirect signaling pathways, the latter possibly involving salicylic acid (Ye and Steudle, 2006; Boursiac et al., 2008).
As key antioxidants, ascorbate and glutathione can be used as biochemical markers of general cell redox state. For instance, leaf glutathione status is clearly influenced by intracellular H2O2 availability (Mhamdi et al., 2010). A comprehensive appraisal of the extensive literature on the response of major antioxidants to drought is beyond the scope of this discussion. However, based on our knowledge of this literature and our own experiments (Bartoli et al., 2005), we conclude that these antioxidants remain relatively unaffected by drought until stress-induced senescence is triggered. At this point, the major change is a marked decrease in the abundance of these antioxidants rather than a change in their redox state, although a decrease in ascorbate during seed desiccation is accompanied by an accumulation of oxidized glutathione (GSSG) with total glutathione remaining relatively constant (Colville and Kranner, 2010). In leaves undergoing relatively mild drought, the lack of accumulation of GSSG contrasts with some other stresses, in which glutathione oxidation is evident. In itself, this observation suggests either that oxidative stress plays a relatively minor role during drought or at least that the antioxidative and reductant-generating systems have sufficient capacity to keep ROS-sensitive redox pools largely in the reduced state. Nevertheless, measurements using a redox-sensitive GFP suggest that the cytosol becomes more oxidized during drought (Jubany-Mari et al., 2010). Evidence suggests that these proteins report mainly on the glutathione redox potential (Meyer et al., 2007), suggesting that drought decreases either the total cytosolic glutathione pool or the GSH:GSSG ratio. This indicates a change in thiol-disulfide status that is compatible with a role in oxidative stress signaling.
Although mild drought does not seem to cause a marked change in the status of key antioxidants measured in whole-tissue extracts, shifts between compartments cannot be discounted. A key factor may be subcellular compartmentation of oxidants and of changes in redox state. For instance, increases in cytosolic (and therefore nuclear) glutathione redox potential during drought may be limited by transfer of GSSG to the vacuole or, perhaps, the apoplast, where it can be degraded by γ-glutamyl transpeptidase-dependent pathways. Vacuolar GSSG accumulation is triggered in CAT-deficient plants and probably involves class C ATP-binding cassette (ABCC) transporters (Queval et al., 2011; Noctor et al., 2013). Interestingly, two ABCC proteins (ABCC4 and ABCC5) have been implicated in stomatal regulation (Klein et al., 2004), with the function of ABCC5 linked to transport of inositol hexakisphosphate from the cytosol to the vacuole (Nagy et al., 2009).
SOURCES AND SITES OF ROS IN DROUGHT: INSIGHTS FROM TRANSCRIPTOMIC PATTERNS
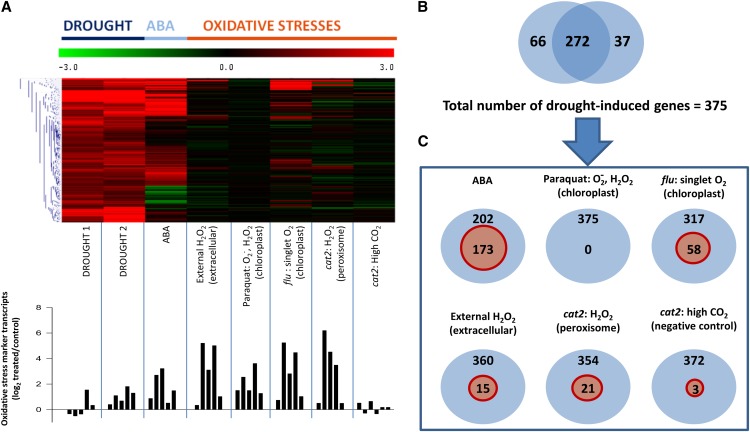
Despite intensive research over many years, key questions remain concerning how important ROS are in drought and which sites contribute to ROS production in these conditions. As discussed above, the ongoing difficulties of accurately quantifying ROS production and accumulation at specific sites contribute to this uncertainty. Transcriptomic signatures may provide useful pointers. To analyze how important ROS-triggered events are in drought responses, we investigated whether oxidative stresses linked to ROS differing in site specificity affected the expression of drought-induced genes. Data sets from two recent studies reporting drought transcriptomes (Bhaskara et al., 2012; Pandey et al., 2013) were chosen that showed considerable overlap (Fig. 5A). Of 375 genes that were induced >2-fold in at least one of the studies, 272 genes were induced to this level in both (Fig. 5B). None of the 103 genes induced in only one experiment showed antagonistic behavior in the other (Supplemental Table S3). The observed overlap suggests that this suite of genes is a reasonably robust indicator of drought-inducible genes in Arabidopsis. Indeed, almost one-half (173) of the 375 genes were induced >2-fold by ABA treatment (Fig. 5).
Figure 5.
Analysis of drought-inducible gene expression in responses to redox perturbation. A, Heatmap of drought-induced genes extracted from Genevestigator and the response of these genes to ABA or oxidative stress (top) and histogram showing expression of oxidative stress marker genes after the different treatments (bottom). Data are shown as log2 values compared with Col-0 (the wild type or untreated). Experimental details are given in Supplemental Table S2. Red and green on the heatmap indicate induction and repression according to the color scale shown at the top. The five genes for which data are shown in the bottom histogram are as follows (left to right): APX1, GSTU24, UGT75B1, UGT73B5, and GPX6 (for values, see Supplemental Table S3C). B, Overlap of induced genes (cutoff, 2-fold) in the two drought experiments; 375 genes were induced >2-fold in at least one of the experiments (Supplemental Table S3A). C, The number of these 375 genes that were induced >2-fold by the different oxidative stresses (indicated in red circles within the outer blue circles). Col-0, Ecotype Columbia 0 of Arabidopsis. [See online article for color version of this figure.]
To examine the potential influence of different ROS in the drought response, we analyzed how many of the drought-induced genes were induced by different oxidative stress conditions. The responses of the drought-induced genes to different oxidative stresses were extracted from Genevestigator (Hruz et al., 2008). Data were available for externally supplied H2O2 (Davletova et al., 2005), paraquat (which mainly stimulates light-dependent production of superoxide and H2O2 in the chloroplast), the flu mutant (excess singlet oxygen production in the chloroplast; Laloi et al., 2007), and the photorespiratory cat2 mutant (excess H2O2 in the peroxisomes; Queval et al., 2012). It cannot be excluded that differences observed between the treatments are partly caused by developmental stage, treatment time, stress intensity, or another factor (details of conditions used for the different data sets are given in Supplemental Table S2). Nevertheless, an examination of known oxidative stress-responsive genes suggests that ROS signaling was active in all of the conditions (Fig. 5A, bottom frame). Five genes encoding two antioxidative enzymes that are up-regulated by oxidative stress and three highly ROS-inducible genes showed a fairly similar expression pattern in response to the different treatments (Fig. 5A). The five oxidative stress marker genes showed some induction by drought and, particularly, ABA treatment (Fig. 5A, bottom frame).
Analysis of the sensitivity of the 375 drought-induced genes to different oxidative stresses (Supplemental Table S3, A and B) revealed the following. First, paraquat did not induce any of these genes to the 2-fold level. Second, approximately 15% of them were induced by the flu mutation. Finally, external H2O2 and CAT deficiency (cat2) induced a lower number, although the values were higher than the number induced in cat2 at high CO2 compared with the wild type at high CO2 (Fig. 5; Supplemental Table S3). The last condition abolishes the main source of oxidative stress in cat2 (photorespiration), and can therefore be considered a negative control. Hence, the comparison indicates that the number of drought-induced genes induced in cat2 in air, by H2O2, or, especially, in the flu mutant are of physiological significance. Of the drought-associated genes that were induced by these three oxidative stresses, 57% to 72% were also induced by ABA (Supplemental Table S3B). These percentages are higher than the overall proportion of the drought-induced genes that were induced by ABA (173 of 375; 46%). This is consistent with a close relationship between oxidative stress and ABA in ROS-dependent drought responses.
A striking implication of the analysis shown in Figure 5 is that of the ROS produced in the chloroplast, superoxide and H2O2 generated as part of the water-water cycle (Fig. 1B, site 2) contribute much less to drought-induced gene expression than singlet oxygen (Fig. 1B, site 3). Moreover, the data suggest that the source of H2O2 leading to drought-induced gene expression could be peroxisomal photorespiratory metabolism (Fig. 1B, site 1) or apoplastic metabolism (Fig. 2). Drought-responsive genes that were induced in flu and cat2, or by treatment with H2O2 but not paraquat, notably include ANAC (for Arabidopsis NAM, ATAF1, ATAF2, CUC2) and DREB (for Dehydration Response Element Binding protein) transcription factors, UGT73b5 (for UDP-glycosyl transferase 73b5), and GSTU4 (Supplemental Table S3A). Several ANACs are induced by photorespiratory H2O2, and analysis of the subcellular compartmentation of five of them after oxidative stress suggests that ANAC032 is the only one exclusively located in the nucleus (Inzé et al., 2012). DREB2A is induced by a range of abiotic stresses, and interacts with JUNGBRUNNEN1, another ROS-responsive NAC transcription factor, to regulate senescence and longevity (Wu et al., 2012). UGT73B5 has been implicated in redox regulation linked to pathogenesis, although its substrate remains to be definitively identified (Simon et al., 2013). As noted above, GSTU4 is the only one of the 302 redox-associated genes that is clearly induced by drought and all of the related stresses analyzed in this study (Fig. 3). Although both conjugase and peroxidase activities have been detected in the recombinant protein (Dixon et al., 2009), the physiological function of GSTU4 is not yet known.
CONCLUSION AND PERSPECTIVES
Chloroplast production of ROS has long been proposed as a major driver of redox signaling or damage during drought. Bearing in mind the constraints of experiments performed under controlled conditions, which may differ considerably from the natural environment, analysis of transcriptomic data sets supports this view while raising one important point with respect to the regulation of electron transport. Any overreduction of the chloroplast stroma (e.g. increased NADPH:NADP+ ratios) should favor patterns of gene expression that are more akin to the singlet oxygen profile rather than that generated by paraquat. That drought effects on the transcriptome are most closely mimicked by singlet oxygen and photorespiratory H2O2 is consistent with (1) current views of photosynthetic control (Joliot and Johnson, 2011), (2) the importance ascribed to singlet oxygen as the most active ROS produced in chloroplasts (Triantaphylidès et al., 2008), (3) conclusions that the Mehler reaction is a minor alternative pathway compared with photorespiration during drought (Cornic and Briantais, 1991), and (4) the likely capacities of H2O2 generation at the two sites (Noctor et al., 2002). Extracellular H2O2 may be particularly important in systemic long-distance signaling (Miller et al., 2009).
Although transcriptomic data can provide useful signposts, further analyses are required to confirm the robustness of the effects shown in Figure 5. A key point is the time dependence of ROS signaling, which is likely to be a highly dynamic process. Another issue is that although the past decade has witnessed an ever-growing focus on programmed production of ROS, the major sources of ROS in drought may be pathways whose main physiological function is not to generate ROS. Gene expression is only one of many levels at which drought may influence ROS production. It would be very interesting to compare overlap between drought- and ROS-induced changes at different levels of regulation, from the transcriptome through the proteome to the metabolome and fluxome. Such analyses would undoubtedly identify novel and probably unforeseen interactions.
Given that drought seems to cause less evident changes in cell redox state than some other stresses (e.g. many biotic stresses), decreased water availability may involve a progressive, rather than an acute, oxidative stress. Nonetheless, based on the centrality of ROS in stress, and the view that their effects are mainly negative, overexpression of single enzymes associated with the complex antioxidative system continues to receive attention as a strategy to ameliorate stress resistance in plants. Indeed, encouraging results of such manipulations on tolerance to drought and related stresses continue to be published, although we are not aware of any plants that have yet been introduced into the field for commercial purposes.
The obvious outstanding issue in research on ROS remains: How are ROS perceived by the cell? Redox proteomics are likely to be key approaches here. Cys S-glutathionylation and S-nitrosylation are just two of the possible reversible oxidative modifications that may be involved in redox signaling during drought (Colville and Kranner, 2010). Based on reports even for single proteins (Palmieri et al., 2010), such approaches are likely to uncover a complex web of modifications. There is a need for accurate quantification of redox modifications within the cellular environment, given that few of them are likely to be all of nothing, especially during relatively mild oxidative stresses. It remains to be seen how many modifications are mediated by ROS that escape the antioxidative system and how many are mediated via oxidant-triggered modulation of the status of thiol-dependent antioxidative/signaling systems (Fig. 4).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Drought response of redox-associated genes.
Supplemental Table S2. Details of studies from which the expression data shown in the figures and other supplemental tables were obtained.
Supplemental Table S3. Expression levels of drought-induced genes in different conditions of oxidative stress.
Supplementary Material
Glossary
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- ABA
abscisic acid
- RuBP
ribulose-1,5-bisphosphate
- NPQ
nonphotochemical quenching of chlorophyll fluorescence
- AOX
alternative oxidase
- GSH
reduced glutathione
- APX
ascorbate peroxidase
- GPX
glutathione peroxidase
- GST
glutathione S-transferase
- PRX
peroxiredoxin
- TRX
thioredoxin
- SOD
superoxide dismutase
- GRX
glutaredoxin
- GSSG
oxidized glutathione
- ABCC
class C ATP-binding cassette
Footnotes
This work was supported by the French Agence Nationale de le Recherche Cynthiol project (Orsay Laboratory, project no. ANR12–BSV6–0011).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P. (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18: 656–660 [DOI] [PubMed] [Google Scholar]
- Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A. (2010) Plant amine oxidases “on the move”: an update. Plant Physiol Biochem 48: 560–564 [DOI] [PubMed] [Google Scholar]
- Asada K. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141: 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Guaimet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou F, Foyer CH. (2005) Ascorbate content of wheat leaves is not determined by maximal l-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28: 1073–1081 [Google Scholar]
- Bhaskara GB, Nguyen TT, Verslues PE. (2012) Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160: 379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C. (2008) Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56: 207–218 [DOI] [PubMed] [Google Scholar]
- Brooks MD, Sylak-Glassman EJ, Fleming GR, Niyogi KK. (2013) A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proc Natl Acad Sci USA 110: E2733–E2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys H, Inzé D. (2013) The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol 162: 1768–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville L, Kranner I. (2010) Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul 62: 241–255 [Google Scholar]
- Cornic G, Briantais J-M. (1991) Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183: 178–184 [DOI] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R. (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho MHC. (2013) Drought stress and reactive oxygen species. Production, scavenging and signalling. Plant Signal Behav 3: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. (2002) A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111: 471–481 [DOI] [PubMed] [Google Scholar]
- Dietz KJ. (2011) Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal 15: 1129–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R. (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BB, Hideg E, Krieger-Liszkay A. (2013) Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid Redox Signal 18: 2145–2162 [DOI] [PubMed] [Google Scholar]
- Ford KL, Cassin A, Bacic A. (2011) Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance. Front Plant Sci 2: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60: 455–484 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119: 355–364 [Google Scholar]
- Foyer CH, Noctor G. (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. (1997) Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem 272: 18515–18517 [DOI] [PubMed] [Google Scholar]
- Gao X, Yuan HM, Hu YQ, Li J, Lu YT. (2014) Mutation of Arabidopsis CATALASE2 results in hyponastic leaves by changes of auxin levels. Plant Cell Environ 37: 175–188 [DOI] [PubMed] [Google Scholar]
- Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147: 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg T, Juul T, Auzina A, Gwizdz S, Malolepszy A, Van Der Kelen K, Dam S, Bressendorff S, Lorentzen A, Roepstorff P, et al. (2013) Catalase and NO CATALASE ACTIVITY1 promote autophagy-dependent cell death in Arabidopsis. Plant Cell 25: 4616–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G. (2013b) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal 18: 2106–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Mhamdi A, Chaouch S, Noctor G. (2013a) Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ 36: 1135–1146 [DOI] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MMR, Pereira A. (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154: 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler T, Steudle E. (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: Model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51: 2053–2066 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé A, Vanderauwera S, Hoeberichts FA, Vandorpe M, Van Gaever T, Van Breusegem F. (2012) A subcellular localization compendium of hydrogen peroxide-induced proteins. Plant Cell Environ 35: 308–320 [DOI] [PubMed] [Google Scholar]
- Iqbal A, Yabuta Y, Takeda T, Nakano Y, Shigeoka S. (2006) Hydroperoxide reduction by thioredoxin-specific glutathione peroxidase isoenzymes of Arabidopsis thaliana. FEBS J 273: 5589–5597 [DOI] [PubMed] [Google Scholar]
- Joliot P, Johnson GN. (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubany-Mari T, Alegre-Batlle L, Jiang K, Feldman LJ. (2010) Use of a redox-sensing GFP (c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Lett 584: 889–897 [DOI] [PubMed] [Google Scholar]
- Juul T, Malolepszy A, Dybkaer K, Kidmose R, Rasmussen JT, Andersen GR, Johnsen HE, Jørgensen JE, Andersen SU. (2010) The in vivo toxicity of hydroxyurea depends on its direct target catalase. J Biol Chem 285: 21411–21415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Apel K. (2013) Singlet oxygen-mediated signaling in plants: Moving from flu to wild type reveals an increasing complexity. Photosynth Res 116: 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Geisler M, Suh SJ, Kolukisaoglu HU, Azevedo L, Plaza S, Curtis MD, Richter A, Weder B, Schulz B, et al. (2004) Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J 39: 219–236 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Marquez-Garcia B, Njo M, Beeckman T, Goormachtig S, Foyer CH. (2014) A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ 37: 488–498 [DOI] [PubMed] [Google Scholar]
- Marshall A, Aalen RB, Audenaert D, Beeckman T, Broadley MR, Butenko MA, Caño-Delgado AI, de Vries S, Dresselhaus T, Felix G, et al. (2012) Tackling drought stress: Receptor-like kinases present new approaches. Plant Cell 24: 2262–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Meyer T, Hölscher C, Schwöppe C, von Schaewen A. (2011) Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant J 66: 745–758 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G. (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197–4220 [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A. (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol Plant 134: 237–245 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA. (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20: 1708–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarakshina Borisova MM, Kozuleva MA, Rudenko NN, Naydov IA, Klenina IB, Ivanov BN. (2012) Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim Biophys Acta 1817: 1314–1321 [DOI] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH. (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy R, Grob H, Weder B, Green P, Klein M, Frelet-Barrand A, Schjoerring JK, Brearley CA, Martinoia E. (2009) The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J Biol Chem 284: 33614–33622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. (2000) Homeostasis of adenylate status during photosynthesis in a fluctuating environment. J Exp Bot 51: 347–356 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Queval G, Foyer CH. (2013) Regulating the redox gatekeeper: vacuolar sequestration puts glutathione disulfide in its place. Plant Physiol 163: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic SD, Driscoll S, Novitskaya L, Foyer CH. (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot (Lond) 89: 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Daudi A, Butt VS, Bolwell GP. (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236: 765–779 [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Radwan S, Peterson A, Zhao P, Badr AF, Xiang C, Oliver DJ. (2007) Characterization of the extracellular γ-glutamyl transpeptidases, GGT1 and GGT2, in Arabidopsis. Plant J 49: 865–877 [DOI] [PubMed] [Google Scholar]
- Osmond CB, Grace SC. (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46: 1351–1362 [Google Scholar]
- Palmieri MC, Lindermayr C, Bauwe H, Steinhauser C, Durner J. (2010) Regulation of plant glycine decarboxylase by S-nitrosylation and glutathionylation. Plant Physiol 152: 1514–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N, Ranjan A, Pant P, Tripathi RK, Ateek F, Pandey HP, Patre UV, Sawant SV. (2013) CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genomics 14: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Kodaira KS, Maruyama K, Mizoi J, Tran LS, Fujita Y, Morimoto K, Shinozaki K, Yamaguchi-Shinozaki K. (2011) SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant Physiol 157: 1900–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Hager J, Gakière B, Noctor G. (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J Exp Bot 59: 135–146 [DOI] [PubMed] [Google Scholar]
- Queval G, Jaillard D, Zechmann B, Noctor G. (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34: 21–32 [DOI] [PubMed] [Google Scholar]
- Queval G, Neukermans J, Vanderauwera S, Van Breusegem F, Noctor G. (2012) Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ 35: 374–387 [DOI] [PubMed] [Google Scholar]
- Rahantaniaina MS, Tuzet A, Mhamdi A, Noctor G. (2013) Missing links in understanding redox signaling via thiol/disulfide modulation: How is glutathione oxidized in plants? Front Plant Sci 4: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M. (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, Lambers H, Medrano H, Berry JA, Flexas J. (2005) Effects of water stress on respiration in soybean leaves. Plant Physiol 139: 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Villamor JG, Verslues PE. (2011) Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157: 292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Langlois-Meurinne M, Didierlaurent L, Chaouch S, Bellvert F, Massoud K, Garmier M, Thareau V, Comte G, Noctor G, et al. (November 24, 2013) The secondary metabolism glycosyltransferases UGT73B3 and UGT73B5 are components of redox status in resistance of Arabidopsis to Pseudomonas syringae pv. tomato. Plant Cell Environ http://dx.doi.org/10.1111/pce.12221 [DOI] [PubMed] [Google Scholar]
- Smirnoff N. (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125: 27–58 [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060 [Google Scholar]
- Steinhorst L, Kudla J. (2013) Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol 163: 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwand BJ, Kieber JJ. (2010) The role of receptor-like kinases in regulating cell wall function. Plant Physiol 153: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Millar AH. (2002) Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 277: 42663–42668 [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917 [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, De Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, et al. (2010) Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22: 2660–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi BN, Bhatt I, Dietz KJ. (2009) Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 235: 3–15 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC. (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14: 6805–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voothuluru P, Yamaguchi M, Zhu J, Cho IJ, Oliver MJ, Simmonds J, Sharp RE (2011) Cell wall proteomics and apoplastic ROS: novel insights into root growth adaptation to water stress [abstract no. P13018]. In 2011 Annual Meeting of the American Society of Plant Biologists, August 6–10, 2011, Minneapolis, MN. American Society of Plant Biologists, Rockville, MD, p 518 [Google Scholar]
- Wang J, Vanlerberghe GC. (2013) A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol Plant 149: 461–473 [DOI] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Steudle E. (2006) Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ 29: 459–470 [DOI] [PubMed] [Google Scholar]
- Zander M, Chen S, Imkampe J, Thurow C, Gatz C. (2012) Repression of the Arabidopsis thaliana jasmonic acid/ethylene-induced defense pathway by TGA-interacting glutaredoxins depends on their C-terminal ALWL motif. Mol Plant 5: 831–840 [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, de Neergaard E, Collinge DB, Thordal-Christensen H. (1998) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol 117: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.